Abstract
Objective
To confirm the overall benefit of drug eluting stents (DES), to evaluate the effect of different DES, and to assess the global safety of DES compared with bare stents through a meta‐analysis of randomised controlled trials.
Methods
Randomised controlled trials comparing sirolimus and derivates or paclitaxel and derivates eluting stents versus bare stents. Binary restenosis and major adverse cardiac events (MACE) were chosen as primary end points. Death, Q wave myocardial infarction (MI), and stent thrombosis up to 12 months' follow up were also analysed.
Results
MACE overall occurrence was highly reduced with DES from 19.9% to 10.1% (odds ratio (OR) 0.46, 95% confidence interval (CI) 0.41 to 0.52, p < 0.001). A significant heterogeneity (p < 0.001) was found between subgroups according to the drug: MACE OR was 0.28 (95% CI 0.22 to 0.35) in the sirolimus subgroup and 0.62 (95% CI 0.53 to 0.73) in the paclitaxel subgroup. Restenosis was also highly reduced from 31.7% with bare stents to 10.5% with DES (OR 0.25, 95% CI 0.22 to 0.29, p < 0.001) with a similar heterogeneity between subgroups. Mortality, Q wave MI, and stent thrombosis were not significantly different between DES and control group, whereas Q wave MI and stent thrombosis tended to be more frequent with paclitaxel.
Conclusion
This meta‐analysis confirms the overall benefit of DES on restenosis and MACE with significant heterogeneity between drugs, suggesting higher efficacy of sirolimus eluting stents. Additional data with longer follow up and in high risk populations are needed to clarify issues on stent thrombosis.
Keywords: drug eluting stents, meta‐analysis, coronary stenting, sirolimus, paclitaxel
Since the advent of coronary balloon angioplasty, prevention of restenosis has been the unfulfilled objective of many studies. Despite the impact of stent implantation on angiographic and clinical outcomes, in‐stent restenosis has remained the major limitation of catheter based intervention.1,2,3,4
Recently, drug eluting stents (DES) have been hailed as the new and only effective means to prevent restenosis. Two DES have proved effective in large randomised trials: the sirolimus eluting stent Cypher (Cordis/Johnson & Johnson, Miami Lakes, Florida, USA) and the polymer based paclitaxel eluting stent Taxus (NIRx; Boston Scientific Corp, Natick, Massachusetts, USA). However, concerns have also been raised about the safety of these DES, leading to a US Food and Drug Administration (FDA) advisory notice and strong debates in the interventional cardiology community.5,6 By aggregating all controlled trials we sought to evaluate the global effect of DES on major adverse cardiac events (MACE) and restenosis and the specific effect of paclitaxel (and analogues) and sirolimus (and analogues) eluting stents. This meta‐analysis was intended also to determine whether DES increased stent thrombosis and hard clinical events, knowing the limitations of such a meta‐analysis.
METHODS
Trials searching
We reviewed randomised trials comparing paclitaxel or sirolimus eluting stents (and analogues of both drugs) with bare metal stents. We searched Medline from January 1996 up to September 2005 and the Cochrane controlled trials register. The key words used were “drug eluting stents”, “sirolimus”, “everolimus”, “rapamycin”, “paclitaxel”, “taxane”, “taxol”, and “clinical trial”. In addition, we identified relevant abstracts and presentations at the annual meetings of the American Heart Association, the American College of Cardiology, the European Society of Cardiology, and Transcatheter Cardiovascular Therapeutics from January 2000 to September 2005. Expert slide presentations were consulted on line from tctmd.com to complete data from abstracts. Internet based sources of information on the results for clinical trials in cardiology (http://www.theheart.org and http://www. tctmd.com) were also searched. Lastly we manually searched the reference sections of each retrieved article.
Inclusion criteria and outcome measurement
Paclitaxel is an antimicrotubular agent and was first developed as an antineoplastic agent. We pooled in the same subgroup polymer or non‐polymer based paclitaxel as well as taxane eluting stents.7,8 Sirolimus is an immunosuppressant macrolide antibiotic first used to prevent allograft rejection. We pooled in this second subgroup sirolimus, everolimus, and biolimus (ABT 578) eluting stents.9,10
For this meta‐analysis, studies were selected if sirolimus or paclitaxel derivatives coated stents were randomly assigned versus bare stent, with at least six months' follow up duration. To avoid bias of non‐publication in this analysis we decided to include at first analogues of both sirolimus and paclitaxel in each related group.
In studies in which doses or kinetics of drug delivery from the stent differed, the subgroups were pooled together in a first step analysis. In a second step analysis we excluded analogues to compare only sirolimus eluting stent (Cypher) with polymer based paclitaxel eluting stent (Taxus).
Two reviewers (CR and PSP) independently extracted the data. Disagreements were resolved by consensus.
The two primary end points of this meta‐analysis were angiographic binary restenosis (restenosis > 50% of the luminal diameter) at 6–9 months of follow up and MACE, which was a composite of death, myocardial infarction (MI), and revascularisation. Table 1 reports definitions and characteristics of the trials, and table 2 presents the clinical and angiographic characteristics. We also collected the numbers of deaths, Q wave and non‐Q wave MIs, and stent thrombosis in all studies. When information was not available for Q wave MI or stent thrombosis or when Q wave MI was not differentiated from non‐Q wave MI, the studies were not used for these end points. These clinical end points were recorded at between 6–12 months according to each clinical trial follow up. Data were extracted in duplicate. We recalculated absolute numbers when percentages were reported.
