Introduction of anti‐retroviral combination therapy has profoundly altered both the course and prognosis of the disease in HIV infected persons. Recent data, however, have raised concerns that anti‐retroviral combination therapy is associated with premature manifestation of coronary artery disease.1 In particular, protease inhibitors have been linked to metabolic changes such as insulin resistance, abnormalities in lipid metabolism and lipodystrophy, and increased coronary artery calcification. While previous studies have reached conflicting conclusions about the incidence of myocardial infarction, the most substantial database recently provided by Friis‐Møller and co‐workers demonstrated an increased incidence in HIV infected persons receiving protease inhibitors or non‐nucleoside reverse transcriptase inhibitor‐containing therapy.2
One of the postulated mechanisms of pro‐atherogenic effects of protease inhibitors is the promotion of atherosclerotic lesion formation by an increase in CD36‐dependent cholesteryl ester accumulation in macrophages. Additionally, hypercholesterolaemia promotes a CD36‐dependent and endothelial nitric oxide synthase mediated endothelial dysfunction. Endothelial dysfunction is associated with future risk of adverse cardiovascular events.3 Impaired endothelial function was previously shown in HIV infected persons receiving protease inhibitor therapy.4 The effect of statins (hydroxy‐methyl‐glutaryl coenzyme A reductase inhibitors) in anti‐retroviral combination therapy associated dyslipidaemia remains to be determined. As most statins are metabolised by the cytochrome P450 3A4 isoform, and thus interfere with the metabolism of many anti‐retroviral drugs, resulting in increased toxicity, cytochrome P450 independent statins, such as pravastatin, may be advantageous.
Hence, the present study aimed to evaluate the effects of pravastatin on endothelial function and plasma lipid profile in persons on protease inhibitor‐containing anti‐retroviral combination therapy.
METHODS
This was a randomised, crossover, double blind, placebo controlled interventional trial investigating pravastatin 40 mg daily and matching placebo for eight weeks each. Laboratory, clinical, and endothelial function parameters were assessed at baseline and after each treatment period. An additional ultrasound measurement was performed at four weeks. Inclusion criteria were HIV infection, and protease inhibitor‐containing anti‐retroviral combination therapy for at least four months, which was unchanged for two months. Exclusion criteria were cholesterol < 5 mmol/l, statins, or angiotensin converting enzyme (ACE) inhibitors, diabetes mellitus, and acute coronary syndromes within the previous four weeks. Concomitant medication remained unchanged throughout the study course. Each patient gave written informed consent and the study was approved by the local ethics committee of the University Hospital of Zurich.
Brachial artery endothelium dependent flow mediated dilation (FMD), induced by inflation of a wrist cuff to suprasystolic pressure for five minutes, and endothelium independent vasodilation (0.4 mg glyceryl trinitrate sublingually) were assessed by a high resolution ultrasound vessel wall tracking device with a 10 MHz linear array transducer (ESAOTE AU‐5, WTS‐2, Pie Medical, Maastricht, The Netherlands). Vessel diameter was recorded every 15 seconds for five minutes.
All data are presented as medians and interquartile range as 25th to 75th centile. For changes of FMD and lipid parameters comparing pravastatin to placebo, Wilcoxon signed rank test was used. For comparison of baseline characteristics Mann‐Whitney test was used. Significance was accepted at p < 0.05. Results of linear univariable and multivariable regression analysis are expressed as regression coefficients and standard error of the mean (SEM).
RESULTS
Twenty nine participants (median age 43 years, 79% male) were included for final analysis. Baseline patients' characteristics between the two treatment arms did not differ. HIV parameters were: previous clinical AIDS, 10 of 29 (34%) patients; median CD4 lymphocyte nadir and baseline count, 93 × 103 (range, 45–245) and 484 (328–633) cells/ml, respectively; median duration of anti‐retroviral therapy was 4.8 (2.3–5.9) years; median baseline HIV‐1 RNA below 50 copies/ml. During the study, no progression of HIV infection was observed.
