Abstract
Objective
To investigate the role of endothelial function, inflammatory markers, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) in patients with impaired chronotropic response during exercise test.
Methods
86 subjects were enrolled. Treadmill exercise test was conducted according to the modified Bruce protocols. Brachial ultrasound was used to measure endothelium dependent flow mediated vasodilatation (FMD). Chronotropic incompetence was defined as either failure to achieve 85% of the age predicted maximum heart rate or a low chronotropic index (< 0.8).
Results
Of the 86 patients, 20 (23%) exhibited chronotropic incompetence. The patients were divided into three groups according to chronotropic index: group 1, < 0.8 (n = 20); group 2, 0.8–1.0 (n = 26); and group 3, > 1.0 (n = 40). Patients with impaired chronotropic response had significantly lower FMD than those with higher chronotropic response (mean (SD) 2.8 (1.9)% v 5.0 (2.8)% v 5.3 (2.5)%, p = 0.002, for groups 1, 2, and 3, respectively). Serum concentrations of high‐sensitivity C reactive protein (hsCRP), monocyte chemoattractant protein‐1 (MCP‐1), and NT‐proBNP were significantly higher in group 1 than in groups 2 and 3 (hsCRP: 19 (12) v 9 (6) v 9 (6) mg/l, p < 0.05; MCP‐1: 140 (51) v 133 (60) v 108 (46) pg/ml, p = 0.046; NT‐proBNP: 4760 (1980) v 3710 (850) v 3910 (1060) mg/l, p = 0.019, respectively). In addition, chronotropic index was significantly related to FMD (r = 0.380, p = 0.001) and inversely related to hsCRP (r = −0.267, p = 0.013). By multivariate analysis, impaired chronotropic response was significantly related to endothelial dysfunction (p = 0.012).
Conclusion
Patients with impaired chronotropic response to graded exercise had endothelial dysfunction, enhanced systemic inflammation, and higher NT‐proBNP concentrations. These findings may partly explain the mechanism of chronotropic incompetence as a predictor of cardiovascular risk and increased mortality.
Keywords: chronotropic incompetence, endothelial dysfunction, high sensitivity C reactive protein, monocyte chemoattractant protein 1, NT‐proBNP
An impaired heart rate (HR) response to exercise, also known as chronotropic incompetence, has been shown to predict cardiovascular risk and is strongly linked to cardiac mortality.1,2 Lauer et al2 showed that both failure to achieve 85% of the age predicted maximum HR and chronotropic incompetence were associated with adverse risk profiles and thallium perfusion defects. Wiens et al3 and Bruce et al4 also proved that peak HRs of patients with coronary artery disease were significantly lower than those of normal subjects at maximal exercise and that impaired chronotropic response was a relatively specific predictor for coronary artery disease.
Impaired HR response to exercise may be a protective effect against early cardiac ischaemia or be a reduced HR variability with adverse outcome.3,5 However, the exact mechanisms by which chronotropic incompetence during exercise predicts coronary artery disease risk or increased mortality remain unclear. The present study was designed to test the hypothesis that impaired HR response to exercise is associated with endothelial dysfunction and increased systemic vascular inflammation.
METHODS
Study population
The study population was composed of 86 consecutive patients with symptoms of typical or atypical chest pain who were referred for exercise testing and agreed to receive brachial ultrasonography for endothelial function evaluation and blood sampling between July 2003 and June 2004. Patients were excluded if they had a history of cardiac surgery (including coronary artery bypass grafting and valve replacement surgery), myocardial infarction, hospitalisation due to heart failure, congenital heart disease, valvar heart disease (moderate to severe aortic or mitral regurgitation), malignant hypertension, or significant endocrine, hepatic (total bilirubin > 27 μmol/l), or renal disease (serum creatinine > 177 μmol/l). Patients taking β blockers or digoxin were also excluded from the study.
