Diastolic heart failure (HF) has emerged over the last two decades as a separate clinical entity. Approximately half of the patients presenting with symptoms of congestive HF exhibit a near normal left ventricular (LV) systolic function at rest, which is thought to be caused by a predominant abnormality in diastolic function. It is generally considered to have a somewhat better prognosis than systolic HF, but frequency of hospitalisations is comparable in systolic and diastolic HF.1 Prevalence of diastolic HF increases with age and is higher in women. It is associated with hypertension, hypertrophy, diabetes, ageing and ischaemia.2 Despite the recognition of its importance, definition and diagnostic criteria of diastolic dysfunction and diastolic HF remain controversial.
DEFINITIONS
The cardiac cycle encompasses systole and diastole. Regardless of the exact time limits of these periods, it is generally accepted that systolic function relates with the ability of the ventricle to contract and eject, while diastolic function reflects its ability to relax and fill. Diastolic dysfunction therefore refers to a disturbance in ventricular relaxation, distensibility or filling—regardless of whether the ejection fraction (EF) is normal or depressed and whether the patient is asymptomatic or symptomatic.3 If a patient, with preserved EF and diastolic dysfunction, exhibits symptoms of effort intolerance and dyspnoea, especially if there were evidence of venous congestion and oedema, the term diastolic HF is used.4
PHYSIOLOGY OF DIASTOLIC FUNCTION
Determinants of diastolic function (table 1) include myocardial relaxation and passive properties of the ventricular wall, such as myocardial stiffness, wall thickness and chamber geometry (size or volume). Other determinants include the structures surrounding the ventricle, the left atrium, pulmonary veins and mitral valve, and heart rate. Except for heart rate these other determinants are extrinsic to the ventricle and therefore normally not considered as true causes of ventricular diastolic dysfunction or failure. Moreover, diagnosis of diastolic HF implies exclusion of these determinants as the cause of ventricular filling disturbances.3
Table 1 Determinants of diastolic function.
| • Myocardial relaxation |
| - load |
| - inactivation (calcium homeostasis, myofilaments, energetics) |
| - non‐uniformity |
| • Passive properties of ventricular wall |
| - myocardial stiffness (cytoskeleton, extracellular matrix) |
| - wall thickness |
| - chamber geometry |
| • Other determinants |
| - structures surrounding the ventricle (pericardium, lungs, remaining cardiac chambers) |
| - left atrium, pulmonary veins and mitral valve |
| - heart rate |
Relaxation
Relaxation is the process whereby the myocardium returns to an unstressed length and force. In the normal heart it comprises the major part of ventricular ejection, pressure fall and the initial part of rapid filling. LV pressure fall is therefore the haemodynamic manifestation of myocardial relaxation and its analysis allows adequate description of the course of myocardial relaxation (see later).
Myocardial relaxation is modulated by load, inactivation and non‐uniformity.5 Effects of load on relaxation depend on its type (preload versus afterload), magnitude, duration and timing in the cardiac cycle at which it occurs.6 When imposed early in the cardiac cycle a mild to moderate afterload elevation will, in the normal heart, delay the onset and accelerate the rate of pressure fall (compensatory response). On the contrary, a severe afterload elevation or an afterload elevation that occurs later in ejection will induce a premature onset and a pronounced slowing of pressure fall, even in a healthy heart (decompensatory response). Such slowing might lead to incomplete relaxation and therefore to elevation of filling pressures, a phenomenon that is exacerbated when preload is elevated.7 As pronounced hypertension represents a heavy afterload to the LV, this mechanism might contribute to exacerbation of diastolic dysfunction and congestion in acute hypertensive crisis.8
Myocardial inactivation relates to the processes underlying calcium extrusion from the cytosol and cross‐bridge detachment. Determinants of myocardial inactivation, listed in table 2, therefore include mechanisms related to calcium homeostasis and myofilament regulators of cross‐bridge cycling. Decreased concentrations or activity of the sarcoplasmic reticulum calcium ATPase pump (SERCA) can slow the removal of calcium from the cytosol. Increased levels or activity of phospholamban, a SERCA‐inhibitory protein, can also impair relaxation. Increased cAMP, resulting from β adrenergic stimulation or inhibition of cardiac phosphodiesterase, phosphorylates phospholamban to remove its inhibitory effect on SERCA. The net effect is an improvement in diastolic relaxation. Pathological LV hypertrophy secondary to hypertension or aortic stenosis results in decreased SERCA and increased phospholamban, again leading to impaired relaxation. Similar changes are seen in the myocardium of patients with hypertrophic or dilated cardiomyopathy. Interestingly, concentrations of SERCA decrease with age, coincident with impaired diastolic function.1 As ATP hydrolysis is required for myosin detachment from actin, calcium dissociation from Tn‐C, and active sequestration of calcium by the SR, energetic factors must also be taken in consideration. Modification of any of these steps, the myofilament proteins involved in these steps, or the ATPase that catalyses them can alter diastolic function.9 It is therefore not surprising that ischaemia leads to impaired relaxation.
