The correct diagnosis of a wide complex tachycardia (WCT)—QRS duration > 120 ms—remains a challenge despite numerous established criteria for the differentiation of ventricular from supraventricular tachycardia (SVT) with aberrant conduction. Making the correct diagnosis is important for the acute as well as long term management of patients with WCT. The objective of the present review is to discuss the major causes as well as clinical and electrophysiologic criteria of WCT (table 1) in patients without structural heart disease.
Table 1 Causes of wide complex tachycardias (WCTs) in patients without structural heart disease.
| Monomorphic configuration |
| Supraventricular tachycardia (SVT) |
| • Bundle branch block (figs 1, 2) |
| - functional (RBBB more often than LBBB) |
| - pre‐existing |
| - rate related |
| • Antidromic (i.e. retrograde conduction over AV node; figs 3, 4) |
| • Non‐specific conduction delay |
| - class I or class III antiarrhythmic drugs |
| - electrolyte imbalance |
| Ventricular tachycardia (VT) |
| • LBBB, inferior axis (fig 7): idiopathic right ventricular VT |
| • RBBB, superior axis (fig 8): idiopathic left ventricular VT |
| • Pacemaker mediated VT |
| Polymorphic configuration |
| Supraventricular tachycardia |
| • Atrial fibrillation with pre‐excitation (fig 3) |
| Ventricular tachycardia |
| • Torsade de pointes (long QT syndrome) (fig 9) |
| • Brugada syndrome |
| • Catecholaminergic polymorphic VT (fig 10) |
| • Short QT syndrome |
All WCTs should be treated as if the rhythm was VT until proven otherwise.
AV, atrioventricular; LBBB, left bundle branch block; RBBB, right bundle branch block.
Broad categories of WCTs include ventricular tachycardia (VT), SVT with abnormal intraventricular conduction, and ventricular paced rhythms. A lack of underlying structural heart disease does neither exclude a VT nor imply a benign prognosis. However, if a patient has had similar episodes during previous years, SVT is more likely than VT. Termination of a tachycardia by the Valsalva manoeuvre or adenosine injection also suggests a supraventricular origin, although some VT can also be terminated by these manoeuvres (for example, fascicular VT).
A WCT in a patient who is alert and haemodynamically stable is not necessarily of supraventricular origin. The clinical presentation depends on the haemodynamic consequences it produces. These depend partly on tachycardia rate, the degree of myocardial dysfunction, the circumstances and suddenness of initiation, and autonomic factors. Physical examination in a patient presenting with WCT may indicate haemodynamic distress (low blood pressure, heart failure or cardiogenic shock). When cardiac output and blood pressure are maintained and/or when the tachycardia is short lived, the arrhythmia may present as palpitations, breathlessness or just discomfort.
WIDE COMPLEX SUPRAVENTRICULAR TACHYCARDIAS
Intraventricular conduction delay can result from heart rate changes, as well as from fixed pathological lesions in the conduction system. In patients with pre‐existing or “fixed” (present during the normal baseline rhythm) bundle branch block (BBB), any SVT results in a broad complex tachycardia. However, rate related and/or “functional” (present only during tachycardia) BBB may also result in WCT. Functional aberration results from sudden increases in cycle length when parts of the His‐Purkinje system are partially or wholly inexcitable. Functional right bundle branch block (RBBB) occurs more frequently than functional left bundle branch block (LBBB) because of the longer refractoriness of the former.1 Sometimes, discrete variations in cycle length change a broad to a narrow complex tachycardia and thereby facilitate the correct diagnosis (fig 1). A sudden short long cycle length variation lengthens the refractoriness of the His‐Purkinje system and an abrupt long‐to‐short cycle length change shortens the refractoriness of the His‐Purkinje system refractoriness.2 Functional BBB may persist for several successive impulses because the bundle branch that is blocked antegradely may be activated transseptally via its contralateral counterpart, a process known as linking phenomenon.3 As the duration of the refractory period is a function of the immediately preceding cycle length (the longer this cycle length, the longer the subsequent refractory period), abrupt cycle length variations (that is, long‐to‐short or short‐to‐long) predispose to the occurrence of functional BBB—for example, in atrial fibrillation, which is known as Ashman phenomenon4 (fig 2).
