Abstract
Secreted proteins may serve as major targets in the immune response to mycobacteria. To identify potentially secreted Mycobacterium leprae antigens, antisera specific for culture filtrate proteins of Mycobacterium tuberculosis were used to screen a panel of recombinant antigens selected previously by leprosy patient sera. Four potentially secreted antigens were identified by this approach, and one was recognized by antibodies specific for MPT32, a secreted M. tuberculosis protein. The DNA coding for the M. leprae antigen, which we have designated 43L, was isolated and characterized and found to encode a 25.5-kDa protein that is preceded by a consensus signal peptide of 39 amino acids. The N-terminal amino acid sequence of 43L shows 50% homology with the 20 known N-terminal amino acids of MPT32, and 47% homology was found with the N terminus of a 45/47-kDa antigen complex identified in Mycobacterium bovis BCG. These findings indicate that 43L represents an antigen related to MPT32 and the M. bovis BCG 45/47-kDa complex and that 43L is likely to be a protein secreted by M. leprae. Purified recombinant 43L protein is recognized by antibodies and T cells from healthy contacts and leprosy patients, illustrating that secreted proteins are of importance in the immune response to M. leprae.
Full text
PDF

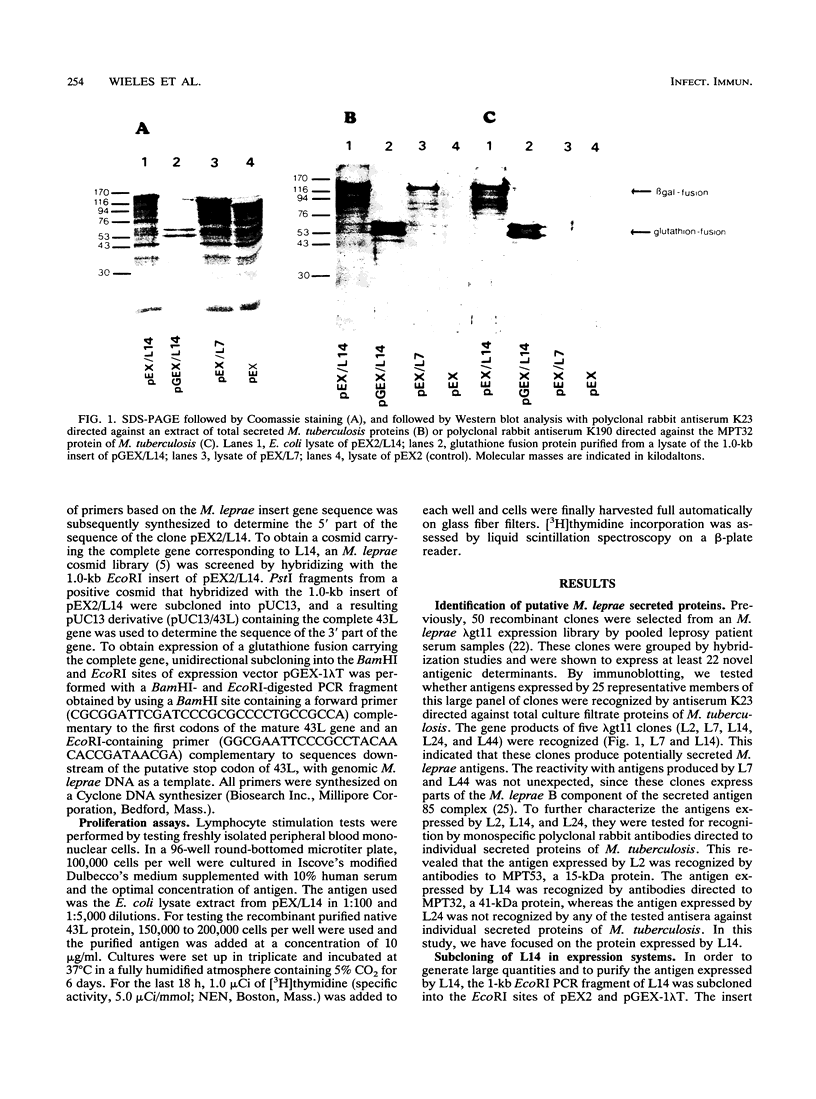