Table 2 Determinants of myocardial inactivation.
| • Ca2+ homeostasis |
| - Ca2+ concentration |
| - sarcolemmal and SR Ca2+ transport |
| - modifying proteins (phospholamban, calmodulin, calsequestrin) |
| • Myofilaments |
| - Tn‐C Ca2+ binding |
| - Tn‐I phosphorylation |
| - Ca2+ sensitivity |
| - α/β‐MHC ATPase ratio |
| • Energetics |
| - ADP/ATP ratio |
| - ADP and Pi concentration |
ADP, adenosine diphosphate; ATP, adenosine triphosphate; MHC, myosin heavy chain; SR, sarcoplasmic reticulum; Tn, troponin.
During isovolumetric relaxation, re‐extension of one ventricular segment is accompanied by post‐systolic shortening of another segment. The ventricle remains isovolumic but changes its shape and produces intraventricular volume displacement. Asynchronous early segment re‐extension and regional non‐uniformity induce early onset and slower rate of ventricular pressure fall5,6 and might contribute to the diastolic disturbances observed in coronary heart disease and with intraventricular conduction disturbances.
Passive properties
Passive properties of the ventricular wall are influenced by myocardial stiffness, wall thickness and chamber geometry. Determinants of myocardial stiffness include factors intrinsic to the cardiomyocytes themselves (cytoskeleton) and the extracellular matrix (ECM). The cardiomyocyte cytoskeleton is composed of microtubules, intermediate filaments (desmin), microfilaments (actin), and endosarcomeric proteins (titin, α‐actinin, myomesin, and M‐protein). Changes in some of these cytoskeletal proteins have been shown to alter diastolic function.9
Most of the elastic force of the cardiomyocytes is now thought to reside in the macromolecule titin, whereas contributions of microtubules (tubulin) and intermediate filaments (desmin) appear < 10% at operating sarcomere lengths.10 Titin is expressed as varying isoforms that impart different mechanical properties, and this likely plays a role in altering passive stiffness in failing hearts. Titin can also be post‐translationally modified by Ca2+ (even in the diastolic range) and by phosphorylation, blurring notions of passive versus active tone.10 Phosphorylation of sarcomeric proteins by pKA was recently shown to normalise increased stiffness of cardiomyocytes from patients with diastolic HF.11
Changes in the structures within the ECM can also affect diastolic function. Myocardial ECM is composed of: (1) fibrillar protein (for example, collagen types I and III, and elastin); (2) proteoglycans; and (3) basement membrane proteins (for example, collagen type IV, laminin, and fibronectin). Fibrillar collagen apparently is the most important component within the ECM contributing to the development of diastolic HF.9 The role played by other fibrillar proteins (basement membrane proteins and proteoglycans) remains largely unexplored.
ECM fibrillar collagen, particularly in terms of its amount, geometry, distribution, degree of cross‐linking, and ratio of collagen type I/type III, is often altered in disease processes that alter diastolic function. The regulatory control of collagen biosynthesis and degradation includes: (1) transcriptional regulation by physical (for example, preload and afterload), neurohumoral (for example, renin–angiotensin–aldosterone and sympathetic nervous systems), and growth factors; (2) post‐translational regulation, including collagen cross‐linking; and (3) enzymatic degradation. Collagen degradation is under the control of matrix metalloproteinases (MMPs).9 Changes in either synthesis or degradation and their regulatory processes have been shown to alter diastolic function and lead to the development of diastolic HF. In addition, it is now increasingly recognised that quality of collagen (specifically cross‐linking and glycation) plays a key role in translating quantity into myocardial stiffness.10 Recent demonstration that 16 weeks of treatment with a glucose cross‐link breaker decreased LV mass and improved diastolic filling and quality of life in patients with diastolic HF12 further reinforces this view.