Before the study, each patient's chart was reviewed and an interview was conducted to gather data on symptoms, medications, coronary risk factors, previous cardiac events, smoking habit, exercise habits, family history, and other systemic diseases. Chronic smoking was defined as a history of smoking for ⩾ 1 pack year. Exercise habits were defined as presence or absence of regular exercise (duration ⩾ 30 minutes, frequency ⩾ 3 times a week) in a patient's day to day life. Body mass index was calculated as weight (kg) divided by height (m2). Cardiovascular medications were classified as non‐dihydropyridine calcium channel blockers (for example, diltiazem, verapamil), dihydropyridine calcium channel blockers (for example, nifedipine, felodipine), angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, antiplatelet agents (aspirin, ticlopidine, clopidogrel), nitrates, and statins. Blood biochemistry was analysed for lipid profiles, fasting sugar, and creatinine after 12 hour overnight fasting. All patients gave written informed consent.
Exercise testing
Treadmill exercise testing was conducted according to the modified Bruce protocols used in our laboratory. Participants were encouraged to exercise until they attained at least 90% of their age predicted maximum HR or until fatigue or medical contraindication occurred. Data on symptoms, HR, and blood pressures were collected and an ECG was recorded before exercise, at the end of each exercise stage, at peak exercise, one minute after the cessation of exercise, and every minute thereafter during the recovery period. During the study, an ischaemic response was considered present if there was more than 1 mm of horizontal or downsloping ST segment depression 80 ms after the J point or more than 1 mm of additional ST segment elevation in leads without pathological Q waves.2 All data were entered on to a computer database for final analysis.
Chronotropic incompetence
Chronotropic incompetence was defined as failure to achieve 85% of the age predicted HR. Because this method may be confounded by effects of age, physical fitness, and resting HR, chronotropic response was also assessed by calculating the ratio of the percentage of HR reserve used to the percentage of metabolic reserve used at peak exercise, as described elsewhere.1,6 For any given stage of exercise, the percentage metabolic reserve used was determined as [(METsstage − METsrest)]/[(METspeak − METsrest)] × 100, where METs refers to metabolic equivalents of oxygen consumption, stage refers to any given stage of exercise, and peak refers to peak exercise. The value of METspeak refers to actual oxygen consumption noted, not a theoretical peak value. In an analogous fashion, the percentage of HR reserve used is equal to [(HRstage − HRrest)]/[(HRpeak − HRrest)] × 100. As in previous studies, in a group of healthy adults, the ratio of the percentage of HR reserve used to the percentage of metabolic reserve used during exercise was approximately 1.0 (95% confidence interval 0.8 to 1.3).6 Thus, a ratio ⩽ 0.8 can be defined as chronotropic incompetence, and the cut off point of 0.8 was used to define chronotropic incompetence as in previous studies.2,7 The chronotropic index has also been shown not to be related to physical activity and functional capacity and not to be affected by exercise protocol or during which stage of exercise measurements are taken.1,8
Endothelium dependent flow mediated vasodilatation
Endothelium dependent flow mediated vasodilatation (FMD) was assessed with a 7.5 MHz linear array transducer (Hewlett Packard Sonos 5500, Andover, Massachusetts, USA) to scan the brachial artery in longitudinal section, as described previously.9,10 All patients were asked to fast, refrain from smoking, and withhold all medications for 12 hours before the endothelial function test. To minimise mental stress, care was taken to make the patients as comfortable as possible, and the procedure was performed in a quiet air conditioned room (22–25°C). The left arm was stabilised with a cushion and a sphygmomanometric cuff was placed on the forearm. A baseline image was acquired and blood flow was estimated by time averaging the pulsed Doppler velocity signals obtained from a mid artery sample volume. Then the cuff was inflated to at least 50 mm Hg above systolic pressure to occlude arteries for five minutes and released abruptly. Post‐occlusion diameters were obtained at 60, 80, 100, and 120 seconds after deflation. A mid artery pulsed Doppler signal was obtained immediately on cuff release and no later than 15 seconds after cuff deflation to assess hyperaemic velocity. FMD was calculated as the maximum post‐occlusion diameter relative to the averaged pre‐occlusion diameter.
Endothelium independent glyceryl trinitrate mediated vasodilatation
At least 10 minutes of rest was given after the reactive hyperaemia before another image was acquired to reflect the re‐established baseline conditions. Diameter was measured at least three times at 3−4 minute intervals after 0.6 mg sublingual glyceryl trinitrate (GTN) administration. The maximum FMD and GTN mediated vasodilatation diameters were determined as the average of the three consecutive maximum diameter measurements after reactive hyperaemia and GTN use, respectively. FMD and GTN mediated vasodilatation were then calculated as the percentage change in diameter compared with baseline. An experienced operator who was blinded to all clinical data took all measurements of endothelial function.