Table 1 Trials included in the meta‐analysis.
| Study and reference | Drug | Follow up | Antiplatelet treatment* | Definition of percutaneous revascularisation† | Angiographic binary restenosis definition | Death |
|---|---|---|---|---|---|---|
| Trials in the sirolimus (and analogues) subgroup | ||||||
| RAVEL38 | Sirolimus | 1 year | Clopidogrel or ticlopidine 8 weeks | TLR: clinically driven or evidence of restenosis | In‐target lesion including proximal and distal edge; at 6 months | Any |
| SIRIUS39 | Sirolimus | 1 year | Clopidogrel 3 months | TLR: clinically driven and restenosis >70% | In segment zone including margins 5 mm distal and proximal; at 8 months | Any |
| E‐SIRIUS40 | Sirolimus | 9 months | Clopidogrel or ticlopidine 2 months | TLR: clinically driven | In lesion including 5 mm proximal and distal edge; at 8 months | Any |
| C‐SIRIUS41,42 | Sirolimus | 1 year | Clopidogrel 2 months | TLR: clinically driven including stenosis >70% | In‐stent lesion; at 8 months | Any |
| FUTURE I/II45 | Everolimus | 6 months | NA | TLR | In segment; at 6 months | Any |
| SES‐SMART59 | Sirolimus | 8 months | Clopidogrel 2 months | TLR | In segment; at 8 months | Any |
| SCANDSTENT44 | Sirolimus | 1 year | NA | TLR | In‐stent lesion; at 6 months | Any |
| DIABETES43 | Sirolimus | 9 months | NA | TLR | In segment; at 9 months | Any |
| ENDEAVOR II10 | Biolimus | 1 year | NA | TVR | Any | |
| Trials in the paclitaxel (and analogues) subgroup | ||||||
| SCORE46 | QP2 is 7‐hexanoyltaxol | 1 year | Ticlopidine or clopidogrel 1–6 months | TLR | In stent; at 6 months | Cardiac death |
| TAXUS I47,48 | Slow release polymer based paclitaxel | 1 year | Clopidogrel 6 months | TVR: indication not reported, only Q wave MI | In‐stent lesion (edges excluded); at 6 months | Any |
| ASPECT52 | Non‐polymer based paclitaxel low or high dose | 6 months | Clopidogrel or ticlopidine 1 or 6 months, or cilostazol | TLR: ischaemia driven | Including proximal and distal references; at 4–6 months | Any |
| TAXUS II48,49 | Slow or moderate release polymer based paclitaxel | 1 year | Clopidogrel (or ticlopidine) 6 months | TVR: indication not reported | In total analysis segment including 5 mm proximal and distal edge; at 6 months | Cardiac death |
| TAXUS IV48,60 | Slow release polymer based paclitaxel | 9 months | Clopidogrel for 6 months | TVR: ischaemia driven | In analysis segment including proximal and distal edge; at 9 months | Cardiac death |
| ELUTES55 | Non‐polymer based paclitaxel 4 doses | 1 year | Clopidogrel 3 months | TLR: clinically driven | In stent; at 6 months | Any |
| DELIVER53,54 | Non‐polymer based paclitaxel | 1 year | Clopidogrel 3 months | TVR | In segment including 5 mm proximal and distal; at 8 months | Cardiac death |
| PATENCY56 | Non‐polymer based paclitaxel | 9 months | Clopidogrel 3 month | TLR | In stent? At 9 months | Any |
| TAXUS V61 | Slow release polymer based paclitaxel | 9 months | NA | TVR: indication not reported | In stent? At 9 months | Cardiac death |
| TAXUS VI62 | Moderate release polymer based paclitaxel | 9 months | NA | TVR: indication not reported | In segment? At 9 months | Cardiac death |
*In addition to aspirin; †revascularisation was one component (the most frequent) of the composite end point (major adverse cardiac events).
ACC, American College of Cardiology; ASPECT, Asian paclitaxel eluting stent clinical trial; C‐SIRIUS, Canadian sirolimus coated balloon expandable stent in the treatment of patients with de novo coronary artery lesions; CV, cardiovascular; DIABETES, diabetes and sirolimus eluting stent trial; ELUTES, European evaluation of paclitaxel eluting stent; ENDEAVOR, randomised, controlled trial of the Medtronic Endeavor drug (ABT‐578) eluting coronary stent system versus the Taxus paclitaxel eluting coronary stent system in de novo native coronary artery lesions; E‐SIRIUS, European sirolimus coated balloon expandable stent in the treatment of patients with de novo coronary artery lesions; FUTURE, first use to underscore reduction in restenosis with everolimus; MI, myocardial infarction; NA, not available; PATENCY, paclitaxel coated logic stent for the cytostatic prevention of restenosis; RAVEL, randomised study with sirolimus coated BX velocity balloon expandable stent in the treatment of patients with de novo native coronary lesions; SCANDSTENT, randomised multicentre comparison of sirolimus versus bare metal stent implantation in complex coronary lesions; SCORE, study to compare restenosis rate between quest and quads‐QP2; SES‐SMART, randomised comparison of a sirolimus eluting stent and a standard stent in the prevention of restenosis in small coronary arteries; SIRIUS, sirolimus eluting balloon expandable stent in the treatment of patients with de novo native coronary artery lesions; TAXUS, treatment of de novo coronary disease using a single paclitaxel eluting stent; TLR, target lesion revascularisation; TVR, target vessel revascularisation.
