Abstract
Objective
To assess the impact on observed mortality of the British Cardiac Society (BCS) definition of myocardial infarction (MI) in 11 UK hospitals.
Design
Prospective observational registry.
Setting
11 adjacent hospitals in the West Yorkshire region.
Patients
2484 patients with the acute coronary syndrome (ACS) were identified during a six month period (28 April to 28 October 2003). Demographic, clinical, and treatment variables were collected on all patients. Deaths were monitored through the Office of National Statistics. Patients were categorised into three groups according to the BCS definition of MI: ACS with unstable angina (UA), ACS with myocyte necrosis, and ACS with clinical MI.
Results
30 day mortality was 4.5%, 10.4%, and 12.9% (p < 0.001) in the ACS with UA, ACS with myocyte necrosis, and ACS with clinical MI groups, respectively. At six months the mortality for patients in the groups ACS with clinical MI and ACS with myocyte necrosis was similar (19.2% v 18.7%), being higher than for ACS with UA (8.6%). Same admission percutaneous coronary intervention was similar in groups with clinical MI and myocyte necrosis (11.1% v 10.7%, respectively) as was coronary artery bypass grafting (2.6% v 2.7%, respectively). However, these two groups differed significantly in the prescribing of secondary prevention (aspirin, 79% v 69%; statins, 80% v 68%; β blockers, 66% v 53%; and angiotensin converting enzyme inhibitors, 65% v 53%; p < 0.001).
Conclusions
At 30 days the new BCS categories for MI predict three distinct outcomes. However, within a contemporary UK population this was no longer apparent at six months, as mortality for patients with ACS with myocyte necrosis had risen to the same level as those for patients with ACS with clinical MI. One possible explanation for this is the apparent under use of drugs known to improve prognosis after traditionally defined MI.
Keywords: acute myocardial infarction, prognosis, acute coronary syndrome, epidemiology, guidelines
In September 2000 the common disease state of myocardial infarction (MI) was redefined by the American College of Cardiology (ACC)/European Society of Cardiology (ESC).1 Under this new definition, the measurement of cardiac troponins became the new reference standard for diagnosing myocardial injury. After years of applying the World Health Organization2,3 definition, scientists and health care providers alike are only just beginning to come to terms with rapid developments in this area. The key areas of progress that have resulted in this change of definition are the ability to measure the cardiac specific markers cardiac troponin T and cardiac troponin I (TnI), which can detect small amounts of myocardial necrosis, and the widespread understanding that MI and unstable angina (UA) generally constitute a single heterogeneous disease state collectively renamed the acute coronary syndrome (ACS).
Recently, the British Cardiac Society (BCS) Working Group commissioned a report4 aimed at helping to establish a consistent nomenclature for the definition of MI, recommending a diagnostic threshold to distinguish patients with MI from patients with other forms of ACS, and recommending a strategy for establishing a reference standard for troponin assays. Concern was raised that the ACC/ESC definition was overly dependent on the measurement of troponin despite significant lack of precision, accuracy, and comparability between the available assays. Others have also made this criticism and highlighted the fact that no available troponin assay fulfils the ACC/ESC requirements for a cut off value at the 99th centile for a normal population while also having good precision indicated by a coefficient of variation (CV) of < 10%.5,6,7 In this context we aimed at exploring the ease of use, demographic features, treatment, and mortality associated with applying the BCS definition of MI to a contemporary UK population of patients with ACS.
