Early atherosclerotic lesions are characterised by a relative abundance of inflammatory cells such as activated T lymphocytes, indicating involvement of immunoinflammatory process in the pathogenesis of atherosclerosis.1 One of the antigens stimulating recruitment of these T cells is believed to be heat shock protein (HSP). HSP60 (human) has been shown to enhance production of proinflammatory cytokines such as tumour necrosis factor α, interleukin 12, and interleukin 15 and to mediate monocyte adhesion to endothelial cells. In a large population based study, Xu et al2 have shown a correlation between soluble HSP60 (sHSP60) and the severity of carotid atherosclerosis. The association of sHSP60 in patients with coronary artery disease (CAD), however, remains to be clarified.
Human HSP60 has been shown to require functional toll‐like receptor 4 (TLR4), a receptor involved in innate immunity, for stimulating production of tumour necrosis factor α and macrophages. Recently, two polymorphisms in the TLR4 gene, Thr399Ile and Asp299Gly, have been associated with lower concentrations of proinflammatory cytokines and a reduced extent or progression of carotid atherosclerosis.3
We hypothesised that serum sHSP60 would be associated with CAD and interact with TLR4 variants, determining the severity of prevalent CAD. The present study was designed to assess the association of sHSP60 with TLR4 polymorphisms with the severity of CAD.
METHODS
A prospective study of 329 consecutive patients admitted for elective coronary artery bypass graft surgery under the care of one surgical unit between August 2002 and November 2003 was carried out. The median age of the cohort was 65 years (range 40–80 years) with a male to female ratio of 4:1. Exclusion criteria were a recent history of myocardial infarction; unstable angina; infection; associated valvar heart disease; a history of inflammatory disorders, such as systemic lupus erythematosus; and use of immunosuppresive drugs such as steroids. The presence of cardiovascular risk factors was assessed by standard methods, as detailed previously.2 The study was approved by the ethics committee at St George's Hospital Medical School and informed consent was obtained.
All the coronary angiograms were comprehensively analysed based on two descriptive concepts: transverse severity (stenosis) and longitudinal severity (expressed as extent index), adopted from Bogaty et al.4 Preoperatively, two 5 ml aliquots of blood were collected in EDTA vials. The serum sHSP60 concentrations were analysed by using a slight modification of a sandwich enzyme linked immunosorbent assay (ELISA) described previously.2 The genotyping was conducted by digesting the polymerase chain reaction products with restriction endonucleases and electrophoresing them on a 3% Aquapor HM gel to determine the TLR4 alleles.3 C reactive protein (CRP) and high and low density lipoprotein cholesterol were also measured.
Baseline characteristics were reported as geometric mean (SD) for normally distributed continuous variables, number (%) for discrete variables, and median (interquartile range) for continuous variables that were not normally distributed. Serum HSP60 and CRP were normalised by loge transformation. The Pearson correlation test was used to assess the association between sHSP60, CRP, and the CAD severity indices (stenoses and extent index). Variables that were significantly associated with coronary artery severity in univariate analysis and other established CAD risk factors were modelled into a multifactorial logistic regression equation to assess their interactions. Comparisons were made with the first quartile as the baseline. Patients were classified according to genotype combination. The interaction between genotype and sHSP60 and their effect on the severity of CAD was tested by two way analysis of covariance, with genotype as a fixed factor and sHSP60 concentration as a covariate. A value of p ⩽ 0.05 (two tailed) was considered significant.
RESULTS
Detailed angiographic evaluation of all the patients (n = 329) showed that 216 (65%) had three vessel disease, 89 (27%) had two vessel disease, and only 24 (8%) had one vessel disease, mainly affecting the left anterior descending coronary artery. The median CAD extent index was 1.2 (interquartile range 0.8–1.69) and the median number of stenoses was 10 (6–15).
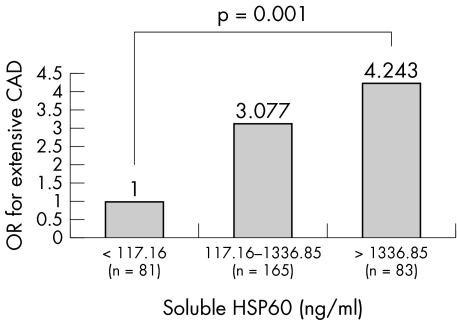
The median sHSP60 concentration in the study cohort was 428 ng/ml, with an interquartile range of 117.6–1336.86 ng/ml. Eighty five (26%) patients had concentrations > 1000 ng/ml. The correlation between extent index and the sHSP60 concentration was significant (r = 0.39, p < 0.0001). Table 1 summarises the observed associations with CAD extent index (longitudinal severity). Figure 1 shows the increase in odds ratio for extensive CAD as we move across the first quartile through the second to third quartile to the fourth quartile of sHSP60 concentrations. The number of stenoses and sHSP60 concentration were also significantly correlated (r = 0.30, p < 0.0001). Multivariate analysis confirmed the association of sHSP60 with both extent index (longitudinal severity: odds ratio 1.35, 95% confidence interval 1.15 to 1.57, p = 0.0002) and stenoses (transverse severity: odds ratio 1.30, 95% confidence interval 1.09 to 1.46, p = 0.001) of CAD, independent of other risk factors.