In addition to post‐translational modifications of titin, other evidence suggests that diastolic stiffness is actively modulated. Cross‐bridge interaction occurs even at low diastolic calcium producing resting muscle tone. Modifications of myofilament calcium sensitivity by HF might also alter active tone.10 This includes changes associated with PKA (or PKG) phosphorylation of myosin light chain 2 and TnI. In this setting, nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) increase resting diastolic cell length as a result of PKG‐mediated phosphorylation of myofilaments, and in patients with dilated cardiomyopathy administration of intracoronary substance P (NO stimulator) decreases LV stiffness.7 Furthermore, myocardial stiffness is modulated by load and endothelin‐1 (ET‐1). Load elevations acutely increase LV stiffness,10 while ET‐1 acutely decrease myocardial stiffness.13
Heart rate
Heart rate (HR) influences myocardial oxygen demand and coronary perfusion time. Rapid HR increases the former and decreases the latter, so that ischaemic diastolic dysfunction might occur even in the absence of coronary disease, especially in hypertrophic hearts. Additionally, fast HR may shorten diastole to an extent that prevents relaxation from being complete between beats, resulting in increased filling pressures and therefore diastolic dysfunction. This may occur at lower HR in failing hearts, which contrary to normal hearts, may exhibit a flat or even negative relaxation velocity‐versus‐HR relationship, so that as HR increases, relaxation rate does not increase or even decreases.9
EVALUATION OF DIASTOLIC FUNCTION
Relaxation
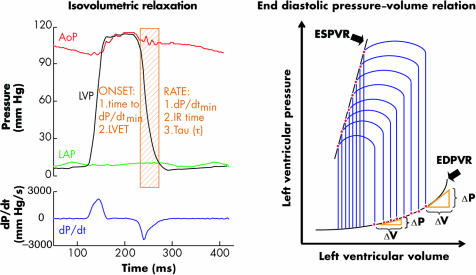
As outlined above, ventricular pressure fall is the haemodynamic manifestation of myocardial relaxation. Its analysis includes measurement of rate and timing of its onset in the cardiac cycle (fig 1, left panel). Timing of LV pressure (LVP) fall can be estimated from the time interval between end diastole and dP/dtmin or the time between aortic valve opening and closure (LVET). Indices of rate of LVP fall include dP/dtmin, isovolumic relaxation time (IRT) and the time constant τ.6 DP/dtmin is an instantaneous value that reflects maximal velocity of LVP fall. IRT is the time interval between aortic valve closure or dP/dtmin and mitral valve opening. It is influenced not only by relaxation rate but also by LVP at aortic valve closure and mitral valve opening. The time constant of isovolumic relaxation, tau (τ, ms), is the most widely used index to evaluate the rate of LVP fall. Tau is inversely related to the rate of LVP fall, becoming shorter when LVP fall accelerates and longer when LVP fall slows. Most methods assume that LVP fall follows a monoexponential course and can be described with the formula: Pt = (P0‐P∞)e‐t/τ+P∞ where Pt is LVP at time t; P0 is LVP at dP/dtmin (time 0). P∞ is the asymptotic pressure, to which relaxation would lead if completed without LV filling. P∞ is negative in normal ventricles, which means that the non‐filling ventricle develops diastolic suction. According to this formula, τ corresponds to the time it takes for LVP to fall to 1/e (∼36%) of its initial value. The formula also indicates that LVP fall, and therefore relaxation, will be 97% complete 3.5*τ after dP/dtmin.6
Figure 1 Haemodynamic evaluation of diastolic function. Left panel: Analysis includes rate and timing of onset of left ventricular (LV) pressure fall in the cardiac cycle. AoP, LVP, LAP, aortic, left ventricular, and left atrial pressures, respectively; dP/dt, first derivative of LVP; dP/dtmin, peak rate of LVP fall; IR, isovolumetric relaxation. Right panel: LV pressure–volume loops at various preload and afterload levels. The end systolic pressure–volume relation (ESPVR) is used to evaluate LV contractility, while the end diastolic pressure–volume relation (EDPVR) allows quantification of chamber stiffness. The operating stiffness at any point along a given EDPVR is equal to the slope of a tangent drawn to the curve at that point (ΔP/ΔV). Operating stiffness changes with filling; stiffness is lower at smaller volumes and higher at larger volumes (volume dependent change in diastolic pressure and stiffness).