Measurement of high‐sensitivity C reactive protein, monocyte chemoattractant protein‐1, and N‐terminal pro‐brain natriuretic peptide
All participants underwent blood sampling before endothelial function measurement and exercise testing. After 12 hour overnight fasting, all patients had a venous blood sample taken for measurement of high‐sensitivity C reactive protein (hsCRP), monocyte chemoattractant protein‐1 (MCP‐1), and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). The blood samples were centrifuged at 3000 rpm for 10 minutes immediately after collection and then the serum samples were kept frozen at −70°C until analysis. hsCRP concentration was determined with the use of a latex enhanced immunophelometric assay (Dade Behring, Marburg, Germany). MCP‐1 in serum was quantified by a sandwich enzyme immunoassay technique (human MCP‐1, Quantikine; R&D Systems, Wiesbaden, Germany) according to the manufacturer's protocol. The serum NT‐proBNP was determined by a sandwich immunoassay (enzyme immunometric assay) with two antibodies (Cortez Diagnostics, Calabasas, California, USA).11 All the procedures were carried out according to the manufacturers' instructions. Each standard and each serum sample were analysed two times, and the mean value was used for all subsequent analysis.
Statistical analysis
The primary end point of this study was endothelium dependent FMD. All data were expressed as mean (SD). A value of p < 0.05 was considered to indicate significance. Differences in baseline characteristics of underlying diseases, smoking habit, exercise habits, and medications were compared by the χ2 test. The three groups were compared by analysis of variance for continuous variables and Kruskal‐Wallis test for normally distributed variables. The degree of association between the independent variables age, sex, body mass index, current smoking, hypertension, diabetes mellitus, serum lipid profiles, fasting glucose, systolic blood pressure, diastolic blood pressure, pulse pressure, resting HR, total exercise time, HR after exercise, chronotropic index, hsCRP, MCP‐1, and NT‐proBNP was measured by means of simple linear regression and multiple regression analyses. The SPSS 9.0 (SPSS, Chicago, Illinois, USA) software package was used for statistical analysis.
RESULTS
Patients' baseline and exercise characteristics
A total of 86 patients (31–78 years, mean (SD) age 59 (14) years; 80% men) were enrolled in the study. The 86 patients were then divided into three groups according to chronotropic index: group 1, < 0.8 (n = 20); group 2, 0.8–1.0 (n = 26); group 3, > 1.0 (n = 40). Twenty patients (23%; group 1) had a low chronotropic incompetence response (chronotropic index < 0.8) during the exercise test, including 16 patients (19%) who failed to reach 85% of their age predicted maximum HR in this study. Table 1 shows the baseline characteristics of the 86 participants.
Table 1 Baseline characteristics and medication use of 86 studied patients in three groups.
| Group 1 (n = 20) | Group 2 (n = 26) | Group 3 (n = 40) | p Value | |
|---|---|---|---|---|
| Age (years) | 57 (18) | 57 (14) | 60 (12) | 0.637 |
| Men | 17 (85%) | 22 (85%) | 30 (75%) | 0.642 |
| Body mass index (kg/m2) | 25 (3) | 26 (3) | 25 (2) | 0.465 |
| Current smoking | 6 (30%) | 7 (27%) | 9 (23%) | 0.821 |
| Exercise habits | 3 (15%) | 4 (15%) | 6 (15%) | 1.000 |
| Systemic hypertension | 10 (50%) | 9 (35%) | 16 (40%) | 0.608 |
| Diabetes mellitus | 2 (10%) | 1 (4%) | 2 (5%) | 0.707 |
| Hypercholesterolaemia* | 7 (35%) | 15 (58%) | 18 (45%) | 0.300 |
| Lipid profile (mmol/l) | ||||
| Total cholesterol | 5.10 (0.9) | 5.67 (1.2) | 5.23 (0.9) | 0.132 |
| Triglycerides | 1.87 (1.1) | 2.65 (4.8) | 1.65 (5.5) | 0.613 |
| High density lipoprotein | 1.24 (0.4) | 1.24 (0.3) | 1.14 (0.3) | 0.364 |
| Fasting glucose (mmol/l) | 6.10 (2.2) | 5.72 (1.7) | 5.38 (0.6) | 0.180 |
| Medication use | ||||
| Non‐dihydropyridine CCB | 2 (10%) | 5 (19%) | 3 (8%) | 0.387 |
| Dihydropyridine CCB | 2 (17%) | 3 (12%) | 7 (18%) | 0.781 |
| ACE or ARB | 5 (25%) | 3 (12%) | 9 (23%) | 0.475 |
| Antiplatelet agent | 6 (30%) | 5 (19%) | 3 (8%) | 0.076 |
| Nitrate | 5 (25%) | 5 (19%) | 4 (10%) | 0.271 |
| Statin | 3 (15%) | 4 (15%) | 3 (8%) | 0.511 |
Values are mean (SD) or number (%).