PATIENTS AND METHODS
The study was designed as a prospective observational registry of patients admitted with ACS to 11 adjacent UK hospitals. Over a six month period (28 April to 28 October 2003) 6715 potential cases of ACS were identified from the 11 UK hospitals. After evaluation of medical records, ECGs, and results of cardiac troponin and creatine kinase (CK) assays, 2499 patients with confirmed cases of ACS were identified and recruited into the study. Patients with potential ACS were identified from coronary care unit registers and from biochemistry lists of requests for cardiac troponin and CK measurement. All patients were enrolled in the study regardless of age or of medical or surgical speciality. The search strategies were complementary—that is, a significant number of cases of ACS that were later validated would have been missed if only one of the search strategies, such as searching coronary care unit registers, had been employed. All patients regardless of age or consultant team were enrolled in the study. The study was approved by a multicentre research ethics committee and the local research ethics committee from each hospital. Patients provided written consent for evaluation of their medical notes and monitoring of their health status through the Office of National Statistics. They also consented to storage of a blood sample (taken routinely 12–24 hours after onset of symptoms) and to measurement of cardiac TnI, together with other cardiac biomarkers.
Patients were potentially eligible if they were admitted to hospital either through casualty or directly to the ward with an admission diagnosis of suspected ACS. Appropriateness of inclusion was judged by a specialist cardiology research nurse in conjunction with a cardiology research registrar (RD and NK), also taking into account the views and opinions of the attending medical team. This decision was based firstly on clinical context and secondly on the results of cardiac biomarkers. Specifically, patients were included in the study if they fulfilled a revised ESC/ACC definition of MI—raised cardiac troponin concentration above the 10% CV taken 12–24 hours after the onset of symptoms or raised CK concentration above twice the upper limit of normal—accompanied by at least one of the following: (1) ischaemic symptoms; (2) development of pathological Q waves on the ECG; (3) ECG changes indicative of ischaemia; (4) delivery of primary coronary angioplasty; and (5) compatible postmortem findings. Furthermore, we included patients with diagnosed ACS on clinical grounds (that is, clinical history, examination, ECG, angiography, or postmortem findings) when biomarkers were not available (for example, owing to very early death) and when troponin concentrations were negative based on the 10% CV threshold. Patients were excluded if they did not provide consent or if they were judged not to have a diagnosis of ACS. Our intention was to include a wide range of consenting “real world” patients with ACS into the study.
A 150 item case record form listing demographic, clinical, and treatment variables was completed for each patient according to a standardised operations manual and entered on to a computer database. Only one event was included (the first presentation with an ACS during the recruitment window) and patients transferred to a tertiary centre were counted only once for the index admission. Clinical characteristics on admission were taken from the following sources in order of preference: emergency department medical notes, admitting medical team's first clerking, and nursing notes.
Ten experienced research nurses and two cardiology registrars (RD and NK) abstracted data from the medical notes. The quality of data abstraction from case notes and data entry into the computer database was formally assessed. Data were checked for completeness and consistency, and queries were generated at regular intervals during the course of the study. Fifty five per cent of randomly generated cases were verified by double data entry in key fields. All 11 centres were visited to confirm that screening methods and source data were adequate.
Patients were categorised into three groups according to the BCS definition based on the following criteria:
ACS with UA (Accu TnI ⩽ 0.06 µg/l; 10% CV)
ACS with myocardial necrosis (Accu TnI > 0.06–0.5 µg/l)
ACS with clinical MI (Accu TnI > 0.5 µg/l or CK twice the upper limit of the normal reference range).
Two cardiology registrars (RD and NK) and a consultant cardiologist (ASH) allocated patients to groups based on data available from clinical history, serial ECGs, and cardiac troponin and CK results.
Cardiac TnI was biochemically analysed with the Beckman Coulter Ltd Accu TnI assay by an immunometric technique. Cardiac troponin was measured in six local hospital laboratories routinely using the Accu TnI assay and at a central core laboratory (Leeds General Infirmary) for five other local hospitals. For this study we used the 10% CV for the assay, which corresponds to an Accu TnI result of 0.06 ng/ml. Accu TnI was measured for 1975 patients and for the remaining 509 patients CK was measured as part of the patients' routine care. The 509 patients whose cardiac TnI was not measured were identified on the basis of either ST elevation on the presenting ECG or a CK concentration twice the upper limit of normal. A CK concentration of 400 U/l (twice the upper limit of normal) was used as the MI diagnostic threshold. Current local guidelines within West Yorkshire indicate that patients with ST elevation MI (STEMI) do not require a cardiac troponin measurement to confirm a diagnosis. A diagnosis of STEMI can be based on clinical history, ECG changes, and from CK results.