Table 2 Clinical and angiographic characteristics.
| Trial | Mean age (years) | Proportion with diabetes | Proportion with previous MI | Reference diameter (mm) | Lesion length (mm) |
|---|---|---|---|---|---|
| RAVEL | 60.7 | 45/238 (19%) | 86/238 (36%) | 2.62 | 9.6 |
| SIRIUS | 62 | 279/1058 (26%) | 318/1058 (31%) | 2.8 | 14.4 |
| E‐SIRIUS | 62.3 | 81/351 (23%) | 147/349 (42%) | 2.55 | 15 |
| C‐SIRIUS | 60.5 | 24/100 (24%) | 45/100 (45%) | 2.63 | 13.5 |
| FUTURE I/II | 63.5 | 17/107 (16%) | 17/107 (16%) | 2.98 | 10.3 |
| SES‐SMART | 62 | 63/257 (24.5%) | 75/257 (29%) | 2.2 | 11.85 |
| SCANDSTENT | 62.7 | 22/322 (6.8%) | 64/322 (20%) | 2.9 | 18 |
| DIABETES | 66.5 | 100% | 74/160 (46%) | 2.34 | 14.9 |
| ENDEAVOR II | 62 | 239/1197 (20%) | 491/1197 (41%) | 2.6 | 14.22 |
| SIROLIMUS subgroup | 62 | 770/3790 (20.3%) | 1317/3788 (35%) | 2.58 | 16.50 |
| SCORE | 62.2 | 53/260 (20%) | 105/260 (40%) | 3 | 11.87 |
| TAXUS I | 64.9 | 11/59 (19%) | 18/59 (30.5%) | 2.96 | 11.29 |
| ASPECT | 60 | 35/177 (20%) | 44/177 (42%) | 2.92 | 10.87 |
| TAXUS II | 60.1 | 58/536 (11%) | 160/536 (30%) | 2.75 | 10.5 |
| TAXUS IV | 62.5 | 316/1314 (24%) | 397/1314 (30%) | 2.75 | 13.4 |
| ELUTES | 60 | 30/192 (16%) | 66/192 (34%) | 2.96 | 10.8 |
| TAXUS V | 63 | 357/1156 (30%) | 333/1156 (29%) | 2.69 | 17 |
| TAXUS VI | 62.6 | 89/446 (20%) | NA | 2.79 | 20.6 |
| DELIVER | 62.3 | 299/1041 (29%) | 275/1041 (26%) | 2.81 | 11.36 |
| PATENCY | NA | 12/60 (20%) | NA | 2.89 | NA |
| PACLITAXEL subgroup | 61 | 1260/5241 (24%) | 1398/4735 (29%) | 3 | 17 |
Statistical analysis
We used different calculation methods for estimation of the overall treatment effect (coated stents versus bare stents) based on odds ratio (OR). Results are presented as the OR calculated by the Mantel‐Haenszel method.11 ORs are reported with 95% confidence intervals (CIs). A χ2 test for association on the pooled estimate of OR was performed and significance was set at p = 0.05. For each trial we calculated the OR for MACE, binary restenosis, death, Q wave MI, and stent thrombosis.
In the first step of the meta‐analysis a fixed effect model was used. Heterogeneity between trials was tested by a χ2 procedure. When heterogeneity was present a random effect model was then used and sources of heterogeneity were studied.
To test publication bias, we constructed funnel plots and analysed robustness. The number of neutral trials (OR = 1) needed to be added to the meta‐analysis to induce a non‐significant overall effect was calculated. Lastly, to investigate the relation between baseline risk in the population and treatment effect we studied the so called effect model, which is a the linear regression between event rates in bare stent and DES groups in the different trials, by means of the Walter weighted regression method.12 Meta‐analysis calculation (association test), heterogeneity, funnel plot, robustness analysis, and effect model were performed with EasyMA software (www.spc.univ‐lyon1.fr/easyma.net/).13
RESULTS
Search results
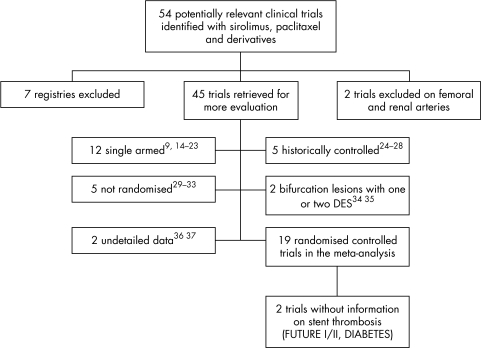
Fifty four studies were identified as potentially relevant. We excluded seven registries and two clinical trials concerning DES in peripheral arteries. Twenty six other studies were excluded: 12 were single armed,9,14,15,16,17,18,19,20,21,22,23 five were historically controlled,24,25,26,27,28 five were not randomised,29,30,31,32,33 two concerned bifurcation lesions with one or two DES,34,35 and two had not enough details for treatment groups or end points (fig 1).36,37
Figure 1 Meta‐analysis profile. DES, drug eluting stents; DIABETES, diabetes and sirolimus eluting stent trial; FUTURE, first use to underscore reduction in restenosis with everolimus.