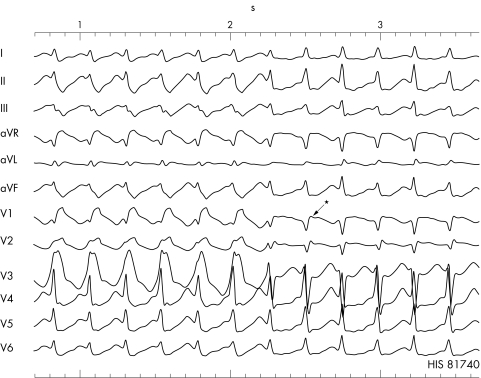
Figure 1 Episode of a wide complex tachycardia with a right bundle branch block (RBBB) configuration, which spontaneously changes to a narrow complex SVT in the presence of a slight decrease in cycle length. Please note that the retrograde P wave is seen slightly after the QRS complex (pseudo r' wave in lead V1 and the pseudo s wave in leads II, III, and aVF (*), which suggest typical atrioventricular (AV) nodal re‐entrant tachycardia.
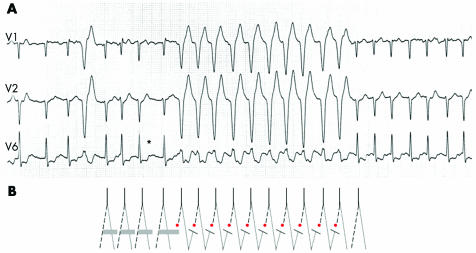
Figure 2 (A) Atrial fibrillation with ventricular aberration resulting from the Ashman phenomenon, and probably concealed transseptal conduction. After a long pause (*) the refractory period of the left bundle branch is prolonged which results in 10 RS complexes with left bundle branch block morphology. The aberration is probably perpetuated by concealed transseptal conduction (“linking phenomenon”) from the right into the left bundle with block of antegrade conduction of the subsequent impulses in the left bundle. (B) The solid line represents the His bundle; the dashes (dots) represent the left (right) bundle. The solid horizontal bars represent the refractory period.
Pre‐excitated tachycardias in Wolff‐Parkinson‐White syndrome account for a minority of WCT. In these tachycardias ventricular activation occurs predominantly or exclusively via an accessory pathway. Variations in pre‐excitation (fig 3) along with cycle length changes are caused by atrial fibrillation conducted over the pathway, whereas a monomorphic picture of pre‐excitation is almost always due to a re‐entrant tachycardia (with the exception of an atrial tachycardia such as atrial flutter conducted over the accessory pathway). In the presence of atrial fibrillation, rapid ventricular rates caused by atrioventricular (AV) conduction over an accessory pathway (fig 3) may cause ventricular fibrillation and sudden cardiac death.5 If the accessory pathway is the antegrade limb in the re‐entrant process, the tachycardia is termed antidromic. The retrograde limb is mostly the normal pathway but may also be another accessory pathway. In general, an antidromic AV re‐entrant tachycardia is seen much less commonly than an orthodromic tachycardia. As accessory pathways connect atrial and ventricular muscle at the level of the AV junction, the ECG of pre‐excitated SVT can be indistinguishable from a VT originating at the base of the ventricles.
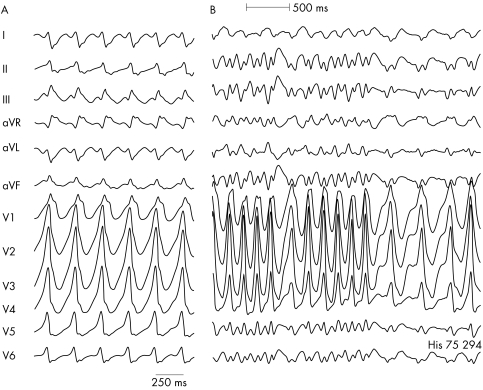
Figure 3 Antidromic tachycardia (A) over a left sided accessory pathway in a patient with Wolff‐Parkinson‐White syndrome. (B) Atrial fibrillation with rapid conduction over the pathway in the same patient (shortest RR interval 200 ms).