*Total cholesterol ⩾5.18 mmol/l.
ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
No significant differences were found between the three groups in terms of age, sex, body mass index, smoking status, exercise habits, underlying diseases (hypertension, diabetes), or medications. Table 2 lists the results of the exercise test in the three groups of patients. No significant differences were found in resting blood pressure and pulse pressure between the three groups of patients. However, patients with impaired chronotropic response were shown to have lower resting HR, lower peak HR, shorter exercise duration, and attenuated HR recovery at the third minute after peak exercise (p < 0.05). Moreover, more patients in group 1 than in group 2 and group 3 (30% v 19% v 8%, p = 0.076) tended to terminate the exercise test due to effort chest pain or ECG changes.
Table 2 Exercise test results of 86 studied patients in three groups.
| Group 1 (n = 20) | Group 2 (n = 26) | Group 3 (n = 40) | p Value | |
|---|---|---|---|---|
| Blood pressure at rest (mm Hg) | ||||
| Systolic | 129 (21) | 134 (20) | 132 (18) | 0.534 |
| Diastolic | 75 (9) | 77 (8) | 81 (10) | 0.068 |
| Pulse | 53 (18) | 57 (17) | 52 (15) | 0.379 |
| Resting HR (beats/min) | 69 (14) | 77 (13) | 82 (16) | 0.009 |
| HR after exercise (1st min) (beats/min) | 96 (18) | 107 (12) | 114 (13) | 0.001* |
| Increased HR at 1st min (beats/min) | 26 (9) | 31 (10) | 32 (8) | 0.114 |
| HR after exercise (3rd min) (beats/min) | 110 (18) | 119 (15) | 131 (18) | 0.001 |
| Increased HR at 3rd min (beats/min) | 41 (10) | 42 (13) | 49 (16) | 0.056 |
| Peak HR (beats/min) | 130 (27) | 157 (14) | 169 (11) | 0.001* |
| Peak metabolic equivalents | 8.0 (2.3) | 9.7 (2.0) | 9.2 (2.2) | 0.027 |
| HR recovery at 1st min (beats/min) | 20 (9) | 24 (15) | 27 (13) | 0.150 |
| HR recovery at 3rd min (beats/min) | 42 (14) | 54 (17) | 56 (17) | 0.010 |
| Total exercise duration (s) | 410 (134) | 515 (118) | 492 (119) | 0.014 |
| Chronotropic index | 0.6 (0.2) | 0.9 (0.1) | 1.2 (0.1 | 0.001 |
| Terminated exercise due to chest pain or ECG change | 6 (30%) | 5 (19%) | 3 (8%) | 0.076 |
Values are mean (SD).
*p<0.05 between groups by Kruskal‐Wallis test.
HR, heart rate.