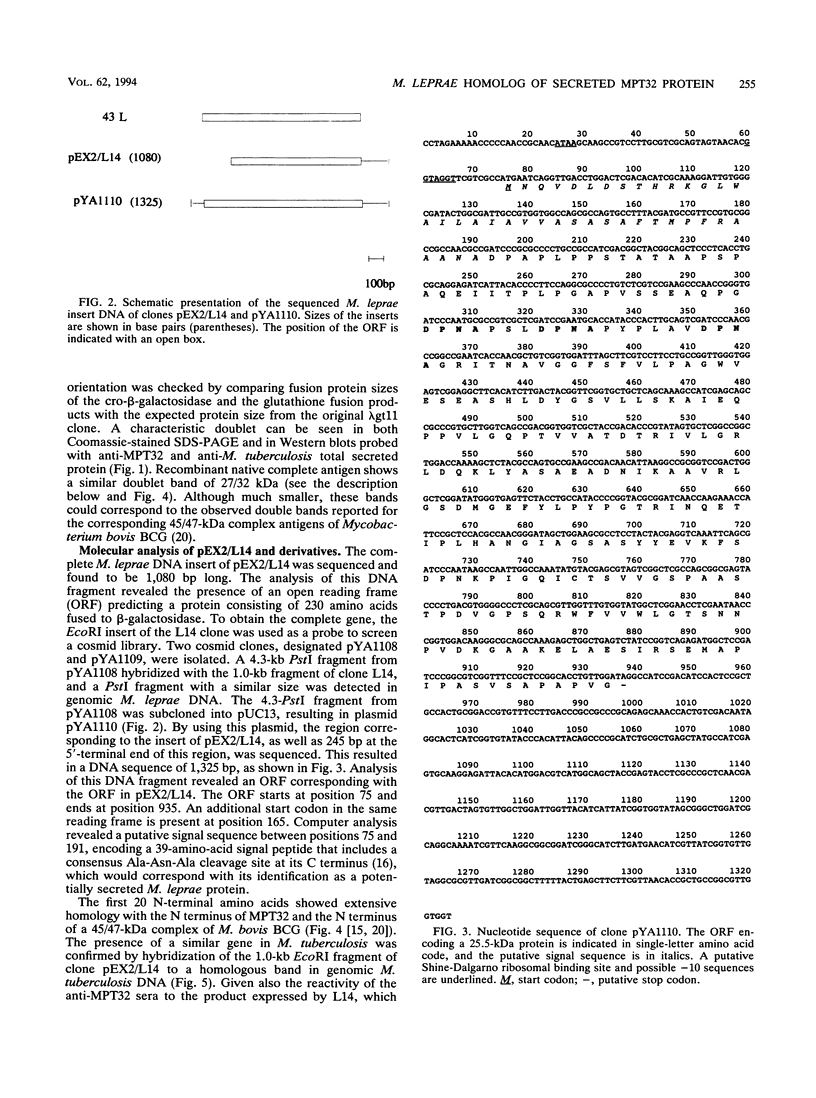


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993 Mar;61(3):844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe T., Harris D., Vordermeier M., Lathigra R., Ivanyi J., Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993 Jan;61(1):260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Launois P., Huygen K., De Bruyn J., N'Diaye M., Diouf B., Sarthouj L., Grimaud J., Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991 Nov;86(2):286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Bajardi A. C., Grisso C. L., Sieling P. A., Alland D., Convit J., Fan X. D., Hunter S. W., Brennan P. J. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992 Jan 1;175(1):275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Pal P. G., Horwitz M. A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992 Nov;60(11):4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain F., Laqueyrerie A., Militzer P., Pescher P., Chavarot P., Lagranderie M., Auregan G., Gheorghiu M., Marchal G. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993 Feb;61(2):742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Esser R. E., Thole J. E., Clark-Curtiss J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect Immun. 1990 May;58(5):1327–1336. doi: 10.1128/iai.58.5.1327-1336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]