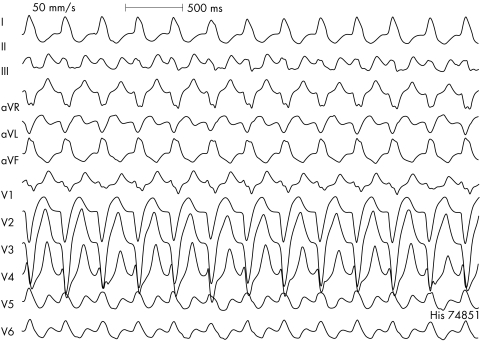
In WCT with an LBBB morphology and a left superior axis (fig 4), one should always consider an atriofascicular (Mahaim) accessory pathway. Patients with these pathways may have episodes of pre‐excitated tachycardia without exhibiting pre‐excitation during sinus rhythm. Narrow QRS complex tachycardias almost never occur because of the absence of retrograde conduction over the accessory pathway. The QRS morphology during tachycardia typically shows a small, short, initial R wave followed by a steep downstroke in ECG lead V1.
Figure 4 Twelve lead ECG recorded during antidromic AV re‐entrant tachycardia using a right atriofascicular (Mahaim) accessory pathway for antegrade conduction. These pathways are typically located along the endocardial surface of the right ventricular free wall with the distal insertion in the apical region of the right bundle branch. Differential diagnosis includes a ventricular paced rhythm as the majority of ventricular pacing leads are situated in the right ventricular apex.
Other mechanisms of ventricular aberration include depressed myocardial conduction as a result of drug effects or electrolyte disturbances such as hyperkalaemia. In addition, high doses of sodium channel blocking antiarrhythmic agents (that is, class IA and class IC drugs) can cause a non‐specific widening of the QRS complex. Class IC drugs can also provoke WCT by slowing an atrial tachycardia rate enough that 1:1 AV conduction can occur (fig 5). In addition, antiarrhythmic agents have also been reported to induce sustained monomorphic VT during exercise testing in young healthy individuals without structural heart disease.
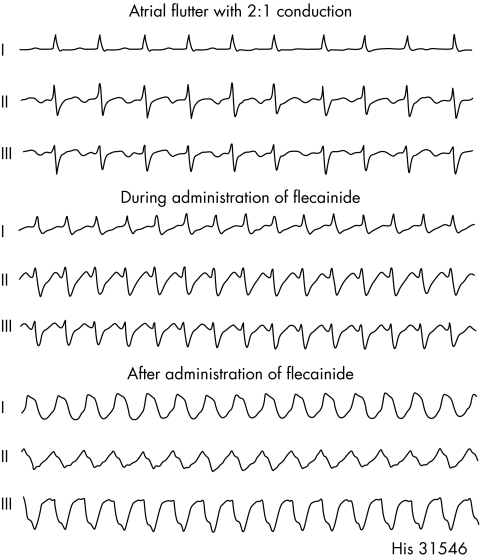
Figure 5 Example of a wide complex tachycardia in the presence of the class IC antiarrhythmic agent flecainide in a patient with atrial flutter. Flecainide causes this tachycardia by slowing the atrial flutter rate enough that 1:1 AV conduction occurs.
Management of wide complex SVT ranges from no treatment when episodes are infrequent and non‐sustained to curative therapy with radiofrequency ablation. The introduction of catheter ablation has dramatically changed the approach to patients with SVT. The current approach is to offer the patient who requires long term treatment the option of radiofrequency catheter ablation as curative therapy. Long term pharmacotherapy (that is, in the absence of pre‐excitation AV node blocking agents) is chosen for those patients who decline or fail catheter ablation and for those in whom ablation is considered to carry a high risk of complications, such as an inadvertent AV block.