For comparisons between DES and bare metal stents, 19 randomised controlled trials were selected concerning 8987 patients (4574 with DES and 4413 with bare stents). Seven randomised controlled trials tested sirolimus,38,39,40,41,42,43,44 one tested biolimus,10 one tested everolimus,45 one tested the taxane analogue QP 2,46 five tested polymer based paclitaxel,47,48,49,50,51 and four tested non‐polymer delivered paclitaxel.46,52,53,54,55,56
DES versus bare metal stents
MACE and restenosis
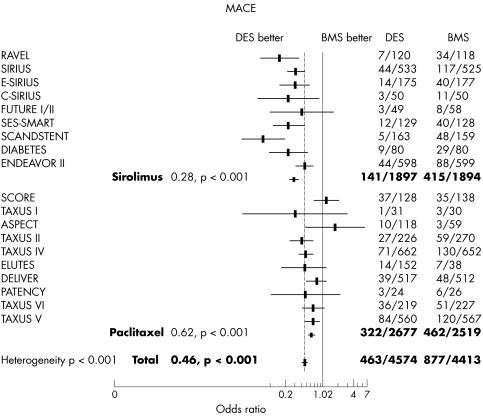
The overall occurrence of MACE was significantly reduced by DES from 19.9% to 10.1% (p < 0.001). Common OR by Mantel‐Haenszel method was 0.46 (95% CI 0.41 to 0.52) (fig 2). We observed a significant heterogeneity (p < 0.001) between subgroups with a larger reduction of MACE in the sirolimus subgroup (7.4% v 21.9%, OR 0.28, 95% CI 0.22 to 0.35) than in the paclitaxel subgroup (12% v 18.3%, OR 0.62, 95% CI 0.53 to 0.73). This heterogeneity remained significant when we kept only sirolimus and polymer based paclitaxel stent trials. In this second step analysis, the MACE rate with the commercialised sirolimus eluting stent was 7.5% versus 25.8% with bare stents (OR 0.23, 95% CI 0.18 to 0.30) and the MACE rate with the commercialised polymer based paclitaxel eluting stent was 11.2% versus 17.9% with bare stents (OR 0.59, 95% CI 0.50 to 0.69). An additional analysis in a random effect model found the sirolimus subgroup OR of 0.26 (95% CI 0.18 to 0.36) and the paclitaxel subgroup OR of 0.64 (95% CI 0.50 to 0.81) with confirmed heterogeneity between the two subgroups (p < 0.001).
Figure 2 Odds ratio and p value for major adverse cardiac events (MACE) with DES versus bare metal stents (BMS) and subgroup analysis. The occurrence of MACE was significantly reduced by DES with a larger reduction in the sirolimus subgroup.
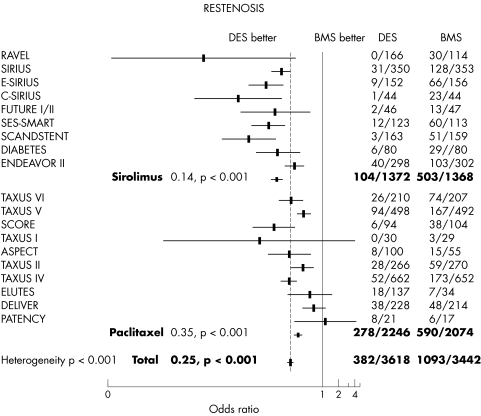
The proportion of patients with complete angiographic follow up was > 85% in all studies except in SCORE (study to compare restenosis rate between quest and quads‐QP2) (74% follow up), SIRIUS (sirolimus eluting balloon expandable stent in the treatment of patients with de novo native coronary artery lesions) (66% follow up), ENDEAVOR II (randomised, controlled trial of the Medtronic Endeavor drug (ABT‐578) eluting coronary stent system versus the Taxus paclitaxel eluting coronary stent system in de novo native coronary artery lesions) (50% of follow up), and DELIVER (43% follow up). Figure 3 shows the restenosis pooled analysis. The overall adjusted rate for angiographic restenosis was 10.5% in the DES group versus 31.7% in the control group (OR 0.25, 95% CI 0.22 to 0.29, p < 0.001). A significant heterogeneity between subgroups was found (p < 0.001). In the sirolimus subgroup, the restenosis adjusted rate was 7.6% with DES versus 36.8% with bare stent (OR 0.14, 95% CI 0.11 to 0.17, p < 0.01). In the paclitaxel subgroup, the restenosis adjusted rate was 12.4% with DES versus 28.4% with bare stent (OR 0.35, 95% CI 0.30 to 0.41, p < 0.001). This heterogeneity between subgroups was found to be significant even when trials with analogues were excluded: restenosis was 6.0% versus 37.9% (OR 0.10, 95% CI 0.08 to 0.13) in the sirolimus group and 12.6% versus 28.0% (OR 0.37, 95% CI 0.31 to 0.43) in polymer based paclitaxel eluting stent group, respectively. Results were also confirmed with a random effect model.
Figure 3 The rate of binary angiographic restenosis was significantly reduced by DES with a larger reduction in the sirolimus subgroup.
The overall adjusted rate for angiographic target lesion revascularisation was 6.2% in the DES group versus 16.6% in the control group (OR 0.36, 95% CI 0.31 to 0.41, p < 0.001). In the sirolimus subgroup, the angiographic target lesion revascularisation adjusted rate was 6.7% with DES versus 16.7% with bare stent (OR 0.20, 95% CI 0.16 to 0.25, p < 0.001). In the paclitaxel subgroup, the angiographic target lesion revascularisation adjusted rate was 8.2% with DES versus 14.7% with bare stent (OR 0.53, 95% CI 0.44 to 0.63, p < 0.001).
Safety
Overall mortality was not significantly different between treatment groups and there was no significant heterogeneity between trials. The overall adjusted percentage was 0.9% with DES versus 1.2% with bare stent (p = 0.92, OR 1.02, 95% CI 0.64 to 1.64) and mortality was not significantly lower with paclitaxel compared with bare stents (fig 4).
Figure 4 Overall mortality at 6–12 months' follow up was not significantly different between treatment groups.
The Q wave MI adjusted percentage was 1.1% with DES versus 0.8% with bare stent (OR 1.25, 95% CI 0.79 to 1.94, p = 0.33) (fig 5) with no significant heterogeneity between trials and more Q wave MI with paclitaxel (p = 0.09).
Figure 5 The overall occurrence of Q wave myocardial infarction (MI) at 6–12 months' follow up was not different between treatment groups.