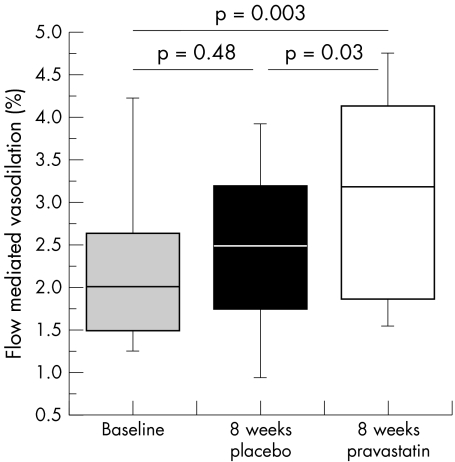
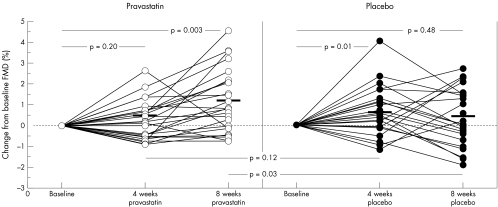
Eight weeks of treatment with pravastatin significantly improved FMD versus both baseline (p = 0.003) and placebo (p = 0.03) (figs 1 and 2, table 1). At four weeks, FMD was not different between pravastatin and placebo (p = 0.12). A mild carryover effect was perceivable: FMD after four weeks of placebo in the group first receiving pravastatin was increased compared to baseline (2.6% v 1.6%, p = 0.01), resembling the preceding eight week pravastatin measure point (2.6%, p = 0.48). In contrast, in patients who first received placebo, FMD was comparable to baseline (2.3% v 2.8%, p = 0.26) after four weeks of placebo. Glyceryl trinitrate induced vasodilation was not influenced by either treatment. Values for plasma creatinine, creatinine kinase, aspartate aminotransferase, alanine aminotransferase, and glucose were within normal limits at baseline and did not change during the study. Laboratory and clinical parameters are summarised in table 1.
Figure 1 Eight weeks of treatment with pravastatin resulted in a significant improvement of flow mediated dilation (FMD).
Figure 2 Individual changes in FMD from baseline resulted in a significant improvement after eight weeks of pravastatin treatment, but not after four weeks of treatment. Placebo did not alter FMD at eight weeks while FMD after four weeks of placebo was slightly increased compared to baseline. This is largely attributable to a carryover effect in the crossover design. At four weeks, change of FMD did not vary between pravastatin and placebo.
Table 1 Comparison of parameters at baseline and after eight weeks of placebo and pravastatin treatment, respectively.
| Baseline n = 29 | Placebo 8 weeks n = 29 | Pravastatin 8 weeks n = 29 | p Value pravastatin v baseline | p Value pravastatin v placebo | |
|---|---|---|---|---|---|
| HIV surrogate markers | |||||
| CD4 cell count (103 cells/ml) | 484 (328–633) | 472 (320–646) | 464 (354–650) | 0.60 | 0.42 |
| HIV‐1 RNA (copies/ml) | 8 (0–37) | 0 (0–22) | 0 (0–18) | 0.57 | 0.33 |
| Clinical parameters | |||||
| Body mass index (kg/m2) | 22.9 (21.4–25.1) | 23.1 (21.3–25.1) | 22.6(21.2–25.4) | 0.53 | 0.93 |
| Systolic blood pressure (mm Hg) | 120 (114–128) | 118 (114–129) | 119 (113–127) | 0.33 | 0.99 |
| Diastolic blood pressure (mm Hg) | 77 (71–83) | 78 (73–83) | 76 (71–84) | 0.41 | 0.95 |
| Heart rate (bpm) | 76 (71–81) | 75 (70–82) | 75 (71–80) | 0.85 | 0.47 |
| Laboratory parameters | |||||
| Total cholesterol (mmol/l) | 6.4 (6.0–7.4) | 6.4 (5.3–7.2) | 5.5 (4.8–6.3) | <0.0001 | 0.001 |
| HDL cholesterol (mmol/l) | 1.2 (1.1–1.6) | 1.2 (1.0–1.6) | 1.3 (1.1–1.4) | 0.90 | 0.38 |
| LDL cholesterol (mmol/l) | 3.7 (2.8–4.2) | 3.9 (2.7–4.4) | 3.0 (2.4–3.7) | 0.001 | 0.01 |
| Triglycerides (mmol/l) | 3.0 (2.1–4.0) | 2.7 (2.1–3.8) | 2.3 (1.6–2.9) | 0.05 | 0.04 |
| Oxidised LDL (IU) | 53 (45–66) | 55 (43–70) | 47 (38–57) | 0.0003 | 0.007 |
| hs CRP (mg/l) | 2.2 (0.7–4.5) | 2.1 (0.8–5.1) | 2.1 (1.0–4.8) | 0.50 | 0.29 |
| Endothelial function | |||||
| % FMD | 2.0 (1.5–2.6) | 2.5 (1.8–3.2) | 3.2 (1.9–4.1) | 0.003 | 0.03 |
| % GTN induced vasodilatation | 11.3 (10.0–14.4) | 11.3 (9.6–16.3) | 10.8 (7.0–15.7) | 0.16 | 0.85 |
| Vessel diameter (mm) | 4.4 (4.1–4.8) | 4.3 (3.8–4.6) | 4.3 (3.9–4.8) | 0.26 | 0.65 |
Data are presented as medians and interquartile range unless otherwise stated
GTN, glyceryl trinitrate; HDL, high density lipoprotein; hs CRP, high sensitivity C reactive protein; LDL, low density lipoprotein.