ELECTROCARDIOGRAPHIC CLUES IN DIFFERENTIAL DIAGNOSIS: SVT VERSUS VT
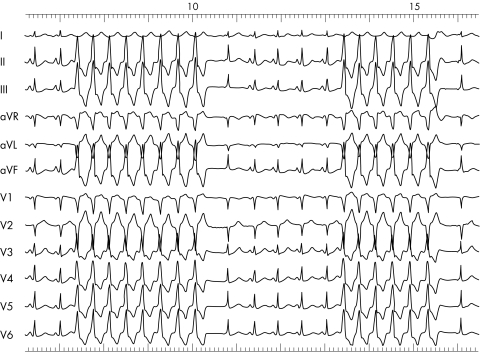
In general, if an ECG showing a WCT does not look like aberration, it is most likely a VT (fig 6). If there is any doubt about the origin of a WCT, the patient should be treated as if the rhythm was VT. The absence of an RS complex in any precordial lead or an interval of the R wave onset to the S wave nadir of more than 100 ms strongly suggest a VT.6 In addition, the following ECG criteria have been suggested to distinguish between VT and SVT with aberration:
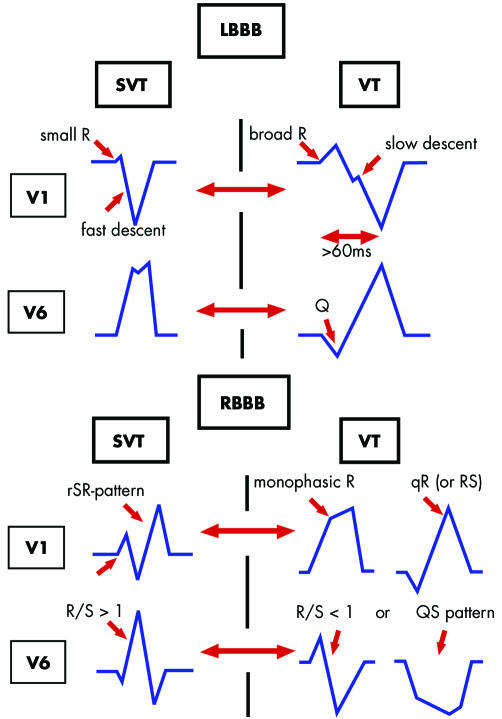
Figure 6 QRS criteria for differential diagnosis in broad complex tachycardia: ventricular tachycardia (VT) versus supraventricular tachycardia (SVT) with left (LBBB) or right (RBBB) bundle branch block.
QRS complex duration—VT is the probable diagnosis when the QRS duration with RBBB is greater than 140 ms, and greater than 160 ms with LBBB morphology.7
QRS axis—A frontal axis between −90° to ±180° cannot be achieved by any combination of bundle branch block and therefore suggests VT. Thus, predominantly negative QRS complexes in leads I, II, and III are a useful criterion for identifying a VT.
Concordant negative ECG patterns in the precordial leads—If all precordial leads are predominantly negative, a VT is the likely diagnosis. If all precordial leads are predominantly positive, the differential diagnosis is an antidromic tachycardia using a left sided accessory pathway or a VT.
QRS morphologies in V1 and V6—In an RBBB pattern, a monophasic R wave, a broad (> 30 ms) R, or a qR in V1 strongly suggest VT. A monophasic R wave, or an S greater than an R in V6, also suggest VT. In the presence of an LBBB pattern, a broad R wave (usually greater than 30 ms) and/or a slow descent to the S wave nadir in V1 and a Q in V6 point towards a VT.
Atrioventricular dissociation
This is one of the most useful criteria for distinguishing VT from SVT. It occurs in 20–50% of VT and almost never in SVT. Atrioventricular dissociation may be diagnosed by a changeable pulse pressure, irregular canon A waves in the jugular veins and a variable first heart sound. It often demands long 12 lead ECG recordings and careful ECG analysis. In addition, about 30% of VTs have 1:1 retrograde conduction. In the presence of AV dissociation, one may also observe fusion beats which may result from the fusion of a P wave conducted to the ventricles.
The 12 lead ECG during VT can be helpful in providing an approximation of the site of origin. In general, VT that have an LBBB‐like morphology in V1 have an exit in the right ventricle or the interventricular septum. A QRS axis that is directed superiorly generally indicates an exit in the inferior wall; an axis directed inferiorly indicates an exit in the anterior (superior) wall. In V2 to V4, dominant R waves usually indicate an exit near the base of the ventricle. In idiopathic right ventricular outflow tract tachycardia (RVOT‐VT), the QRS duration during VT is usually > 140 ms if it originates from the free wall of the RVOT, and < 140 ms if the arrhythmia originates from the septal site of the RVOT. Furthermore, the precordial R wave transition in RVOT‐VT usually occurs in leads V2 through V4 and becomes earlier as the site of origin advances more superiorly along the septum. An R wave transition in lead V2 suggests a site of origin immediately inferior to the pulmonic valve or the left ventricular outflow tract.