Table 1 Associations with coronary artery disease extent index.
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| p Value | Adjusted OR | 95% CI | p Value | |
| Age | 0.02 | 0.98 | 0.95 to 1.02 | 0.31 |
| Male sex | 0.03 | 1.68 | 0.78 to 3.625 | 0.18 |
| Hypercholesterolaemia | 0.06 | 7.89 | 1.68 to 36.95 | 0.009 |
| Hypertension | 0.30 | 0.86 | 0.44 to 1.68 | 0.66 |
| Diabetes | 0.97 | 0.55 | 0.26 to 1.15 | 0.11 |
| Smoking | 0.08 | 1.60 | 0.59 to 4.20 | 0.30 |
| Previous MI | 0.23 | 0.52 | 0.27 to 0.99 | 0.04 |
| Previous CVA | 0.29 | 1.10 | 0.37 to 3.26 | 0.87 |
| Poor LV function | 0.17 | 1.58 | 0.83 to 3.03 | 0.17 |
| BMI | 0.15 | 0.97 | 0.91 to 1.03 | 0.33 |
| Fasting glucose | 0.42 | 1.09 | 0.89 to 1.34 | 0.39 |
| LDL | 0.90 | 1.05 | 0.71 to 1.56 | 0.79 |
| HDL | 0.95 | 1.86 | 0.63 to 5.46 | 0.26 |
| CRP | 0.87 | 1.12 | 0.89 to 1.40 | 0.34 |
| sHSP60 | <0.0001 | 1.35 | 1.15 to 1.57 | 0.0002 |
BMI, body mass index; CI, confidence interval; CRP, C reactive protein; CVA, cerebrovascular accident; HDL, high density lipoprotein; LDL, low density lipoprotein; LV, left ventricular; MI, myocardial infarction; OR, odds ratio; sHSP60, soluble heat shock protein 60.
Figure 1 Progressive increase in odds ratio (OR) for presence of more extensive (greater longitudinal severity) coronary artery disease (CAD) with higher circulating concentrations of serum soluble heat shock protein (HSP) 60 (first quartile compared with second to third and fourth quartiles).
To ensure consistency with previously published studies and to have a genetically homogeneous group, only 241 white patients were assessed for TLR4 polymorphisms. One patient of the 241 could not be genotyped completely and hence was excluded. The four genotypic combinations in the cohort were normal TLR4, Asp299Gly+/Thr399Ile+, Asp299Gly+/Thr399Ile−, or Asp299Gly−/Thr399Ile+ and were present at frequencies of 0.88, 0.10, 0.01, and 0.01, respectively. The observed allele frequencies were in Hardy‐Weinberg equilibrium. Three patients each had Asp299Gly+/Thr399Ile− and Asp299Gly−/Thr399Ile+ allelic combinations and hence were excluded from further statistical analysis. In the remaining group (n = 234), no significant differences were noted in the severity of CAD, prevalence of various cardiovascular risk factors, sHSP60, and CRP between the two TLR4 genotypes.
Analysis of covariance was used for assessing the interaction between TLR4 genotype and sHSP60 concentrations. Most of the observed variation in CAD severity was accounted for by sHSP60. The presence of TLR4 variants (Asp299Gly+/Thr399Ile+) did not alter the severity (transverse or longitudinal) of CAD even in association with sHSP60 and there seems to be no significant statistical interaction between them (F = 0.02, λ = 0.02, p ⩾ 0.20, power = 20).