Passive properties
Chamber stiffness can be quantified by examination of the end diastolic pressure–volume relationship (EDPVR). This relationship is drawn by fitting the lower right corner of multiple pressure–volume (PV) loops obtained at various preloads to an exponential curve (fig 1, right panel). The operating stiffness at any point along a given EDPVR is equal to the slope of a tangent drawn to the curve at that point (ΔP/ΔV). Operating stiffness changes with filling; stiffness is lower at smaller volumes and higher at larger volumes (volume dependent change in diastolic pressure and stiffness). As EDPVR is exponential, the relationship between ΔP/ΔV and pressure is linear. The slope (Kc) of this line is the chamber stiffness constant and can be used to quantify chamber stiffness.4 When overall chamber stiffness is increased, the pressure–volume curve is shifted upwards and to the left, the slope of the ΔP/ΔV‐versus‐pressure relationship becomes steeper, and Kc is increased (volume independent change in diastolic pressure and stiffness). Thus, diastolic pressure can be changed either by a volume dependent change in operating stiffness or by a volume independent change in chamber stiffness.
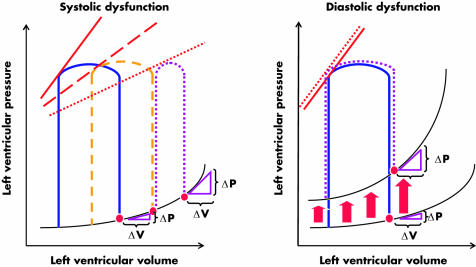
Figure 2 shows that while in systolic dysfunction (left panel) end diastolic pressures are usually elevated by a volume dependent increase in operating stiffness, in diastolic dysfunction (right panel) end diastolic pressures are elevated by a volume independent increase in chamber stiffness. In both panels the blue PV loops represent a cardiac cycle from a normal heart. When systolic dysfunction develops the ventricle uses preload reserve to preserve EF (dashed orange line). When systolic dysfunction progresses, EF (PV loop width) cannot be maintained anymore, even if end diastolic pressures are further increased (dotted purple line). Therefore, in systolic dysfunction increased filling pressures reflect a rightward shift along the same EDPVR and a volume dependent increase in operating stiffness. It should be noted that when ventricular dilatation occurs, the entire EDPVR is right and downward shifted and therefore a volume dependent increase in operating stiffness might occur even if volume independent chamber stiffness is decreased. On the contrary, when diastolic dysfunction occurs EF is preserved and end diastolic pressures are raised due to a left and upward shift of the entire PV relation, reflecting a volume independent increase in chamber stiffness. Note that the slope of the end systolic PV relation (red lines) progressively decreases with systolic dysfunction, but is preserved in diastolic dysfunction.
Figure 2 Effects of systolic dysfunction (left panel) and diastolic dysfunction (right panel) on pressure–volume (PV) loops, and end systolic (ESPVR) and end diastolic PV relations (EDPVR). In both panels the blue PV loops represent cardiac cycles from a normal heart. When systolic dysfunction develops the ventricle uses preload reserve in an attempt to preserve ejection fraction (dashed orange line). When systolic dysfunction progresses, ejection fraction (PV loop width) cannot be maintained even if end diastolic pressures are further increased (dotted purple line, left panel). Therefore, in systolic dysfunction increased filling pressures reflect a rightward shift along the same EDPVR and a volume dependent increase in operating stiffness. On the contrary, when diastolic dysfunction occurs, ejection fraction is preserved and end diastolic pressures are raised due to a left and upward shift of the entire PV relation, reflecting a volume independent increase in chamber stiffness. Note that the slope of the ESPVR (red lines) progressively decreases with systolic dysfunction, but is preserved in diastolic dysfunction.