VENTRICULAR TACHYCARDIA IN PATIENTS WITHOUT STRUCTURAL HEART DISEASE: “IDIOPATHIC” VT
“Idiopathic VT” is a non‐specific term which represents a heterogeneous group of arrhythmias. Patients can be completely asymptomatic or have transient symptoms including palpitations, dizziness, or presyncope; however, these arrhythmias, with the exception of rapid polymorphic VT occurring in the setting of inherited arrhythmic syndromes, are rarely life‐threatening. The underlying mechanisms include re‐entry, triggered activity, and catecholamine‐mediated automaticity. Idiopathic VT can be categorised according to the clinical presentation (non‐sustained versus sustained), precipitating factors (for example, exercise), site of origin (that is, left or right ventricle), by response to antiarrhythmic drugs (for example, adenosine or verapamil), or on the basis of an underlying primary electrical disorder.
VT IN PATIENTS WITHOUT STRUCTURAL HEART DISEASE AMENABLE TO CURATIVE TREATMENTS
Idiopathic ventricular outflow tract tachycardia
This arrhythmia usually originates in the right ventricular outflow tract and manifests as a left bundle branch block VT with an inferior axis (fig 7). It is often seen in younger patients (female > male) without structural heart disease and accounts for up to 70% of idiopathic VT. Although the majority of cases appear to occur sporadically rather than on a familial basis, the condition is generally considered as a “primary electrical disease”. It is important in the differential diagnosis of various entities, in particular mild or subclinical forms of arrhythmogenic right ventricular cardiomyopathy.8 Most data suggest that the underlying mechanism is triggered activity caused by adenylcyclase‐mediated delayed afterdepolarisations. Idiopathic outflow tract tachycardias are usually exertion or stress related arrhythmias. They can also present as recurrent extrasystoles or non‐sustained arrhythmias tending to occur at rest, or provoked only with exercise (Gallavardin's tachycardias). However, these forms may just represent different spectra of the same arrhythmia. Idiopathic outflow tract tachycardias are usually well tolerated, probably because of the preserved ventricular function. Hence, this VT has a favourable long term prognosis when compared with VT in structural heart disease. The arrhythmia is often responsive to treatment with β blockers, sotalol9 or calcium channel blockers and can also be amenable to transcatheter ablation.8,10
Figure 7 Repetitive episodes of an idiopathic right ventricular tachycardia demonstrating the characteristic LBBB pattern with an inferior axis.
Idiopathic left ventricular tachycardia (fascicular VT)
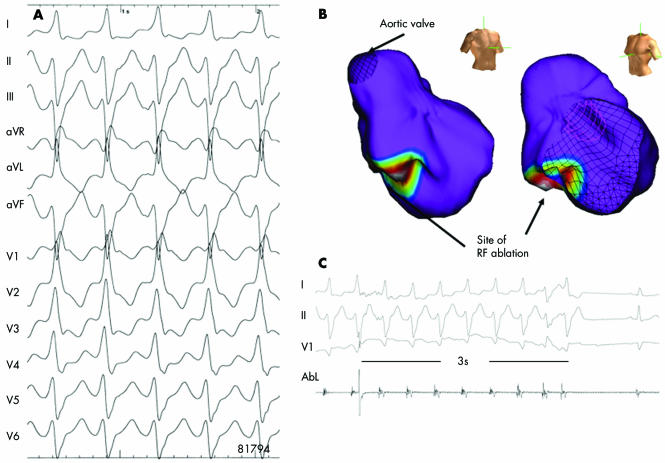
Idiopathic left ventricular tachycardia (ILVT) tends to occur in young, predominantly male patients without structural heart disease.11 The arrhythmia has a relatively narrow (0.10–0.14 s) RBBB morphology with a rapid downstroke of S waves in the precordial leads and a left superior axis (fig 8). ILVT is thought to have a re‐entrant basis or derives from triggered activity secondary to delayed afterdepolarisations. It arises on or near to the septum near the left posterior fascicle. Rarely, VT can arise from the left anterior fascicle and thus produce an RBBB pattern with right axis deviation. These VT are often unresponsive to β blockers, but frequently respond to verapamil (“verapamil sensitive” VT). The prognosis is generally good, but these patients may be highly symptomatic. Catheter ablation (fig 8)12 offers curative therapy and should be considered early in the management of symptomatic patients.