DISCUSSION
In this study, we showed that extracellular serum HSP60 is present in the blood of patients with CAD and that its concentration correlates with the severity of prevalent coronary atherosclerosis. Furthermore, we found this association to be independent of age, sex, CRP, and other established risk factors. Our findings are consistent with previously published seroepidemiological studies on atherosclerosis and may suggest a possible pathogenic role for sHSP60 in the development of CAD.2 Eighty five (26% of 329) patients in our study had concentrations > 1000 ng/ml. Such high concentrations have been observed, in various in vitro studies, to maximally activate macrophages, endothelial cells, and T cells. It is thought that similar inflammatory responses occurring in arterial wall may lead to the development of atherosclerosis.1
In our cohort, the isolated TLR4 haplotype (Asp299Gly+/Thr399Ile−) was present in only three patients. In the study by Kiechl et al,3 this was the combination that resulted in maximal reduction in the progression of carotid atherosclerosis. In our study, this group also had CAD of less severity (mean stenoses 7 (7) compared with normal TLR4 with a mean stenoses 11 (5)). However, the small number preclude any meaningful statistical conclusion. The lack of influence of TLR4 polymorphisms on CAD severity in our study signifies only the lack of a difference between normal TLR4 and Asp299Gly+/Thr399Ile+. These results are in agreement with some studies5,6 but differ from others.3,7 Animal studies also do not support the role for functional TLR4 receptors in the development and progression of atherosclerosis.8
No significant interaction was observed between the Asp299Gly+/Thr399Ile+ TLR4 variant and sHSP60 concentration in determining the severity of CAD (F = 0.02, λ = 0.02, p ⩾ 0.20, power = 20). The variation in CAD severity was accounted for by sHSP60 concentration alone. This observation is consistent with the published work, which shows that the function of the TLR4 receptor produced by the Asp299Gly+/Thr399Ile+ alleles is not reduced.9 Evidence also indicates that the TLR4 receptor complex may not be involved in the binding of human HSP60 by murine macrophages and that the receptor of human HSP60 is different from that of HSP70 and HSP90, which signal through TLR2 and TLR4.10 However, lack of evidence for a significant interaction or association may be accounted for by the small sample size.
In summary, we provide the first evidence confirming the association of sHSP60 concentrations with the severity of CAD. sHSP60, in conjunction with other markers such as CD40/CD40L and CRP, may have a role in identifying patients with more severe CAD.1 These observations are consistent with the involvement of immunity in atherosclerosis and lend further support to the role of HSP60 as an important autoantigen in atherogenesis.
ACKNOWLEDGEMENTS
This work was supported by grants from the British Heart Foundation (PG/03/132) and the Oak Foundation. The authors also acknowledge the support of Mr John Smith, Mr Mazin Sarsam, and Mr Robin Kanagasabay, consultant cardiothoracic surgeons at St George's Hospital, London, for allowing their patients to be recruited for the study. We thank Miss Frances Williams for editorial assistance.
Abbreviations
CAD - coronary artery disease
CRP - C reactive protein
ELISA - enzyme linked immunosorbent assay
HSP - heat shock protein
sHSP60 - soluble heat shock protein 60
TLR4 - toll‐like receptor 4
References
- 1.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Ann Rev Immunol 200422361–403. [DOI] [PubMed] [Google Scholar]
- 2.Xu Q, Schett G, Perschinka H.et al Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 200010214–20. [DOI] [PubMed] [Google Scholar]
- 3.Kiechl S, Lorenz E, Reindl M.et al Toll‐like receptor 4 polymorphisms and atherogenesis. N Engl J Med 2002347185–192. [DOI] [PubMed] [Google Scholar]
- 4.Bogaty P, Brecker S J, White S E.et al Comparison of coronary angiographic findings in acute and chronic first presentation of ischemic heart disease. Circulation 1993871938–1946. [DOI] [PubMed] [Google Scholar]
- 5.Yang I A, Holloway J W, Ye S. TLR4 Asp299Gly polymorphism is not associated with coronary artery stenosis. Atherosclerosis 2003170187–190. [DOI] [PubMed] [Google Scholar]
- 6.Netea M G, Hijmans A, van Wissen S.et al Toll‐like receptor‐4 Asp299Gly polymorphism does not influence progression of atherosclerosis in patients with familial hypercholesterolaemia. Eur J Clin Invest 20043494–99. [DOI] [PubMed] [Google Scholar]
- 7.Edfeldt K, Bennet A M, Eriksson P.et al Association of hypo‐responsive toll‐like receptor 4 variants with risk of myocardial infarction. Eur Heart J 2004251447–1453. [DOI] [PubMed] [Google Scholar]
- 8.Wright S D, Burton C, Hernandez M.et al Infectious agents are not necessary for murine atherogenesis. J Exp Med 20001911437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erridge C, Stewart J, Poxton I R. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the toll‐like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med 20031971787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habich C, Baumgart K, Kolb H.et al The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol 2002168569–576. [DOI] [PubMed] [Google Scholar]