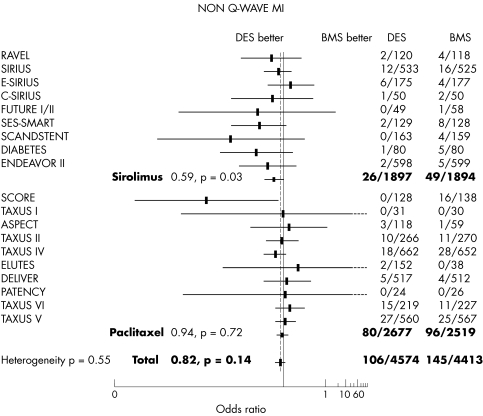
The non‐Q wave MI adjusted percentage was 2.3% with DES versus 3.28% with bare stent (OR 0.82, 95% CI 0.63 to 1.07, p = 0.14) (fig 6).
Figure 6 The overall occurrence of non‐Q wave MI at 6–12 months' follow up was not different between treatment groups.
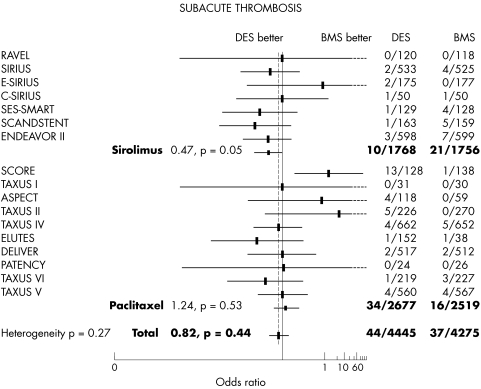
The occurrence of stent thrombosis was 0.7% with DES versus 0.8% with bare stent (OR 0.71, 95% CI 0.41 to 1.25, p = 0.24) without heterogeneity between trials but with a trend to more stent thrombosis in the paclitaxel subgroup (fig 7).
Figure 7 The overall occurrence of stent thrombosis was not different between treatment groups.
The different calculation methods provided similar results on outcomes.
Robustness and sensitivity analysis
The robustness test found that more than 10 negative studies (size determined as the mean of included trials) would be necessary to affect the meta‐analysis results for MACE and binary restenosis.
The sensitivity analysis for MACE and binary restenosis excluding the three main trials did not change the global results.
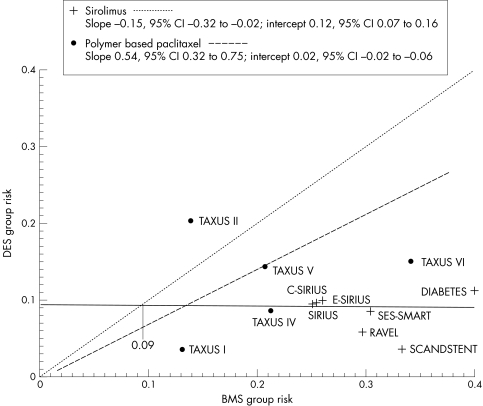
Effect model
To investigate whether the effect of DES on MACE was related to the risk of the studied population we performed an effect model analysis. We excluded analogues as well as the trials testing different doses and kept only studies performed with the two marketed DES. Because of the detected heterogeneity between sirolimus and paclitaxel stents, the effect model was analysed separately for these two types of stents. Figure 8 shows results for MACE. The risk ratio (slope) between bare stents and coated stents is different with paclitaxel and sirolimus. The effectiveness of sirolimus eluting stent (relative risk reduction) improved with increasing event rates in the control groups. This is not the case in the paclitaxel eluting stent group, where the relative risk reduction appeared constant whatever the risk in the bare stent group.
Figure 8 Effect model on MACE for polymer based paclitaxel and sirolimus. CI, confidence interval.
DISCUSSION
DES are the most recent breakthrough in the field of interventional cardiology. Their tremendous impact on restenosis in every single trial lifts one of the last barriers to the use of coronary intervention in patients at high risk of restenosis. However, issues have also been raised about the cost effectiveness, the impact on hard clinical events, and the safety of DES.6 Moreover, among the many drugs developed (actinomycin, oestrogens, batimastat, dexamethasone, angiopeptin, etc) the most convincing evidence has emerged with sirolimus and paclitaxel coated stents, but it is uncertain whether these two DES have the same risk to benefit ratio. Babapulle et al57 conducted a meta‐analysis on the same subject but our updated results are slightly different. Regarding safety concerns we analysed Q wave MI, instead of all MIs, which are mainly enzymatic rises following percutaneous coronary intervention. We believe that Q wave MI is a harder clinical event probably containing underdiagnosed late stent thrombosis. We also included sirolimus or paclitaxel derivatives to enhance the power to detect infrequent event differences. For the efficacy analysis we choose a two step analysis with comparison of commercially available DES as the second step; we found differences between drugs that were graphically displayed by the effect model analysis.
Our meta‐analysis confirms, with good robustness, the very significant reduction of MACE and restenosis by DES compared with bare stents. The heterogeneity between the two subgroups, with larger reductions of these two end points with sirolimus, suggests a more potent effect of sirolimus than of paclitaxel eluting stents. This was confirmed when studies of analogues were excluded from the meta‐analysis, evaluating then only commercially available DES. The type of model (random or fixed) did not affect the results either. Acknowledging that MACE are mainly driven by revascularisation for restenosis, these results for the two end points are consistent.