Figure 8 Non‐contact mapping of an idiopathic left ventricular tachycardia with right bundle branch block, leftward axis (A). The multiple electrode array (MEA) was placed in the left ventricle. The MEA is part of the non‐contact mapping system (EnSite 3000; Endocardial Solutions, Inc). The system permits mapping of a single complex. The MEA, which is filled with a contrast–saline medium, is positioned in the left ventricle (right/left anterior oblique views). The system calculates electrograms from 3000 endocardial points simultaneously by reconstructing far‐field signals. Non‐depolarised myocardium is shown in purple in this three dimensional isopotential map (B). The map also shows the site of earliest depolarisation (white circle). At the distal part of the left posterior fascicle, radiofrequency ablation almost immediately terminated the VT (C), which, thereafter, was no longer inducible.
Bundle branch re‐entry tachycardia
Re‐entry in the His‐Purkinje system (bundle branch re‐entry) accounts for a substantial number of monomorphic VTs in patients with heart failure, but may occasionally also occur in structurally normal hearts. The re‐entry wavefront proceeds down one bundle branch (mostly the RBB), and up the contralateral bundle. This creates a QRS complex that has an LBBB contour and a normal or leftward frontal plane axis. Thus, it meets the electrocardiographic criteria for SVT with aberration, but often AV dissociation is present. Its significance lies in the fact that it can be easily cured by catheter ablation of the right bundle branch.
VT IN PATIENTS WITHOUT STRUCTURAL HEART DISEASE BUT NOT CURRENTLY AMENABLE TO CURATIVE THERAPIES
These arrhythmias are based on changes of ionic transmembrane channels (channelopathy) or of intracellular calcium handling proteins. In recent years, a great many mutations have been identified.
Long QT syndrome
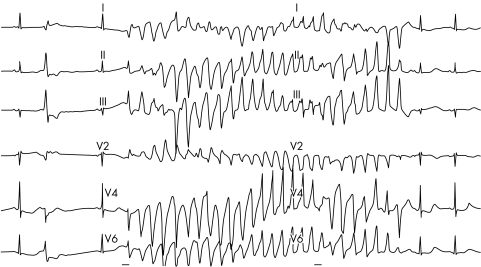
Long QT syndrome (LQTS) is characterised by a prolonged QT interval in the surface ECG, recurrent syncope or sudden death resulting from polymorphic WCTs with a characteristic twisting pattern of the RS complexes around the isoeletric line (that is, torsade de pointes) (fig 9).13 The incidence of LQTS has been estimated as 1:7000 to 1:10 000 live births. More than 250 mutations in seven genes (LQTS 1–7) have been described. Mutations involve genes encoding potassium channels with loss of function (LQT1, 2, 5, and 6, Jervell Lange‐Nielsen (JLN) 1 and 2), sodium channels with gain of function (LQT3) and ankyrin B (LQT4), which acts as a targeting and anchoring molecule for the sodium channel. In 30–40% of all patients with LQTS, no gene defect can be found pointing towards a large heterogeneity of gene loci. The term acquired LQTS14 is reserved for a syndrome similar to the congenital form but caused by exposure to drugs that prolong the duration of the ventricular action potential or to QT prolongation secondary to bradycardia or an electrolyte imbalance. Drugs that prolong the QT interval and thereby predispose to torsade de pointes are listed on websites such as www.longqt.org or www.qtdrugs.org. In congenital LQTS high risk patients should be treated prophylactically using β blockers.15 Prophylactic implantable cardioverter‐defibrillator (ICD) implantation is considered in patients with syncope despite β blocker treatment or in patients with syncope and a family history of sudden death.
Figure 9 Episode of torsade de pointes in a patient with long QT syndrome.
