Abstract
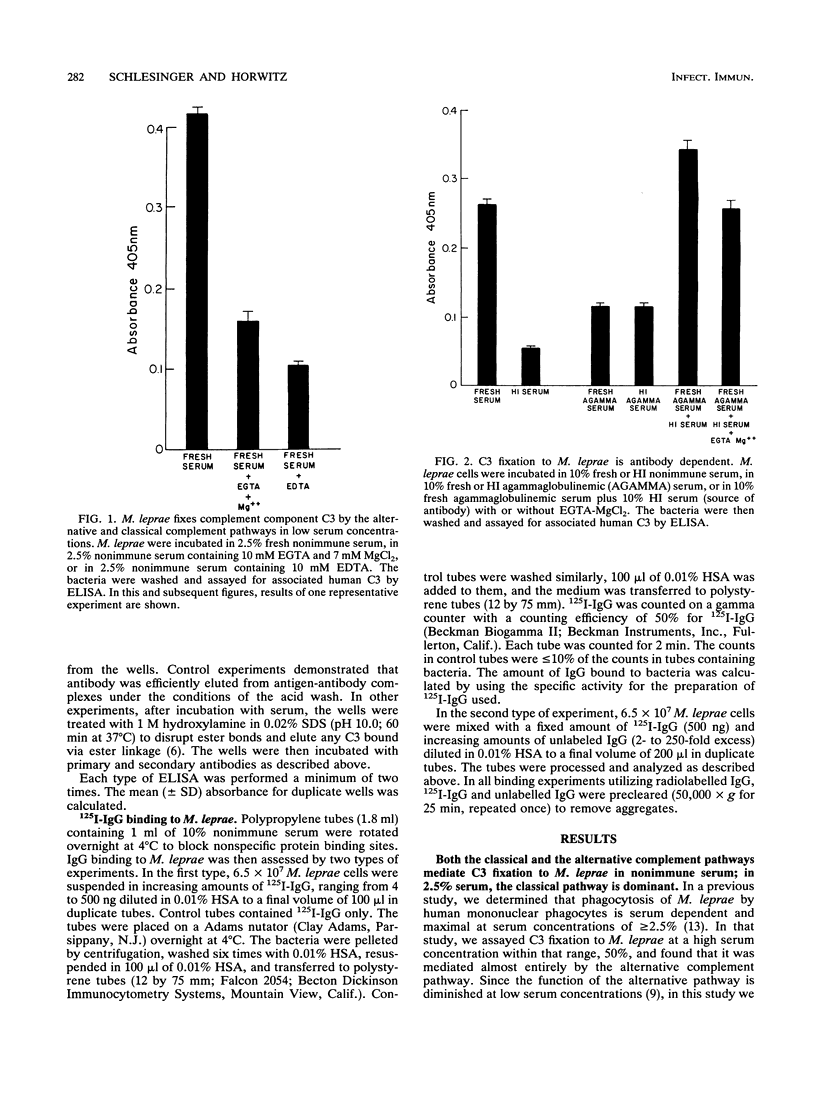
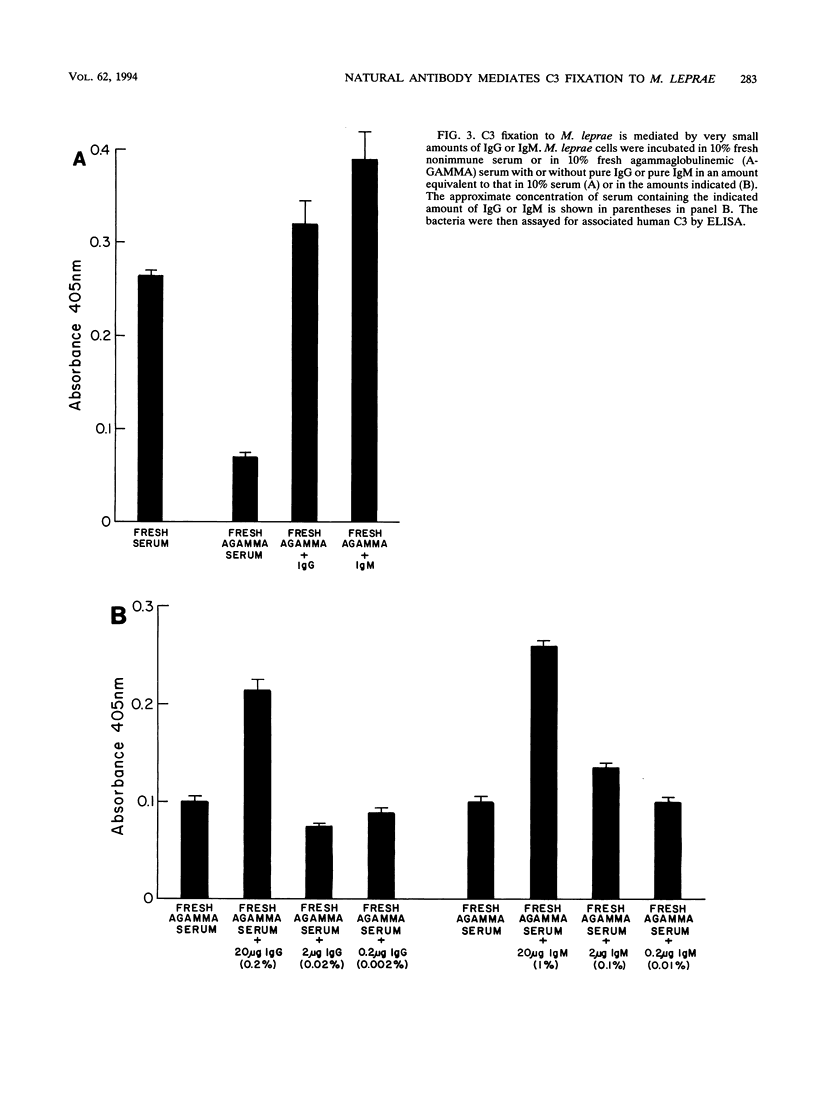
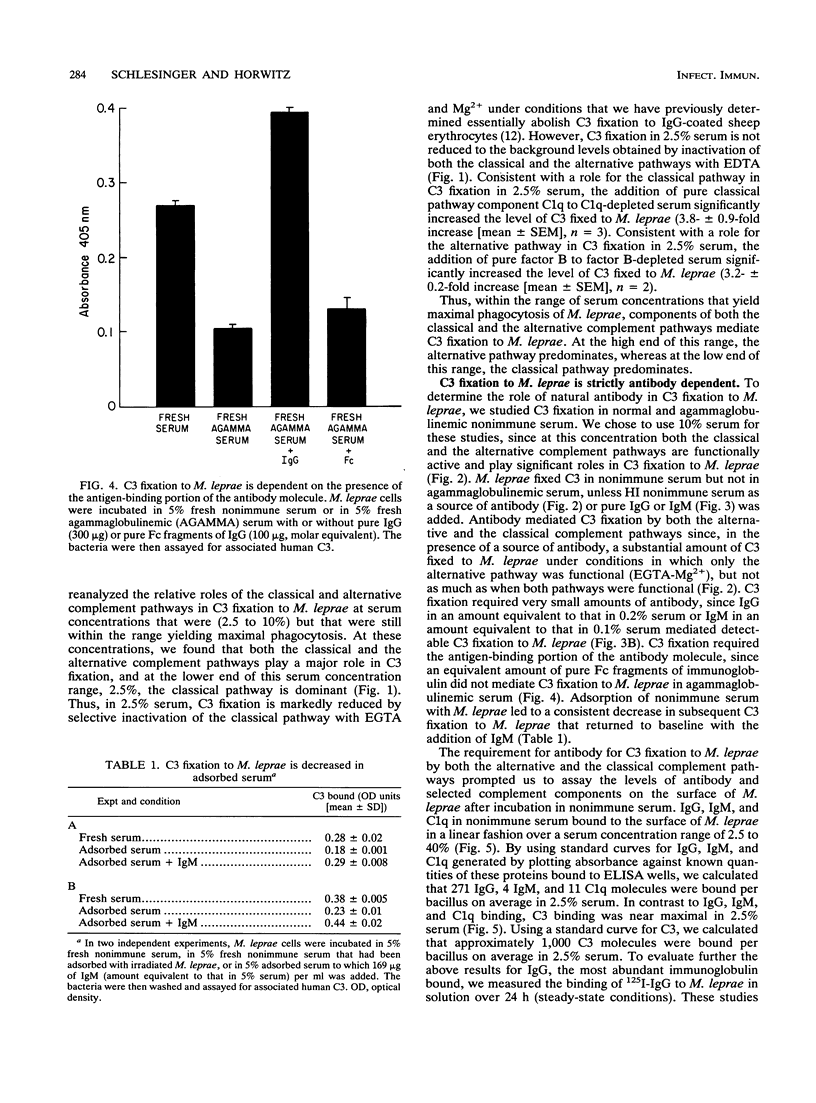
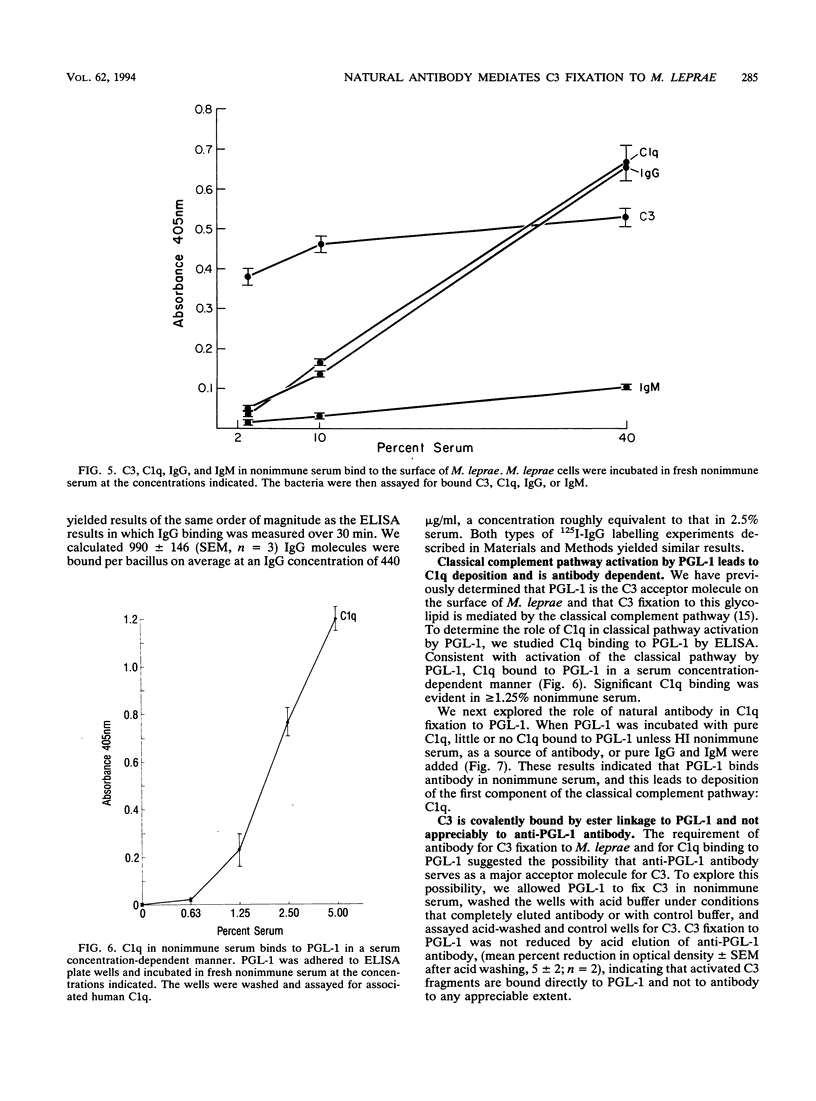
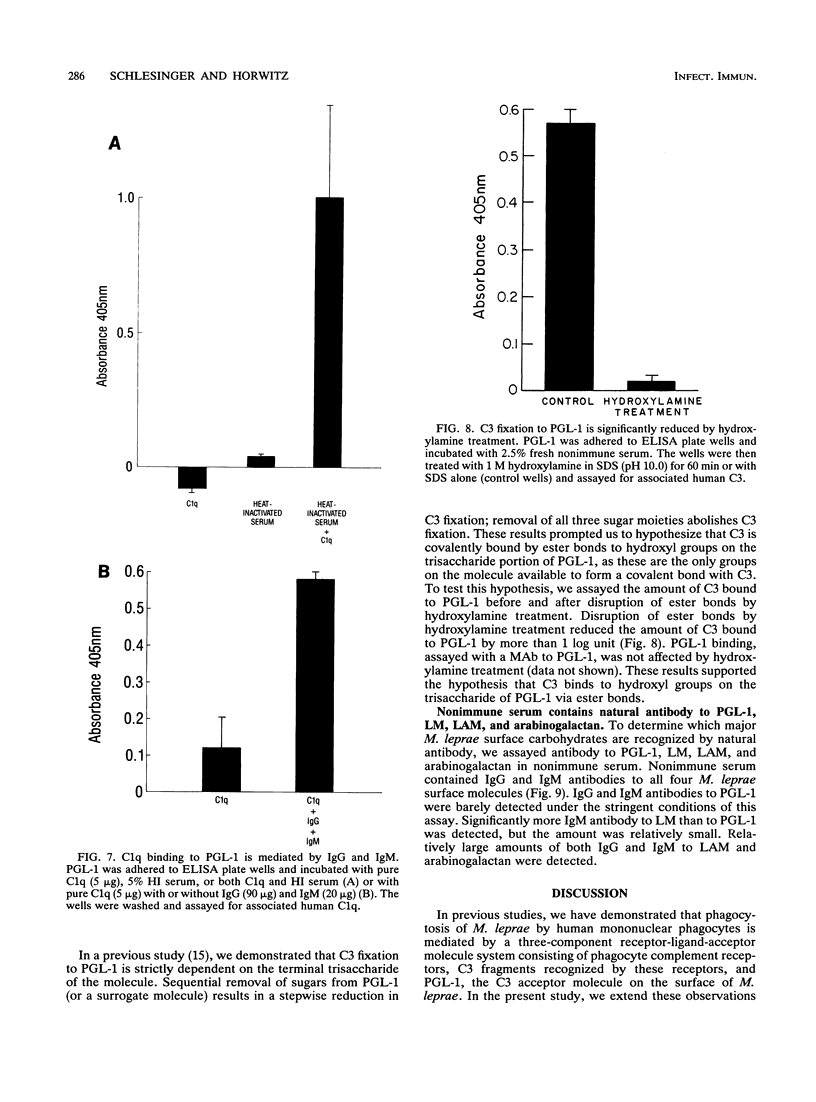
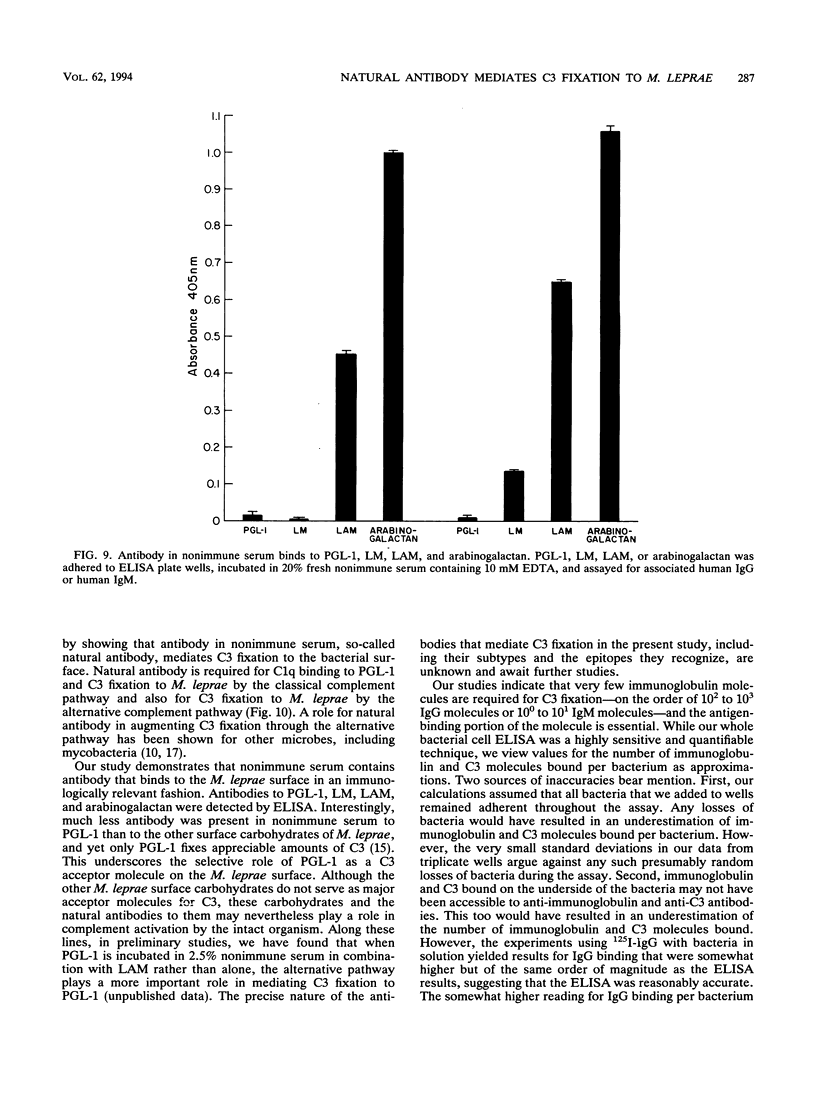
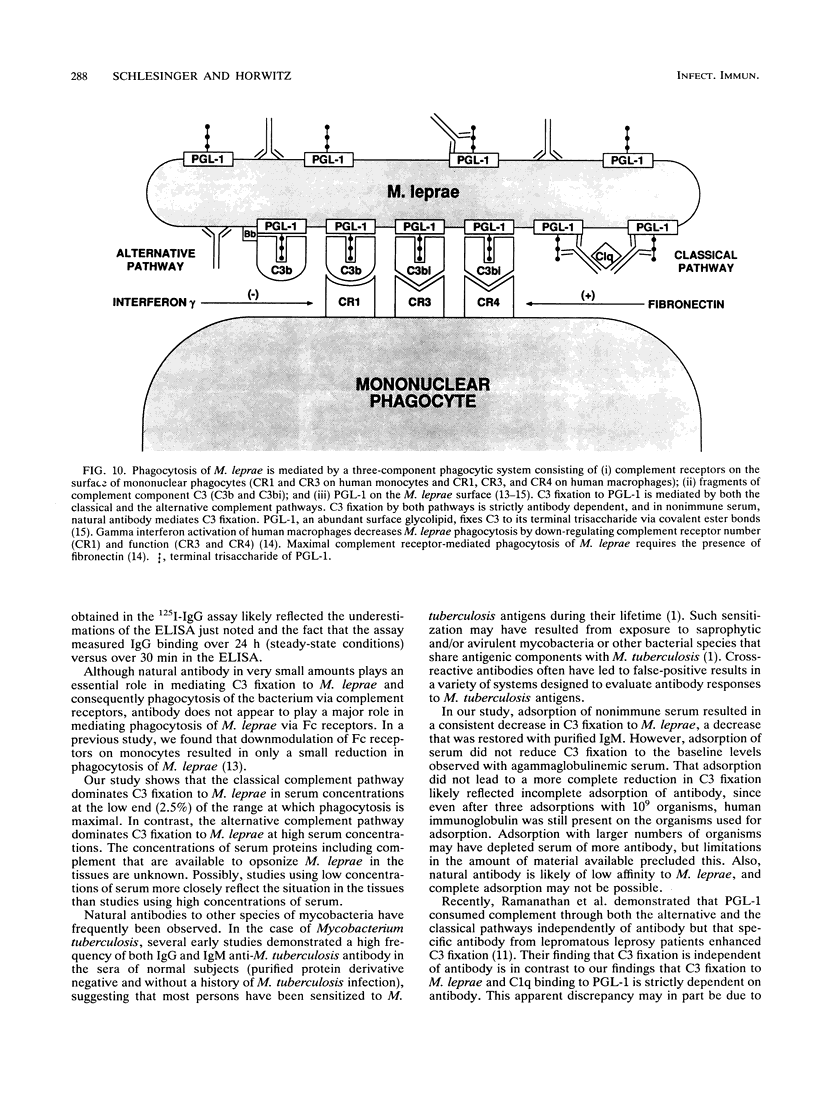
We have previously determined that complement receptors on human mononuclear phagocytes and complement component C3 in nonimmune serum mediate phagocytosis of the intracellular bacterial pathogen Mycobacterium leprae, the agent of leprosy. We have also determined that C3 fixes selectively to the major surface glycolipid of M. leprae, phenolic glycolipid 1 (PGL-1). In this study, we have explored the role of natural antibody in nonimmune serum in C3 fixation and C1q binding to M. leprae and