Data were statistically analysed with SPSS system 11.1 (SPSS Inc, Chicago, Illinois, USA) software. Continuous variables are presented as mean (SD) and categorical variables as frequencies. Groups were compared by the χ2 test for categorical data and analysis of variance for continuous variables. Kaplan‐Meier analysis was used to analyse survival of patients in each of the three groups.
RESULTS
Evaluation of the medical records confirmed ACS in 2499 patients. According to the BCS diagnostic criteria, patients were subsequently divided into three groups as follows: ACS with UA (11%), ACS with myocardial necrosis (21%), and ACS with clinical MI (68%). In 15 patients it was not possible to determine a BCS category because these patients died in hospital before any cardiac markers were measured, with no diagnostic ECG or postmortem findings. These patients were excluded from the analysis.
Table 1 gives demographic and clinical baseline characteristics for the BCS groups. Patients having ACS with myocyte necrosis tended to be older than those in the other groups and more were women (p < 0.001). There was also a trend for patients with ACS with UA to have a history of acute MI and hypercholesterolaemia (defined as a serum cholesterol concentration > 5.1 mmol/l before any treatment with a statin) as compared with the other groups (p < 0.001). Patients having ACS with clinical MI were also more likely than those in the other groups to be current smokers (p < 0.001). A greater percentage of patients underwent percutaneous coronary intervention (PCI) if they had ACS with myocyte necrosis or ACS with clinical MI (10.7% and 11.1%, respectively) than if they had ACS with UA (4.9%). This was found to be significant (p = 0.008).
Table 1 Baseline characteristics by British Cardiac Society category of acute coronary syndrome (ACS).
| ACS with unstable angina (n = 268) | ACS with myocyte necrosis (n = 530) | ACS with clinical MI (n = 1686) | p Value | |
|---|---|---|---|---|
| Age (years) | 69.1 (12.8) | 71.8 (12.6) | 69.7 (13.3) | 0.03 |
| Women | 89 (33%) | 241 (46 %) | 601 (36%) | <0.001 |
| Current smoker | 51 (19%) | 114 (22%) | 509 (30%) | <0.001 |
| Systolic BP (mm Hg) | 142.1 (27.2) | 144.2 (30.5) | 141.4(30.4) | NS |
| Heart rate (beats/min) | 79.9 (23.0) | 87.9 (25.4) | 83.3 (23.4) | <0.001 |
| Medical history | ||||
| Acute MI | 97 (36%) | 155 (29%) | 373 (22%) | <0.001 |
| Hypertension | 133 (50%) | 233 (44%) | 696 (41%) | NS |
| Diabetes mellitus | 52 (19%) | 94 (18%) | 280 (17%) | NS |
| Hypercholesterolaemia | 125 (47%) | 197 (37%) | 514 (31%) | <0.001 |
| Peripheral vascular disease | 25 (9%) | 51 (10%) | 32 (8%) | NS |
| Same admission | ||||
| Angiogram | 55 (21%) | 146 (28%) | 403 (24%) | NS |
| PCI | 13 (5%) | 56 (11%) | 187 (11%) | 0.008 |
| CABG | 6 (2%) | 14 (3%) | 44 (3%) | NS |
| Planned | ||||
| Angiogram | 16 (6%) | 29 (6%) | 123 (7%) | NS |
| PCI/CABG | 7 (3%) | 23 (4%) | 67 (4%) | NS |
| All cause mortality | ||||
| 30 day | 4.5% | 10.4% | 12.9% | <0.001 |
| 6 month | 8.6% | 18.7% | 19.2% | <0.001 |
| Increase 30 day to 6 month | 4.1% | 8.3% | 6.3% | <0.001 |
Data are mean (SD) or number (%).