FMD, GTN mediated vasodilatation, hsCRP, MCP‐1, and NT‐proBNP
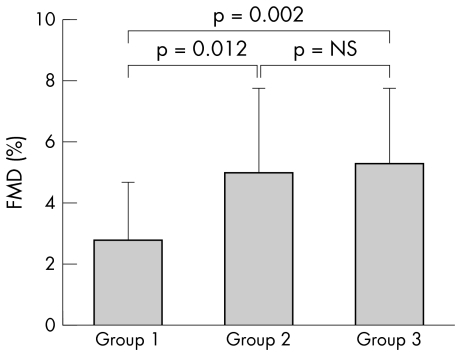
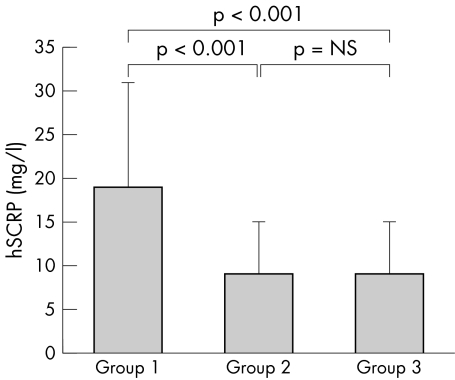
Table 3 lists the mean percentage changes of brachial artery diameters at baseline, during reactive hyperaemia, and after GTN administration, as well as the serum concentrations of hsCRP, MCP‐1, and NT‐proBNP in the three groups. Baseline diameter did not differ significantly in the three groups. Patients in group 1 had significantly lower FMD responses than those in group 2 and group 3 (2.8 (1.9)% v 5.0 (2.8)% v 5.3 (2.5)%, p = 0.002) but not significantly lower GTN mediated vasodilatation (9.6 (4.7)% v 13.2 (6.5)% v 13.1 (6.6)%, p = 0.082). Patients with a lower chronotropic index (group 1) had significantly higher serum concentrations of hsCRP and MCP‐1 than those with a higher chronotropic index (group 2 and group 3) (19 (12) v 9 (6) v 9 (6) mg/l, p < 0.05; 140 (51) v 133 (60) v 108 (46) pg/ml, p = 0.046, respectively). In addition, patients in group 1 were observed to have higher NT‐proBNP concentrations than those in group 2 and group 3 (4760 (1980) v 3710 (850) v 3910 (1060) mg/l, p = 0.019). Figures 1 and 2 compare the percentage changes of FMD and hsCRP between the three groups of patients.
Table 3 Comparison of baseline brachial artery diameter, mean percentage change of diameter in response to FMD and GTN mediated dilatation, and hsCRP and NT‐proBNP in the three groups of patients.
| Group 1 (n = 20) | Group 2 (n = 26) | Group 3 (n = 40) | p Value | |
|---|---|---|---|---|
| Baseline diameter (mm) | 3.9 (0.6) | 3.7 (0.6) | 3.7 (0.7) | 0.658 |
| Maximum diameter (mm) | 4.0 (0.5) | 3.9 (0.6) | 3.9 (0.7) | 0.915 |
| FMD (%) | 2.8 (1.9) | 5.0 (2.8) | 5.3 (2.5) | 0.002 |
| Flow change during hyperaemic phase (%) | 353 (188) | 345 (181) | 360 (173) | 0.948 |
| Baseline diameter before GTN mediated vasodilatation (mm) | 3.9 (0.6) | 3.7 (0.6) | 3.7 (0.7) | 0.465 |
| GTN mediated vasodilation (%) | 9.6 (4.7) | 13.2 (6.5) | 13.1 (6.6) | 0.082 |
| hsCRP (mg/l) | 19 (12) | 9 (6) | 9 (6) | <0.05* |
| MCP‐1 (pg/ml) | 140 (51) | 133 (60) | 108 (46) | 0.046 |
| NT‐proBNP (mg/l) | 4760 (1980) | 3710 (850) | 3910 (1060) | 0.019 |
Values are mean (SD).
*p<0.05 between groups by Kruskal‐Wallis test.
FMD, flow mediated vasodilatation; GTN, glyceryl trinitrate; hsCRP, high sensitivity C reactive protein; MCP‐1, monocyte chemoattractant protein 1; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 1 Comparison of percentage changes of endothelium dependent flow mediated dilatation (FMD) between the groups of patients by post hoc test. Group 1 v group 2, p = 0.012; group 2 v group 3, not significant (NS); group 1 v group 3, p = 0.002; all three groups by analysis of variance, p = 0.002.
Figure 2 Comparison of mean serum concentrations of high sensitivity C reactive protein (hsCRP) between the groups of patients by post hoc test. Group 1 v group 2, p < 0.001; group 2 v group 3, NS; group 1 v group 3, p < 0.001; all three groups by Kruskal‐Wallis test, p < 0.05.