Short QT syndrome
A new syndrome associated with sudden cardiac death in otherwise healthy patients with structurally normal hearts has recently been described, the short QT syndrome (SQTS).16 The prevalence of this syndrome is unknown. Patients with SQTS present with a short QT interval (< 270 ms) on the 12 lead ECG, familial sudden death, and palpitations, syncope, or sudden cardiac arrest due to fast polymorphic VT. Currently, ICD implantation is the only therapeutic option.
Catecholaminergic polymorphic ventricular tachycardia
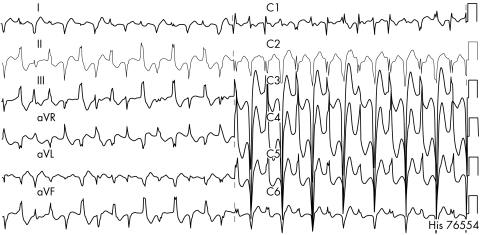
Catecholaminergic polymorphic VT (CPVT) is a clinically and genetically heterogeneous disease. It is characterised by episodes of syncope, or sudden death in response to physiological or emotional stress occurring in structurally normal hearts.17 Documented arrhythmias include bidirectional VT (fig 10), polymorphic VT, and in rare patients catecholaminergic idiopathic VF. A family history of juvenile sudden death and stress induced syncope is present in approximately one third of cases. Mortality is high and reaches up to 30–50% by the age of 30 years. Around 40–60% of the patients with CPVT carry mutations in two calcium handling genes, the cardiac ryanodine receptor gene (RyR2)18 and the calsequestrin 2 gene (CASQ2). Genotype–phenotype analysis showed that men are at higher risk of cardiac events (that is, syncope) and that mutation carriers became symptomatic at a younger age.17 Current treatment of CPVT consists of β adrenergic blockers,17 calcium channel antagonists, antiarrhythmic drugs and/or ICD implantation, mainly based on empiric grounds or the results of serial exercise–pharmacologic testing.
Figure 10 Bidirectional tachycardia after exercise in a patient affected with catecholaminergic polymorphic VT.
Brugada syndrome
In 1992, Brugada et al19 reported a new clinical entity with an RBBB pattern and ST segment elevation in right precordial ECG leads and a high incidence of sudden cardiac death in patients with structurally normal hearts. It manifests with episodes of polymorphic VT, syncope, and cardiac arrest during adulthood at a mean age of 40 years, however, with a large age range. It accounts for approximately 4–12% of sudden deaths and for 20–40% of sudden cardiac arrest in patients without structural heart disease. It predominantly occurs in males and appears to be most prevalent in South‐East Asia and Japan, where the disorder is a leading cause of natural death among young men with an estimated annual mortality rate of 26–38 per 100 000. Diagnostic criteria mainly rely on electrocardiographic abnormalities after exclusion of structural heart disease by detailed cardiac investigation. Brugada syndrome is considered a “channelopathy” which belongs to the group of “primary electrical diseases” of the heart. In about 20–30% of patients with Brugada syndrome, genetic mutations of the α subunit of the cardiac sodium channel (SCN5A) can be identified. Assessment of these mutations in expression systems demonstrated loss of function of the sodium channel (in contrast to the gain of function of the sodium channel in LQT3). The electrocardiographic manifestations of Brugada syndrome may be transient or concealed but can be unmasked or challenged with sodium channel blockers (ajmaline, flecainide, and others), vagotonic stimulation or fever. The diagnostic and prognostic impact of an incidental finding of Brugada‐type ECG signs in asymptomatic individuals without a family history represents a controversial and currently unresolved, yet growing problem in clinical decision making. Currently, ICD implantation is the treatment of choice in secondary and primary prevention of sudden death in high risk patients with Brugada syndrome.20
Wide complex tachycardias: key points
If a wide complex tachycardia (WCT) does not look like aberration, it is most likely a ventricular tachycardia
About 80% of all WCTs are ventricular tachycardias
In patients with known structural heart disease almost all WCTs are ventricular tachycardias
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Myerburg R J, Stewart J W, Hoffman B F. Electrophysiological properties of the canine peripheral A‐V conducting system. Circ Res 197026361–378. [DOI] [PubMed] [Google Scholar]