The effect model analysis displays a different efficacy pattern for the two types of DES. The benefit of paclitaxel eluting stent (relative risk) appears constant whatever the level of risk in the bare stent group, whereas the benefit of sirolimus eluting stent increases with increasing risk in the bare stent group. Such a different effect may rely on a more appropriate pharmacological action of sirolimus against intrastent cell proliferation. Obviously, this potential superiority of sirolimus eluting stents has been confirmed in recent head to head trials and a meta‐analysis of these trials.58
The present meta‐analysis also allows an increase of power to detect small differences that cannot be seen in every trial for rare events. Indeed, in October 2003 the FDA released an advisory notice6 informing physicians about adverse events associated with sirolimus eluting stents. It reported more than 290 cases of thrombosis occurring within the first 30 days of stent implantation and more than 50 reports of apparent hypersensitivity reactions. One month later, the FDA updated this information. We found, compared with bare stents, unfavourable trends for Q wave MI and stent thrombosis with DES driven by paclitaxel studies. However, mortality analysis was reassuring for paclitaxel stents.
For stent thrombosis, the influence of SCORE, which was terminated early due to safety concerns, is important and affects the global result. The SCORE data can have a disproportionate effect, since a major part of the problem was the delivery method, and this is a limitation of the present analysis. No definite conclusion can be drawn but this trend, put into perspective with the FDA report, draws attention to a possible prothrombotic effect. Whether the drug, the dose, the polymer, the coronary anatomy, patients' characteristics, or the play of chance is involved is still unknown. Future studies, registries, and pharmacovigilance surveys on the use of DES in high risk patients will also help to monitor the safety of DES in real life coronary intervention.
As far as mortality is concerned we did not find a significant difference between bare stents and DES. The recent three year follow up report of the RAVEL (randomised study with sirolimus coated BX velocity balloon expandable stent in the treatment of patients with de novo native coronary lesions) study showed a non‐significant increase in death mainly of non‐cardiac origin.38 Prolonged follow up of patients enrolled in DES studies is also required after the standard period of observation for restenosis.
Conclusion
A very significant reduction of MACE and restenosis was observed with DES in this meta‐analysis. Heterogeneity of benefit according to the type of stent was found for these two end points. The effect model on MACE suggests that patients at high risk of restenosis benefit even more from sirolimus eluting stents. More information needs to be collected to evaluate fairly the safety of DES with regard to thrombotic events.
Abbreviations
CI - confidence interval
DES - drug eluting stents
ENDEAVOR - randomised, controlled trial of the Medtronic Endeavor drug (ABT‐578) eluting coronary stent system versus the Taxus paclitaxel eluting coronary stent system in de novo native coronary artery lesions
FDA - Food and Drug Administration
MACE - major adverse cardiac events
MI - myocardial infarction
OR - odds ratio
SCORE - study to compare restenosis rate between quest and quads‐QP2
SIRIUS - sirolimus eluting balloon expandable stent in the treatment of patients with de novo native coronary artery lesions
References
- 1.Fischman D L, Leon M B, Baim D S.et al A randomized comparison of coronary‐stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994331496–501. [DOI] [PubMed] [Google Scholar]
- 2.Serruys P W, de Jaegere P, Kiemeneij F.et al A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 1994331489–495. [DOI] [PubMed] [Google Scholar]
- 3.SoS Investigators Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the stent or surgery trial): a randomised controlled trial. SoS investigators. Lancet 2002360965–970. [DOI] [PubMed] [Google Scholar]
- 4.Serruys P W, Unger F, Sousa J E.et al Comparison of coronary‐artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 20013441117–1124. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Guagliumi G, Farb A.et al Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus‐eluting stent: should we be cautious? Circulation 2004109701–705. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration FDA advises physicians of adverse events associated with Cordis Cypher coronary stent. T03‐71, October 29, 2003. www.fda.gov/bbs/topics/ANSWERS/2003/ANS01257.html (accessed 14 November 2005)
- 7.Sousa J E, Serruys P W, Costa M A. New frontiers in cardiology: drug‐eluting stents: part II. Circulation 20031072383–2389. [DOI] [PubMed] [Google Scholar]
- 8.Sousa J E, Serruys P W, Costa M A. New frontiers in cardiology: drug‐eluting stents: part I. Circulation 20031072274–2279. [DOI] [PubMed] [Google Scholar]
- 9.Sousa J E, Costa M A, Abizaid A C.et al Sustained suppression of neointimal proliferation by sirolimus‐eluting stents: one‐year angiographic and intravascular ultrasound follow‐up. Circulation 20011042007–2011. [DOI] [PubMed] [Google Scholar]
- 10.Gruberg L. ENDEAVOR II. A randomized comparison of the Endeavor ABT‐578 drug‐eluting stent with a bare metal stent for coronary revascularization 2005. http://www.medscape.com/viewarticle/501475