Correlation between chronotropic index and FMD
As table 4 shows, simple linear regression analysis of data from all participants showed that the chronotropic index and HR recovery after exercise (first minute) were positively correlated with FMD (r = 0.380, p = 0.001 and r = 0.213, p = 0.049, respectively). When all univariate baseline parameters were entered into a multiple regression analysis, the results showed that endothelium dependent FMD was significantly related to chronotropic index (p = 0.012) and serum triglyceride concentration (p = 0.011) (table 5).
Table 4 Simple linear regression analysis for determinants of FMD.
| Variable | r | p Value |
|---|---|---|
| Age | −0.131 | 0.228 |
| Chronotropic index | 0.380 | 0.001 |
| hsCRP | −0.267 | 0.013 |
| MCP‐1 | −0.200 | 0.065 |
| NT‐proBNP | −0.174 | 0.108 |
| Total cholesterol | 0.073 | 0.502 |
| Triglycerides | 0.203 | 0.060 |
| High density lipoprotein | 0.094 | 0.389 |
| Fasting glucose | −0.090 | 0.411 |
| Systolic blood pressure | 0.064 | 0.559 |
| Diastolic blood pressure | 0.119 | 0.274 |
| Pulse pressure | 0.007 | 0.950 |
| Resting HR | 0.069 | 0.527 |
| Total exercise time | 0.195 | 0.072 |
| Increased HR at 1st min | 0.119 | 0.275 |
| HR recovery after exercise (1st min) | 0.213 | 0.049 |
Table 5 Multiple regression analysis for determinants of FMD.
| Variable | Coefficient | 95% confidence interval | Standardised coefficient | p Value |
|---|---|---|---|---|
| Chronotropic index | 3.056 | 0.691 to 5.421 | 0.287 | 0.012 |
| Triglycerides | 0.003 | 0.001 to 0.005 | 0.271 | 0.011 |
| MCP‐1 | −0.004 | −0.015 to 0.007 | −0.084 | 0.440 |
| hsCRP | −0.324 | −1.004 to 0.355 | −0.108 | 0.355 |
| NT‐proBNP | −0.001 | −0.005 to 0.004 | −0.011 | 0.919 |
| HR recovery after exercise (1st min) | 0.029 | −0.012 to 0.071 | 0.287 | 0.160 |
DISCUSSION
To the best of our knowledge, this is the first study to show that impaired chronotropic response to exercise is significantly related to endothelial vasodilator dysfunction in patients with suspected coronary artery disease referred for a treadmill exercise test. In addition, patients with chronotropic incompetence during exercise were found to have higher serum concentrations of hsCRP, MCP‐1, and NT‐proBNP than those with a normal chronotropic response to exercise.
Several large scale studies have proved that chronotropic incompetence is independently predictive of all cause mortality, even after the angiographic severity of coronary artery disease or thallium perfusion defects are accounted for.2,12 In the Framingham heart study, Lauer et al1 showed that an attenuated HR response to exercise was associated with higher total mortality and with an increased risk of coronary heart disease presenting as myocardial infarction, angina pectoris, and sudden cardiac death.
It is well known that most myocardial perfusion occurs during the diastolic phase and that, as the HR increases, the diastolic phase is reduced from about 70% of the cardiac cycle at rest to about 20% at maximum HR.13 During exercise, patients with coronary artery disease face an increased oxygen demand and decreased coronary perfusion due to a reduced diastolic phase. Therefore, we may propose that inhibition of HR increase during exercise may be a protective physiological response to avoid excessive myocardial ischaemia. In this study, chronotropic incompetence was shown to be significantly related to endothelial dysfunction and systemic vascular inflammation. Recently, Aronson et al14 also showed that patients with the metabolic syndrome who maintain a high fitness level have much lower CRP concentrations than do those with a low fitness level. Furthermore, the effect of physical fitness on patients with the metabolic syndrome is more pronounced than that in those without the metabolic syndrome. This finding may explain why patients with an impaired HR response to exercise have increased coronary heart disease events and implies that vascular atherosclerosis develops in stages.
It is interesting to find, however, that patients with chronotropic incompetence to exercise test had a lower resting HR than those with a normal HR response during exercise, which was previously thought to be a protective factor against cardiovascular events.7 This finding was also observed in previous clinical studies.2,8 Elhendy et al8 showed that resting HR was significantly lower in patients with chronotropic incompetence than in those without chronotropic incompetence. The reason underlying this phenomenon is unclear, but a decreased response of the heart to β stimulation due to frequent sympathetic hyperactivation may be responsible in these patients.