PGL-1. At serum concentrations within the range at which phagocytosis of M. leprae is maximal, C3 fixation was mediated by both the classical and the alternative complement pathways. At the low end of this serum concentration range (2.5%), C3 fixation was mediated predominantly by the classical pathway. Consistent with a role for both pathways, C3 fixation to M. leprae was enhanced by the addition of either pure C1q to C1q-depleted serum or pure factor B to factor B-depleted serum. C3 fixation to M. leprae was strictly antibody dependent regardless of the serum concentration used. C3 fixation to M. leprae occurred in nonimmune serum but not in agammaglobulinemic serum unless heat-inactivated nonimmune serum or small amounts of pure immunoglobulin G (IgG) or IgM were added. C3 fixation by both the alternative and the classical complement pathways was mediated by antibody, and the antigen-binding portion of the antibody molecule was required. C3, IgG, IgM, and C1q were readily detected on the surface of M. leprae. Consistent with the previously demonstrated exclusive role of the classical complement pathway in C3 fixation to PGL-1, C1q bound to PGL-1 in a dose-dependent fashion; C1q binding was evident in > 1.25% nonimmune serum. C1q binding to PGL-1 was strictly antibody dependent. When PGL-1 was incubated with pure C1q, little or no C1q bound to PGL-1 unless heat-inactivated nonimmune serum or pure IgG or IgM was added. When PGL-1 was incubated in nonimmune serum, C3 bound directly to PGL-1 and not to anti-PGL-1 antibody, since the amount of C3 bound to PGL-1 was not