BP, blood pressure; CABG, coronary artery bypass grafting; MI, myocardial infarction; NS, not significant; PCI, percutaneous coronary intervention.
Table 2 shows the pharmacological treatment of patients admitted with ACS. In‐hospital use of low molecular weight heparin was uniform across the groups (about 75% of patients) with a tendency for unfractionated heparin to be given to patients having ACS with clinical MI. The use of clopidogrel was 44%, 55%, and 52% (ACS with UA, ACS with myocardial necrosis, and ACS with clinical MI, respectively). Only a small proportion of patients received a glycoprotein IIb/IIIa inhibitor as part of their in‐hospital treatment (1% ACS with UA, 7% ACS with myocardial necrosis, and 7% ACS with clinical MI). Intravenous nitrates were given more commonly to patients having ACS with clinical MI than to the other groups (p < 0.001). More potassium channel modulators were given to patients having ACS with UA and ACS with myocardial necrosis. At discharge, secondary prevention prescribing increased across the groups. Patients having ACS with clinical MI were more likely than the other two groups to receive aspirin, β blockers, angiotensin converting enzyme (ACE) inhibitors, and statins (p < 0.001).
Table 2 Pharmacological treatments during hospital stay and at discharge.
| ACS with unstable angina (n = 268) | ACS with myocardial necrosis (n = 530) | ACS with clinical MI (n = 1686) | p Value | |
|---|---|---|---|---|
| In hospital | ||||
| Unfractionated heparin | 8 (3%) | 23 (4%) | 252 (15%) | <0.001 |
| LMWH | 192 (72%) | 401 (76%) | 1230 (73%) | NS |
| Clopidogrel | 118 (44%) | 290 (55%) | 866 (52%) | 0.033 |
| Glycoprotein IIb/IIIa inhibitor | 3 (1%) | 36 (7%) | 121 (7%) | 0.004 |
| Intravenous β blocker | 1 (0.4%) | 5 (1%) | 32 (2%) | NS |
| Calcium antagonist | 77 (29%) | 116 (22%) | 319 (19%) | 0.005 |
| Intravenous nitrate | 12 (5%) | 56 (11%) | 320 (19%) | <0.001 |
| Oral nitrate | 142 (53%) | 262 (49%) | 726 (43%) | 0.007 |
| Potassium channel modulator | 62 (23%) | 115 (22%) | 227 (14%) | <0.001 |
| After discharge | ||||
| β Blocker | 139 (53%) | 277 (53.1 %) | 1106 (66%) | <0.001 |
| Statin | 181 (69%) | 358 (68.5 %) | 1350 (80%) | <0.001 |
| ACE inhibitor | 121 (46%) | 276 (52.8 %) | 1094 (65%) | <0.001 |
| Aspirin | 190 (72%) | 362 (69.1 %) | 1327 (79%) | <0.001 |
| Clopidogrel | 85 (32%) | 224 (42.3%) | 651 (39%) | 0.004 |
ACE, angiotensin converting enzyme; LMWH, low molecular weight heparin.
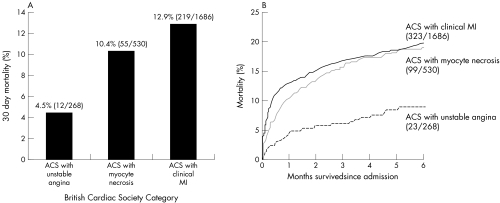
Figure 1 shows Kaplan‐Meier survival curves for the BCS definition groups. Thirty day all cause mortality rates increased across the groups (log rank test, p < 0.001). The mortality was 4.5%, 10.4%, and 12.9% in the ACS with UA, ACS with myocyte necrosis, and ACS with clinical MI groups, respectively). The six month all cause mortality was similar for patients having a diagnosis of ACS with myocyte necrosis and for those having a diagnosis of ACS with clinical MI (18.7% v 19.2%).