Echocardiographic indices
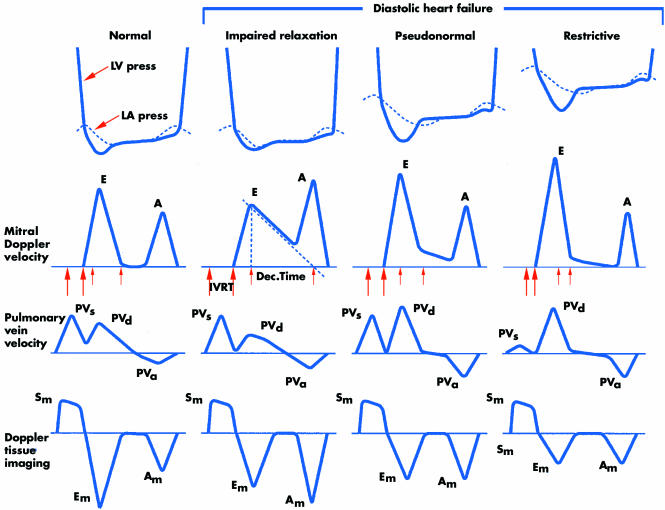
In addition to providing information about LV dimensions and EF, both important for the diagnosis of diastolic HF, echocardiography provides indices of diastolic function.1 The most familiar of these are the mitral inflow velocities; the E and A waves that correspond, respectively, to early flow during LV relaxation and subsequent contribution of atrial contraction. When diastolic function is normal E wave exceeds A wave velocity. Pulmonary vein flow is also measured in two phases, systolic and diastolic, analogous to the x and y descents of the jugular veins.
With impaired relaxation, atrial contraction contributes relatively more to ventricular filling (A>E, with prolonged deceleration of the E wave). This state is common with increasing age and may identify patients at risk for diastolic HF. When LV diastolic pressure increases to the point that atrial contraction contributes little to filling, the E wave again becomes predominant but with rapid deceleration, first in a pseudonormal pattern and ultimately in a restrictive pattern (high E wave velocity, usually more than twice the A wave velocity) (fig 3). Both filling patterns are reliably associated with diastolic dominant pulmonary vein flow in patients over 50 years of age.1
Figure 3 Left ventricular (LV) and left atrial (LA) pressures during diastole, transmitral Doppler LV inflow velocity, pulmonary vein Doppler velocity, and Doppler tissue velocity. A, velocity of LV filling contributed by atrial contraction; Am, myocardial velocity during filling produced by atrial contraction; Dec. Time, e‐wave deceleration time; E, early LV filling velocity; Em, myocardial velocity during early filling; IVRT, isovolumic relaxation time; PVa, pulmonary vein velocity resulting from atrial contraction; PVd, diastolic pulmonary vein velocity; PVs, systolic pulmonary vein velocity; Sm, myocardial velocity during systole. Reproduced from Zile and Brutsaert,9 with permission.
New indices derived from transmitral flow propagation (Vp) and from tissue Doppler velocity (Em) are less sensitive to load and provide new tools to detect early abnormalities or to study non‐invasively the effects of new therapies on diastolic function. For a recent, extensive review on non‐invasive assessment of diastolic function see Quinones.14
DIAGNOSIS
The European Society of Cardiology study group on diastolic HF proposed three obligatory criteria that should be met simultaneously for the diagnosis15: (1) presence of signs or symptoms of congestive HF; (2) normal or only mildly abnormal LV systolic function; and (3) evidence of abnormal LV relaxation, filling, diastolic distensibility or diastolic stiffness. Several criticisms were made to these criteria. First, the requirement of signs or symptoms, instead of signs and symptoms, for the clinical diagnosis of CHF (fulfilment of Framingham criteria had been suggested). Second, the cut off value of 45% for LVEF. Although values in the 40–50% range were used in several studies, Vasan and Levy16 proposed an LVEF > 50%. These authors developed criteria for definite, probable, and possible diastolic HF. Definite diastolic HF requires definitive evidence of HF, LVEF > 50% (evaluated less than 72 hours after the HF event), and evidence by cardiac catheterisation (the European study group also accepts echocardiography) of abnormal LV relaxation, filling, diastolic distensibility, or diastolic stiffness. If catheterisation evidence of diastolic dysfunction is not available they propose a diagnosis of probable diastolic HF, or of possible diastolic HF if LVEF is measured more than 72 hours after the HF event. In fact, LVEF can vary according to when it is determined. For example, in HF secondary to acute transitory myocardial ischaemia or hypertensive crisis, LVEF determined during the first hours can be reduced but at 24 hours it is normal. However, Gandhi and colleagues8 showed that in patients with HF and uncontrolled hypertension differences between LVEF determined in the emergency department and at 72 hours were not significant in those patients who were already clinically stable. Thus, it is not usually essential to determine LVEF during initial decompensation as values obtained in the following days are reliable; the only exception to this rule may be in patients with acute ischaemia.