reduced by acid elution of the antibody. However, the amount of C3 bound to PGL-1 was markedly reduced by hydroxylamine treatment, providing evidence for C3 fixation via a covalent ester bond. Nonimmune serum contained antibody to all four major M. leprae surface carbohydrates. Relative to PGL-1, nonimmune serum contained more antibody to the other surface carbohydrates.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardana E. J., Jr, McClatchy J. K., Farr R. S., Minden P. Universal occurrence of antibodies to tubercle bacilli in sera from non-tuberculous and tuberculous individuals. Clin Exp Immunol. 1973 Jan;13(1):65–77. [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Walsh G. P. Conservation of genomic sequences among isolates of Mycobacterium leprae. J Bacteriol. 1989 Sep;171(9):4844–4851. doi: 10.1128/jb.171.9.4844-4851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- McNeil M., Wallner S. J., Hunter S. W., Brennan P. J. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Mycobacterium tuberculosis are furanoid. Carbohydr Res. 1987 Sep 1;166(2):299–308. doi: 10.1016/0008-6215(87)80065-4. [DOI] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Ramanathan V. D., Parkash O., Tyagi P., Sengupta U., Ramu G. Activation of the human complement system by phenolic glycolipid 1 of Mycobacterium leprae. Microb Pathog. 1990 Jun;8(6):403–410. doi: 10.1016/0882-4010(90)90027-n. [DOI] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991 Sep 15;147(6):1983–1994. [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J Clin Invest. 1990 Apr;85(4):1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phenolic glycolipid-1 of Mycobacterium leprae binds complement component C3 in serum and mediates phagocytosis by human monocytes. J Exp Med. 1991 Nov 1;174(5):1031–1038. doi: 10.1084/jem.174.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw J., Langendijk E. M., Stoner G. L., Belehu A. Humoral immunity in leprosy: immunoglobulin G and M antibody responses to Mycobacterium leprae in relation to various disease patterns. Infect Immun. 1982 Jun;36(3):885–892. doi: 10.1128/iai.36.3.885-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S., Wood W. B., Jr Heat labile opsonins to Pneumococcus. 3. The participation of immunoglobulin and of the alternate pathway of C3 activation. J Immunol. 1972 Jun;108(6):1681–1689. [PubMed] [Google Scholar]