NT‐proBNP, a circulating hormone released from the cardiac ventricles in response to increased cardiac wall stress, had been proved to be a strong predictor of cardiovascular events and congestive heart failure.15,16 The measurement of NT‐proBNP has also been shown to be useful in detecting left ventricular dysfunction.17 In our study, patients with chronotropic incompetence were found to have higher serum concentrations of NT‐proBNP, and this finding suggests that patients with impaired HR responses during exercise have greater ventricular wall stress or may have more intermittent and transient ventricular ischaemia. This consequence may result in sympathetic hyperactivity and ultimately lead to decreased sensitivity of the heart to β adrenergic receptor stimulation.
More and more evidence has shown that the autonomic system has a critical role in regulating cardiovascular function in both healthy and diseased populations.5,18,19 In the present study, patients with impaired chronotropic response to exercise had attenuated HR recovery, which had been proved to be an independent predictor of all cause mortality in several large scale studies.20 Chronotropic incompetence may reflect a modulation of autonomic tone that implies more severe cardiovascular perturbations. Fei et al21 showed that HR variability is significantly decreased in patients with congestive heart failure who have chronotropic incompetence. The low frequency component of spectral HR variability gives a predominant measure of sympathetic activity, and the high frequency is almost exclusively mediated by vagal activity. The low, but not the high, frequency components was significantly lower in patients with chronotropic incompetence.21 The finding that only the low frequency component was significantly reduced suggests that impaired sympathetic activity may be an important factor contributing to chronotropic incompetence. Previous studies have also shown that increased sympathetic tone may lead to endothelial dysfunction.22,23 These data clearly showed the close relation between the autonomic system regulated chronotropic response during exercise and endothelial function. Therefore, patients with impaired chronotropic responses during exercise, reflecting a higher systemic inflammatory status, greater ventricular wall stress, and endothelial dysfunction, are warranted to receive more aggressive antiatherosclerosis treatment and lifestyle modification in preventing further cardiovascular events. However, further studies are needed to support this finding and may focus on whether improving endothelial function can improve chronotropic HR response to exercise.
Study limitations
In this study, some of the relations between chronotropic index and markers of inflammation may have been mediated by other covariates or confounders. Except for the analysis of FMD, the analyses presented are univariate analyses and may not be adequately adjusted.
Conclusion
We found a significant relation between chronotropic incompetence to exercise and endothelial dysfunction. Enhanced vascular inflammation was also noted in this high risk group, and the early stage of vascular injury may account for the impaired chronotropic response to exercise being predictive of increased coronary heart disease risk and long term survival. More aggressive risk factor modification and antiatherosclerosis treatment may be needed in such group of patients.
ACKNOWLEDGEMENTS
The authors thank Wei‐Heng Chi, BS, a statistician, and Shu‐Chuan Lin, BSc, who made it possible to analyse the data in a more meaningful way. This study was partly supported by research grants NSC 93‐2314‐B‐010‐004 from the National Science Councils, Taiwan, ROC, VGH‐239 VGHUST 93‐P3‐15 from Taipei Veterans General Hospital, and CI 93‐12 from the Yen Tjing Ling Medical Foundation, Taipei, Taiwan.