The clinical application of these criteria is limited because of their complexity and the fact that both demand demonstrable abnormalities in diastolic function. Recognising the difficulties inherent in the assessment of the diastolic properties of the heart, Zile and colleagues17 tested the hypothesis that measurements of the LV relaxation and passive stiffness were not necessary to make the diagnosis of diastolic HF. They showed that among patients with CHF diagnosed according to Framingham criteria and LVEF > 50% who undergo a haemodynamic study and Doppler echocardiogram, 100% present at least one diastolic abnormality identified by one or other of these methods. Consequently, the study of diastolic function serves to confirm the diagnosis of diastolic congestive HF rather than establish it, although a better characterisation of the underlying mechanism might help to guide treatment. Furthermore, it was recently shown that diastolic dysfunction (abnormal relaxation and passive stiffness) is the predominant pathophysiological cause of HF in patients who met the diagnostic criteria of definite diastolic HF.18
Increasing evidence suggest that B‐type natriuretic peptide (BNP) and N‐terminal pro‐BNP (NT‐proBNP) might facilitate the differential diagnosis of HF and help to distinguish patients with systolic from those with diastolic HF.3
PROGNOSIS
Prognosis of diastolic HF is slightly less ominous than that of systolic HF, with an annual mortality of 5–8% in those individuals with the former and 10–15% in those with the latter.4 Mortality in the general population without HF and of a similar age is 1% per year. Presence of coronary disease, age and the LVEF cut‐off value are important factors in the prognosis. When patients with ischaemic heart disease are excluded, annual mortality for diastolic congestive HF falls to 2–3%. In patients > 70 years with congestive HF, mortality is similar in systolic and diastolic HF.2,4
TREATMENT
To date, only one large scale monitored randomised clinical trial was undertaken to compare drug versus placebo administration in patients with HF and preserved systolic function (CHARM‐preserved). This trial compared the efficacy of a daily 32 mg dose of candesartan versus a placebo in 3023 patients with chronic HF and LVEF > 40%. After a 36.6 month mean follow up, primary combined outcome incidence (death by cardiovascular cause or admission for congestive HF) was similar in both groups. Data for cardiovascular mortality did not differ, but a moderate impact of candesartan in preventing admissions for congestive HF among patients who have HF and LVEF > 40% was observed.19
Although the moderate benefit of candesartan should be taken into consideration, until data from randomised clinical trials provide new evidence, Zile and Brutsaert9 propose that treatment of diastolic HF must be directed toward symptoms, aetiology and, in the future, underlying mechanisms, as outlined in table 3. Aetiologic factors include hypertension, diabetes, or ischaemia. Angiotensin receptor blockers (ARBs) have proven effective in causing regression of LV hypertrophy (LIFE) and may reduce morbidity, but not mortality (CHARM). Maintenance of sinus rhythm, heart rate control (β blockers, calcium channel blockers) and anti‐ischaemic treatment may be indicated in view of pathophysiological aspects. Diuretics should be administered with caution in patients with symptoms of congestion; digitalis is not useful in the treatment of isolated diastolic HF. The results of ongoing trials (for example, I‐Preserve) may offer new therapeutic options, and evidence based guidelines for the so far often unsatisfactory treatment of diastolic dysfunction/HF are awaited.