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 195922719–748. [PubMed] [Google Scholar]
- 12.Walter S D. Variation in baseline risk as an explanation of heterogeneity in meta‐analysis. Stat Med 1997162883–2900. [DOI] [PubMed] [Google Scholar]
- 13.Cucherat M. EasyMA. Department of Clinical Pharmacology, Cardiological Hospital, Lyon, France, 2002. www.spc.univ‐lyon1.fr/easyma.net/
- 14.Liistro F, Stankovic G, Di Mario C.et al First clinical experience with a paclitaxel derivate‐eluting polymer stent system implantation for in‐stent restenosis: immediate and long‐term clinical and angiographic outcome. Circulation 20021051883–1886. [DOI] [PubMed] [Google Scholar]
- 15.Honda Y, Grube E, de La Fuente L M.et al Novel drug‐delivery stent: intravascular ultrasound observations from the first human experience with the QP2‐eluting polymer stent system. Circulation 2001104380–383. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe K, Serruys P W, Grube E.et al TAXUS III trial: in‐stent restenosis treated with stent‐based delivery of paclitaxel incorporated in a slow‐release polymer formulation. Circulation 2003107559–564. [DOI] [PubMed] [Google Scholar]
- 17.Degertekin M, Regar E, Tanabe K.et al Sirolimus‐eluting stent for treatment of complex in‐stent restenosis: the first clinical experience. J Am Coll Cardiol 200341184–189. [DOI] [PubMed] [Google Scholar]
- 18.Suresh V, Kumar S, Levy D. Early outcome of sirolimus drug‐eluting stents in high‐risk patients undergoing coronary angioplasty [abstract]. Am J Cardiol 200392 Oral abstract from the Transcatheter Cardiovascular Therapeutics Congress 2003 [Google Scholar]
- 19.Tmbattoni D, Antoniucci D, Fabbiacchi F. First clinical experience with the tacrolimus‐eluting Janus carbostent in de novo coronary arteries: the Jupiter I study [abstract]. Am J Cardiol 200392 Oral abstract from the Transcatheter Cardiovascular Therapeutics Congress 2003 [Google Scholar]
- 20.Serruys P. PISCES. http://www.tctmd.com expert presentation, 2003
- 21.Costa M A, Moses J W, Leon M B. Sirolimus‐eluting stent for the treatment of bypass graft disease: the initial U.S. experience [abstract]. J Am Coll Cardiol 20034113A [Google Scholar]
- 22.Hongo Y, Sakurai R, Meredith I.et al Intravascular ultrasound analysis of the new ABT‐578 eluting phosphorylcholine‐coated stent implantation to de novo human coronary lesions: the ENDEAVOR I trial [abstract]. J Am Coll Cardiol 200443A85 [Google Scholar]
- 23.Sakurai R, Hongo Y, Ormiston J.et al The PREFER Trial Investigators. First human experience with the ABT‐578 eluting phosphorylcholine polymer stent: a serial volumetric intravascular ultrasound analysis from the PREFER trial [abstract]. J Am Coll Cardiol 200443A67 [Google Scholar]
- 24.Meie B. SVELTE trial: a multicenter historically controlled study in patients with de novo native coronary artery lesions in small vessels treated with the CYPHER stent. Am J Cardiol 200392 (suppl I) [abstract] [Google Scholar]
- 25.Ong A T, Hoye A, Aoki J.et al Thirty‐day incidence and six‐month clinical outcome of thrombotic stent occlusion after bare‐metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol 200545947–953. [DOI] [PubMed] [Google Scholar]
- 26.De la Torre Hernandez J M, Sainz Laso F, Ruisanchez C.et al Treatment of lesions with a high risk of stenosis. comparative study in 300 patients of rapamycin‐ and paclitaxel‐ eluting polymer‐based stents, and bare metal stents. Rev Esp Cardiol 200558262–269. [PubMed] [Google Scholar]
- 27.Hoye A, Lemos P A, Arampatzis C A.et al Effectiveness of the sirolimus‐eluting stent in the treatment of patients with a prior history of coronary artery bypass graft surgery. Coron Artery Dis 200415171–175. [DOI] [PubMed] [Google Scholar]
- 28.Lemos P A, Saia F, Hofma S H.et al Short‐ and long‐term clinical benefit of sirolimus‐eluting stents compared to conventional bare stents for patients with acute myocardial infarction. Je Am Coll Cardiol 200443704–708. [DOI] [PubMed] [Google Scholar]
- 29.Gerckens U, Buellesfeld L, Horstkotte D.et al Evaluation of a tacrolimus‐eluting coronary stent with nanoporous ceramic coating in treatment of native coronary artery lesions: phase I and II of the PRESENT study. J Am Coll Cardiol 2003417 [Google Scholar]
- 30.Feres F, Munoz J S, Abizaid A. Sirolimus‐eluting stent or intracoronary brachytherapy to treat in‐stent restenosis [bstract]. J Am Coll Cardiol 20034183A [Google Scholar]
- 31.Bartorelli A, Grube E, Blanchard D.et al About the use of the ACHIEVE paclitaxel eluting stent in treatment of restenotic lesions: a subanalysis of the 30‐day safety data of DELIVER II [abstract]. J Am Coll Cardiol 20034173A [Google Scholar]
- 32.Sengottuvel G, Lefevre T, Louvard Y.et al Evaluation of sirolimus‐eluting stents for the treatment of bifurcation lesions: a real world study. J Am Coll Cardiol 200443A36 [Google Scholar]
- 33.Stauffer J‐C E, Seydoux C, Goy J‐J. Is the sirolimus drug eluting stent better than paclitaxel coated stent in “real life” environment? J Am Coll Cardiol 200443A86 [Google Scholar]
- 34.Pan M, Medina A, de Lezo J.et al Drug‐eluting stents for the treatment of bifurcated coronary lesions: a randomized comparison of simple versus complex‐strategy approach. J Am Coll Cardiol 200443A87. [DOI] [PubMed] [Google Scholar]
- 35.Colombo A, Louvard Y, Raghu C.et al Sirolimus‐eluting stents in bifurcation lesions: six‐month angiographic results according to the implantation technique. J Am Coll Cardiol 20034153 [Google Scholar]