Abbreviations
FMD - flow mediated vasodilatation
GTN - glyceryl trinitrate
HR - heart rate
hsCRP - high‐sensitivity C reactive protein
MCP‐1 - monocyte chemoattractant protein‐1
METs - metabolic equivalents
NT‐proBNP - N‐terminal pro‐brain natriuretic peptide
Footnotes
†Also the Cardiovascular Research Centre, Taipei, Taiwan
References
- 1.Lauer M S, Okin P M, Larson M G.et al Impaired heart rate response to graded exercise: prognostic implications of chronotropic incompetence in the Framingham heart study. Circulation 1996931520–1526. [DOI] [PubMed] [Google Scholar]
- 2.Lauer M S, Francis G S, Okin P M.et al Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999281524–529. [DOI] [PubMed] [Google Scholar]
- 3.Wiens R D, Lafia P, Marder C M.et al Chronotropic incompetence in clinical exercise testing. Am J Cardiol 19845474–78. [DOI] [PubMed] [Google Scholar]
- 4.Bruce R A, Gey G O, Cooper M N.et al The Seattle heart watch: initial, clinical, circulatory and electrocardiographic responses to maximal exercise. Am J Cardiol 197433459–469. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H, Venditti F J, Manders E S.et al Reduced heart rate variability and mortality risk in an elderly cohort: the Framingham heart study. Circulation 199490878–883. [DOI] [PubMed] [Google Scholar]
- 6.Go R T, Marwick T H, Maclntyre W J.et al A prospective comparison of rubidium‐82 PET and thallium‐201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med 1990131160–168. [PubMed] [Google Scholar]
- 7.Kannel W B, Kannel C, Paffenbarger R S., Jret al Heart rate and cardiovascular mortality: the Framingham study. Am Heart J 19871131489–1494. [DOI] [PubMed] [Google Scholar]
- 8.Elhendy A, Mahoney D W, Khandheria B K.et al Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol 200342823–830. [DOI] [PubMed] [Google Scholar]
- 9.Corretti M C, Anderson T J, Benjamin E J.et al Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery. J Am Coll Cardiol 200239257–265. [DOI] [PubMed] [Google Scholar]
- 10.Huang P H, Leu H B, Chen J W.et al Usefulness of attenuated heart rate recovery immediately after exercise to predict endothelial dysfunction in patients with suspected coronary artery disease. Am J Cardiol 20049310–13. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen L S, Svanegaard J, Klitgaard N A.et al N‐terminal pro‐brain natriuretic peptide for discriminating between cardiac and non‐cardiac dyspnoea. Eur Heart J 2004663–70. [DOI] [PubMed] [Google Scholar]
- 12.Dresing T J, Blackstone E H, Pashkow F J.et al Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. Am J Cardiol 200086602–609. [DOI] [PubMed] [Google Scholar]
- 13.Ferro G, Spinelli L, Duilio C.et al Diastolic perfusion time at ischemic threshold in patients with stress‐induced ischemia. Circulation 19918449–56. [DOI] [PubMed] [Google Scholar]
- 14.Aronson D, Sella R, Sheikh‐Ahmad M.et al The association between cardiorespiratory fitness and C‐reactive protein in subjects with the metabolic syndrome. J Am Coll Cardiol 2004442003–2007. [DOI] [PubMed] [Google Scholar]
- 15.Galvani M, Ottani F, Oltrona L.et al N‐terminal pro‐brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation 20041101–7. [DOI] [PubMed] [Google Scholar]
- 16.Campbell D J, Woodward M, Chalmers J P.et al Prediction of heart failure by amino terminal‐pro‐B‐type natriuretic peptide and C‐reactive protein in subjects with cerebrovascular disease. Circulation 2005451–6. [DOI] [PubMed] [Google Scholar]
- 17.Hunt P J, Richards A M, Nicholls M G.et al Immunoreactive amino‐terminal pro‐brain natriuretic peptide (NT‐proBNP): a new marker of cardiac impairment. Clin Endocrinol (Oxf) 199747287–296. [DOI] [PubMed] [Google Scholar]
- 18.Cohn J N, Levine T B, Olivari M T.et al Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984311819–823. [DOI] [PubMed] [Google Scholar]
- 19.Robertson D, Johnson G A, Robertson R M.et al Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation 197959637–643. [DOI] [PubMed] [Google Scholar]
- 20.Nishime E O, Cole C R, Blackstone E H.et al Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 20002841392–1398. [DOI] [PubMed] [Google Scholar]
- 21.Fei L, Keeling P J, Sadoul N.et al Decreased heart rate variability in patients with congestive heart failure and chronotropic incompetence. Pacing Clin Electrophsyiol 199619477–483. [DOI] [PubMed] [Google Scholar]
- 22.Hijmering M L, Stroes E S, Olijhoek J.et al Sympathetic activation markedly reduces endothelium‐dependent, flow‐mediated vasodilation. J Am Coll Cardiol 200239683–688. [DOI] [PubMed] [Google Scholar]
- 23.Ghiadoni L, Donald A E, Cropley M.et al Mental stress induces transient endothelial dysfunction in humans. Circulation 20001022473–2478. [DOI] [PubMed] [Google Scholar]