Change of low density lipoprotein (LDL) cholesterol was found to be inversely related to percentage change of FMD (r = 0.36, p = 0.014). This relation remained significant in a multivariable model including treatment sequence and heart rate as independent variables (r = 0.53, p = 0.003).
DISCUSSION
This randomised, double blind investigation demonstrates that not only does statin treatment lower total LDL cholesterol and oxidised LDL cholesterol, but also significantly improves endothelial function in HIV infected persons on protease inhibitors containing anti‐retroviral combination therapy.
HIV infection has become a chronic disease, requiring long term management strategies and greater attention to disease prevention issues. As such, statins are expected to become a cornerstone of coronary artery disease prevention since emerging evidence suggests that HIV infected persons receiving anti‐retroviral combination therapy, particularly protease inhibitor containing regimens, are at increased risk for cardiovascular morbidity and mortality by mechanisms that are not fully understood yet.1,2 Our study provides evidence that statins improve a clinically relevant cardiovascular surrogate end point and further corroborate and extend findings of a recent study by Stein showing a trend towards improvement of FMD in HIV patients. While significant beneficial effects of statins on vascular endothelial function have previously been demonstrated in a range of patient populations with hypercholesterolaemia, the present study extends these results to HIV positive individuals. The decrease in total cholesterol and LDL cholesterol in the present study was considerably less than reported from large lipid lowering studies using identical doses of pravastatin in hypercholesterolaemic subjects. The relatively modest effects on plasma lipids in the present and previous studies in HIV patients may in part be explained by pharmacokinetic interactions. Indeed, in combination with protease inhibitors, plasma concentration of simvastatin increased 30‐fold, while pravastatin concentrations were found to decline by 50%.5
In conclusion, the results of the present study demonstrate that statin treatment beneficially impacts on a clinically relevant surrogate marker of cardiovascular disease. The definitive answer as to the net effect of statins on cardiovascular events in HIV patients can only be provided by large scale prospective randomised clinical trials.
Acknowledgements
The authors would like to thank Manuela Zahno, Pina Scalegno and Susan Hochstrasser for expert technical assistance and patient recruitment. This work was supported by Swiss national research foundation (SNF) grants 3200‐057225 and 3200‐065447, Foundation for Cardiovascular Research Zurich, Switzerland and an educational grant from Bristol‐Myers‐Squibb, Switzerland.
References
- 1.Periard D, Telenti A, Sudre P.et al Atherogenic dyslipidemia in HIV‐infected individuals treated with protease inhibitors. The Swiss HIV cohort study. Circulation 1999100700–705. [DOI] [PubMed] [Google Scholar]
- 2.Friis‐Moller N, Sabin C A, Weber R.et al Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 20033491993–2003. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, Britten M B, Zeiher A M. Prognostic impact of coronary vasodilator dysfunction on adverse long‐ term outcome of coronary heart disease. Circulation 20001011899–1906. [DOI] [PubMed] [Google Scholar]
- 4.Stein J H, Klein M A, Bellehumeur J L.et al Use of human immunodeficiency virus‐1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001104257–262. [DOI] [PubMed] [Google Scholar]
- 5.Fichtenbaum C J, Gerber J G, Rosenkranz S L.et al Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. Aids (London, England) 200216569–577. [DOI] [PubMed] [Google Scholar]