- 2.Denker S, Shenasa M, Gilbert C J.et al Effects of abrupt changes in cycle length on refractoriness of the His‐Purkinje system in man. Circulation 19836760–68. [DOI] [PubMed] [Google Scholar]
- 3.Wellens H J, Durrer D. Supraventricular tachycardia with left aberrant conduction due to retrograde invasion into the left bundle branch. Circulation 196838474–479. [DOI] [PubMed] [Google Scholar]
- 4.Gouaux J L, Ashman R. Auricular fibrillation with aberration simulating ventricular paroxysmal tachycardia. Am Heart J 194734366. [DOI] [PubMed] [Google Scholar]
- 5.Klein G J, Bashore T M, Sellers T D.et al Ventricular fibrillation in the Wolff‐Parkinson‐White syndrome. N Engl J Med 19793011080–1085.Large series on patients with Wolff‐Parkinson‐White syndrome and a history of sudden cardiac death that demonstrated that patients are most susceptible to ventricular fibrillation if they have a history of atrial fibrillation or reciprocating tachycardia, demonstrate rapid conduction over an accessory pathway, or have multiple pathways. [DOI] [PubMed] [Google Scholar]
- 6.Brugada P, Brugada J, Mont L.et al A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991831649–1659.Original paper that reports on the absence of an RS complex in all precordial leads as an easily recognisable and highly specific predictor for the diagnosis of ventricular tachycardia. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar M, Shenasa M, Jazayeri M.et al Wide QRS complex tachycardia. Reappraisal of a common clinical problem. Ann Intern Med 1988109905–912. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell D, Cox D, Bourke J.et al Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur Heart J 200324801–810. [DOI] [PubMed] [Google Scholar]
- 9.Mont L, Seixas T, Brugada P.et al The electrocardiographic, clinical, and electrophysiologic spectrum of idiopathic monomorphic ventricular tachycardia. Am Heart J 1992124746–753.Excellent overview on the clinical spectrum of idiopathic monomorphic VT. [DOI] [PubMed] [Google Scholar]
- 10.Ribbing M, Wasmer K, Monnig G.et al Endocardial mapping of right ventricular outflow tract tachycardia using noncontact activation mapping. J Cardiovasc Electrophysiol 200314602–608. [DOI] [PubMed] [Google Scholar]
- 11.Lin F C, Finley C D, Rahimtoola S H.et al Idiopathic paroxysmal ventricular tachycardia with a QRS pattern of right bundle branch block and left axis deviation: a unique clinical entity with specific properties. Am J Cardiol 19835295–100. [DOI] [PubMed] [Google Scholar]
- 12.Kottkamp H, Chen X, Hindricks G.et al Idiopathic left ventricular tachycardia: new insights into electrophysiological characteristics and radiofrequency catheter ablation. PACE 1995181285–1297. [DOI] [PubMed] [Google Scholar]
- 13.Moss A J, Schwartz P J, Crampton R S.et al The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991841136–1144.One of the first long term follow up reports on long QT syndrome. [DOI] [PubMed] [Google Scholar]
- 14.Haverkamp W, Breithardt G, Camm A J.et al The potential for QT prolongation and proarrhythmia by non‐antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J 2000211216–1231. [DOI] [PubMed] [Google Scholar]
- 15.Priori S G, Aliot E, Blomstrom‐Lundqvist C.et al Task force on sudden cardiac death, European Society of Cardiology. Europace 200243–18. [DOI] [PubMed] [Google Scholar]
- 16.Gussak I, Brugada P, Brugada J.et al Idiopathic short QT interval: a new clinical syndrome? Cardiology 20009499–102. [DOI] [PubMed] [Google Scholar]
- 17.Priori S G, Napolitano C, Memmi M.et al Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 200210669–74.Excellent overview on catecholaminergic polymorphic VT that emphasises the importance and role of genetic testing in these patients. [DOI] [PubMed] [Google Scholar]
- 18.Priori S G, Napolitano C, Tiso N.et al Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001103196–200. [DOI] [PubMed] [Google Scholar]
- 19.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992201391–1396.Outstanding first report of the Brugada brothers on six patients with a new clinical syndrome later referred to as Brugada syndrome. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt L, Probst V, Smits J P.et al Long‐term prognosis of individuals with right precordial st‐segment‐elevation Brugada syndrome. Circulation 2005111257–263. [DOI] [PubMed] [Google Scholar]