- 36.Li S, Fu F, Liu W.et al Randomized study to evaluate sirolimus‐eluting stents implanted at coronary small vessel lesions. Am J Cardiol 200494218Eabstract [Google Scholar]
- 37.Mehran A, Lansky A J, Sousa J E.et al Biolimus A9 drug eluting stent (MATRIX‐stent) in treatment of de novo coronary lesions: first‐in‐man experience (the STEALTH‐I trial). Am J Cardiol 20049469Eabstract [Google Scholar]
- 38.Morice M C, Serruys P W, Sousa J E.et al A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 20023461773–1780. [DOI] [PubMed] [Google Scholar]
- 39.Holmes D R, Jr, Leon M B, Moses J W.et al Analysis of 1‐year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus‐eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004109634–640. [DOI] [PubMed] [Google Scholar]
- 40.Schofer J S M, Gershlick A H.et al Sirolimus‐eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: randomised controlled trial (E‐SIRIUS). Lancet 20033621093–1099. [DOI] [PubMed] [Google Scholar]
- 41.Schampaert E, Cohen E A, Schluter M.et al The Canadian study of the sirolimus‐eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C‐SIRIUS). J Am Coll Cardiol 2004431110–1115. [DOI] [PubMed] [Google Scholar]
- 42.Schampaert E. C‐Sirius, Data ACC 2003. http://www.tctmd.com expert presentation, 2003
- 43.Gruberg L. DIABETES: diabetes and sirolimus‐eluting trial http://www.medscape.com/viewarticle/491691; 2005
- 44.Kelbaek H, Thuesen L, Helqvist S.et alRandomized multicenter comparison of sirolimus vs bare metal stent implantation in complex coronary artery disease: the stenting of coronary arteries in non‐stress/Benestent disease trial. www.tctmd.com. Denmark,2005
- 45.Costa R A, Lansky A J, Mehran R.et al The multicenter evaluation of the everolimus‐eluting stent for inhibition of neointimal hyperplasia: results of the pooled FUTURE I and II trials. J Am Coll Cardiol 200443A12 [Google Scholar]
- 46.Grube E, Lansky A, Hauptmann K E.et al High‐dose 7‐hexanoyltaxol‐eluting stent with polymer sleeves for coronary revascularization: one‐year results from the SCORE randomized trial. J Am Coll Cardiol 2004441368–1372. [DOI] [PubMed] [Google Scholar]
- 47.Grube E, Silber S, Hauptmann K E.et al TAXUS I: six‐ and twelve‐month results from a double‐blind trial on a slow‐release paclitaxel‐eluting stent for de novo coronary lesions. Circulation 200310738–42. [DOI] [PubMed] [Google Scholar]
- 48.Anon Boston Scientific. Taxus Express paclitaxel‐eluting coronary stent system. www.bostonscientific.com/med_specialty/deviceDetail.jsp?task = tskBasicDevice.jsp§ionId = 4&relId = 2,74,75,76&deviceId = 11013 (accessed 14 November 2005)
- 49.Colombo A, Drzewiecki J, Banning A.et al Randomized study to assess the effectiveness of slow‐ and moderate‐release polymer‐based paclitaxel‐eluting stents for coronary artery lesions. Circulation 2003108788–794. [DOI] [PubMed] [Google Scholar]
- 50.Stone G W, Ellis S G, Cox D A. A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004350221–231. [DOI] [PubMed] [Google Scholar]
- 51.Gruber L. TAXUS‐V De Novo: clinical and angiographic results of the Taxus stent in complex lesions. http://www.medscape.com/viewarticle/501427; 2005
- 52.Park S J, Shim W H, Ho D S.et al A paclitaxel‐eluting stent for the prevention of coronary restenosis. N Engl J Med 20033481537–1545. [DOI] [PubMed] [Google Scholar]
- 53.Knopf W, O'Neill W, Midei M. The DELIVER clinical trial. Am J Cardiol 200290 (suppl I) [abstract] [Google Scholar]
- 54.Lansky A J, Costa R A, Mintz G S.et al for the DELIVER Clinical Trial Investigators. Non‐polymer‐based paclitaxel‐coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow‐up of the DELIVER clinical trial. Circulation 20041091948–1954. [DOI] [PubMed] [Google Scholar]
- 55.Gershlick A, De Scheerder I, Chevalier B.et al Inhibition of restenosis with a paclitaxel‐eluting, polymer‐free coronary stent: the European evaluation of paclitaxel eluting stent (ELUTES) trial. Circulation 2004109487–493. [DOI] [PubMed] [Google Scholar]
- 56.Heldman A W, Farhar N, Cummins F. Paclitaxel‐eluting stent for cytostatic prevention of restenosis: PATENCY study follow‐up. Am J Cardiol 200290 (suppl I) [abstract] [Google Scholar]
- 57.Babapulle M N, Joseph L, Belisle P.et al A hierarchical Bayesian meta‐analysis of randomised clinical trials of drug‐eluting stents. Lancet 2004364583–591. [DOI] [PubMed] [Google Scholar]
- 58.Kastrati A, Dibra A, Eberle S.et al Sirolimus‐eluting stents vs paclitaxel‐eluting stents in patients with coronary artery disease: meta‐analysis of randomized trials. JAMA 2005294819–825. [DOI] [PubMed] [Google Scholar]
- 59.Ardissino D, Cavallini C, Bramucci E.et al SES‐SMART Investigators. Sirolimus‐eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA 20042922727–2734. [DOI] [PubMed] [Google Scholar]
- 60.Stone G W, Ellis S G, Cox D A.et al for the TAXUS‐IV Investigators. One‐year clinical results with the slow‐release, polymer‐based, paclitaxel‐eluting Taxus stent: the TAXUS‐IV trial. Circulation 20041091942–1947. [DOI] [PubMed] [Google Scholar]
- 61.Stone G W, Ellis S G, Cannon L.et al Comparison of a polymer‐based paclitaxel‐eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA 20052941215–1223. [DOI] [PubMed] [Google Scholar]
- 62.Gruberg L. Taxus VI: a randomized trial of moderate‐rate‐release, polymer‐based, paclitaxel‐eluting stent for the treatment of longer lesions: 9 month clinical results. http://www.medscape.com/viewarticle/482822; 2005