Table 3 Diastolic heart failure: treatment9.
| Symptom targeted treatments |
| • Decrease pulmonary venous pressure |
| - reduce left ventricular volume |
| - maintain atrial contraction |
| - prevent tachycardia |
| • Improve exercise tolerance |
| • Use positive inotropic agents with caution |
| • Non‐pharmacological treatment |
| - restrict sodium to prevent volume overload |
| - restrict fluid to prevent volume overload |
| - perform moderate aerobic exercise to improve cardiovascular conditioning, decrease heart rate, and maintain skeletal muscle function |
| • Pharmacological treatment |
| - diuretics, including loop diuretics, thiazides, spironolactone |
| - long acting nitrates |
| - β adrenergic blockers |
| - calcium channel blockers |
| - renin–angiotensin–aldosterone antagonists, including angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, and aldosterone antagonists |
| Disease targeted treatments |
| • Prevent/treat myocardial ischaemia |
| • Prevent/regress ventricular hypertrophy |
| Mechanism targeted treatments |
| • Modify myocardial and extramyocardial mechanisms |
| • Modify intracellular and extracellular mechanisms |
Diastolic dysfunction and heart failure: key points
Approximately half of the patients presenting with symptoms of congestive heart failure exhibit a near normal left ventricular (LV) systolic function at rest, which is thought to be caused by a predominant abnormality in diastolic function
Prevalence of diastolic heart failure increases with age and is higher in women. It is associated with hypertension, hypertrophy, diabetes, ageing and ischaemia
Mortality is somewhat lower but morbidity is similar in diastolic versus systolic heart failure
Determinants of diastolic function include myocardial relaxation and passive properties of the ventricular wall
Myocardial relaxation is modulated by load, inactivation and non‐uniformity
Passive properties of the ventricular wall are influenced by myocardial stiffness (cytoskeleton and extracellular matrix), wall thickness and chamber geometry
There is now evidence that diastolic stiffness is actively modulated by load and neurohumoral agents
The time constant of isovolumic relaxation, tau (τ), is the most widely used index to evaluate the rate of left ventricular pressure fall, which is the haemodynamic manifestation of myocardial relaxation
Chamber stiffness can be quantified by examination of the end diastolic pressure–volume relationship
Echocardiographic indices derived from Doppler mitral inflow velocities are load dependent, but this limitation might be compensated by new indices derived from pulmonary venous flow, transmitral flow propagation and tissue Doppler velocity
Virtually all patients with congestive heart failure and a normal ejection fraction exhibit evidence of diastolic dysfunction
Until data from randomised clinical trials provide new evidence, treatment of diastolic heart failure must be directed toward symptoms, aetiology and, in the future, underlying mechanisms
Statin treatment may be associated with lower mortality in patients with diastolic heart failure
Therefore, even if the rationale of their use differs, these principles suggest that drugs recommended for diastolic HF may be the ones recommended for systolic dysfunction. For example, β blockers are now recommended for the treatment of both systolic and diastolic HF. In diastolic HF, however, β blockers are used to decrease heart rate, increase the duration of diastole, and modify the haemodynamic response to exercise. In systolic HF, β blockers are used chronically to increase inotropic state and modify LV remodelling. In systolic HF, β blockers must be titrated slowly and carefully over an extended time period. This is generally not necessary in diastolic HF. Diuretics are used in the treatment of both systolic and diastolic HF. However, the doses of diuretics used to treat diastolic HF are generally smaller than the doses used in systolic HF. Some drugs are used only to treat either systolic or diastolic HF, but not both. For example, calcium channel blockers have no place in the treatment of systolic HF, but have been considered potentially useful in the treatment of diastolic HF.9
Conceptually, an ideal therapeutic agent should target the underlying mechanisms that cause diastolic HF. Therefore, a therapeutic agent might improve calcium homeostasis and energetics, blunt neurohumoral activation and decrease myocardial stiffness. Fortunately, some pharmaceutical agents that fit these design characteristics are already in existence, and many more are under development. Unfortunately, randomised, double blind, placebo controlled, multicentre trials that examine the efficacy of these agents used either singly or in combination have been slow to develop.
Two recent pilot studies opened new perspectives for diastolic HF treatment. In the first one, 16 weeks treatment with ALT‐711, a glucose crosslink breaker, resulted in a decrease in LV mass and improvements in LV diastolic filling and quality of life in elderly patients with diastolic HF.12 This drug targets increased myocardial stiffness caused by collagen cross‐linking. This pathophysiological mechanism of diastolic HF was not tested previously in HF. The other study gathered evidence that statin treatment may be associated with lower mortality in patients with diastolic HF,20 by mechanisms that still remain speculative. This was the first study showing a beneficial effect of a drug on mortality of diastolic HF patients.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Angeja B G, Grossman W. Evaluation and management of diastolic heart failure. Circulation 2003107659–663. [DOI] [PubMed] [Google Scholar]
- 2.Owan T E, Redfield M M. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 200547320–332.Excellent review with emphasis on the epidemiology of diastolic heart failure. [DOI] [PubMed] [Google Scholar]
- 3.Gaasch W H, Zile M R. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 200455373–394. [DOI] [PubMed] [Google Scholar]
- 4.Zile M R, Brutsaert D L. New concepts in diastolic dysfunction and diastolic heart failure. Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 20021051387–1393.First part of an excellent and comprehensive review about the basic and clinical features of diastolic dysfunction and failure. [DOI] [PubMed] [Google Scholar]
- 5.Brutsaert D L, Sys S U. Relaxation and diastole of the heart. Physiol Rev 1989691228–1315. [DOI] [PubMed] [Google Scholar]
- 6.Leite‐Moreira A F, Gillebert T C. The physiology of left ventricular pressure fall. Rev Port Cardiol 2000191015–1021. [PubMed] [Google Scholar]
- 7.Leite‐Moreira A F, Correia‐Pinto J. Load as an acute determinant of end‐diastolic pressure‐volume relation. Am J Physiol Heart Circ Physiol 2001280H51–H59. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi S K, Powers J C, Nomeir A M.et al The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 200134417–22. [DOI] [PubMed] [Google Scholar]
- 9.Zile M R, Brutsaert D L. New concepts in diastolic dysfunction and diastolic heart failure. Part II: causal mechanisms and treatment. Circulation 20021051503–1508.Second part of an excellent and comprehensive review about the basic and clinical features of diastolic dysfunction and failure. [DOI] [PubMed] [Google Scholar]
- 10.Kass D A, Bronzwaer J G, Paulus W J. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 2004941533–1542.Excellent review with emphasis on the basic mechanisms underlying diastolic dysfunction and failure. [DOI] [PubMed] [Google Scholar]
- 11.Borbely A, van der Velden J, Papp Z.et al Cardiomyocyte stiffness in diastolic heart failure. Circulation 2005111774–781. [DOI] [PubMed] [Google Scholar]
- 12.Little W C, Zile M R, Kitzman D W.et al The effect of alagebrium chloride (ALT‐711), a novel glucose cross‐link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 200511191–195.Pilot study showing the benefits, in patients with diastolic heart failure, of a cross‐link breaker which influences a mechanism outside the classic therapeutic targets for systolic heart failure. [DOI] [PubMed] [Google Scholar]
- 13.Leite‐Moreira A F, Bras‐Silva C, Pedrosa C A.et al ET‐1 increases distensibility of acutely loaded myocardium: a novel ETA and Na+/H+ exchanger‐mediated effect. Am J Physiol Heart Circ Physiol 2003284H1332–H1339. [DOI] [PubMed] [Google Scholar]
- 14.Quinones M A. Assessment of diastolic function. Prog Cardiovasc Dis 200547340–355.Excellent review with emphasis on the non‐invasive assessment of diastolic function. [DOI] [PubMed] [Google Scholar]
- 15.European Study Group on Diastolic Heart Failure How to diagnose diastolic heart failure. Eur Heart J 199819900–1003.Landmark consensus document on the diagnostic criteria of diastolic heart failure. [DOI] [PubMed] [Google Scholar]
- 16.Vasan R S, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 20001012118–2121. [DOI] [PubMed] [Google Scholar]
- 17.Zile M R, Gaasch W H, Carroll J D.et al Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001104779–782.Landmark study showing that most patients with heart failure and normal ejection fraction have diastolic dysfunction. [DOI] [PubMed] [Google Scholar]
- 18.Zile M R, Baicu C F, Gaasch W H. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 20043501953–1959. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Pfeffer M A, Swedberg K, for the CHARM Investigators and Committees et al Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved trial. Lancet 2003362777–781.First clinical trial showing the benefits of a pharmacologic intervention in patients with diastolic heart failure. Candesartan had a moderate impact in preventing admissions for CHF in these patients. [DOI] [PubMed] [Google Scholar]
- 20.Fukuta H, Sane D C, Brucks S.et al Statin therapy may be associated with lower mortality in patients with diastolic heart failure. A preliminary report. Circulation 2005112357–363.Pilot study showing for the first time that a pharmacologic intervention might decrease mortality of patients with diastolic heart failure. [DOI] [PubMed] [Google Scholar]