Figure 1 (A) Thirty day mortality according to British Cardiac Society category. (B) Kaplan‐Meier survival curves for events from admission to six months.
Patients having a diagnosis of ACS with clinical MI were further classified as having non‐ST elevation MI (NSTEMI) and STEMI based on serial ECGs, together with cardiac troponin and CK results. The presence of ST elevation or bundle branch block and an appropriate rise in biomarker(s) was used to distinguish those events categorised as STEMIs. NSTEMI events were indicated by all other ECG patterns together with an appropriate rise in biomarker(s). The mortality at 30 days was 11.8% for patients with NSTEMI and 14.4% for patients with STEMI; at six months the mortality was identical for patients having ACS with myocyte necrosis, NSTEMI, or STEMI (18.7%, 19.1%, and 19.2%).
DISCUSSION
Previous studies have shown that patients identified by troponin rise but normal CK have a worse six month outcome than do patients with MI defined by conventional criteria.8 This observation has very important pathological and clinical implications, as it suggests that patients with ACS with myocyte necrosis are at greater risk of reinfarction or completion of infarction. Consequently, patients with ACS with myocyte necrosis merit treatment and investigation strategies that are at least as aggressive as those given to patients with ACS with clinical MI.9 In addition to other treatments, the task force on the management of acute coronary syndromes of the ESC advocate angiography and treatment with glycoprotein IIb/IIIa inhibitors for all patients with ACS and a detectable troponin rise but without ST elevation on the admission ECG. This group also advocate wider treatment with the same four drugs (aspirin, β blockers, statins, and ACE inhibitors) proved to be effective in secondary prevention after NSTEMI/STEMI. Routine administration of these drugs to patients previously classified as having UA cannot be directly supported by the randomised clinical trial evidence base, yet is viewed by this and other groups as an important strategy for risk reduction.
In a contemporary UK cohort of patients, we have both confirmed and further extended the observation of delayed increases in total mortality for patients with NSTEMI by showing that a similar effect is seen also for patients referred to as having ACS with myocyte necrosis. The increase in total mortality between 30 and 180 days was highest for this initially intermediate risk group (8.3% as compared with 4.1% with UA and 6.3% with clinical MI). At 30 days all cause mortality of patients with ACS and UA is distinct from that seen for ACS with myocyte necrosis and ACS with clinical MI (4.5%, 10.4%, and 12.9%, respectively). By six months the mortality of patients having ACS with myocyte necrosis is seen to rise to the same rate as for ACS with clinical MI (18.7% and 19.2%, respectively) despite presumed lower levels of myocyte necrosis during the index event. Consequently, this is the first report regarding the survival outcomes for patients who have been categorised by the new BCS Working Group MI terminology.4
The evidence suggests that cardiac troponin concentrations correlate with mortality in patients with ACS.10 With this in mind, one would expect patients with ACS having a smaller troponin rise (ACS with myocardial necrosis) to have a lower mortality than that of patients with ACS having a greater troponin rise (ACS with clinical MI). As stated above, we did not observe this in our cohort where total mortality at six months was the same for patients with ACS with either myocardial necrosis or clinical MI. The likely explanation for this is later recurrence or completion of the infarction process in the group with initial evidence of more limited myocyte necrosis. However, we also observed a more conservative approach to the use of secondary prevention (aspirin, β blockers, statins, and ACE inhibitors) in managing patients having a diagnosis of ACS with myocardial necrosis than in patients with ACS with clinical MI. This strongly suggests one key strategy for improving the outcome of these patients. Important baseline differences between the groups must also be acknowledged, which may partially account for these results. For example, compared with patients with ACS with clinical MI, more of the patients with ACS with myocyte necrosis were women and this group tended to be older, to have a history of MI, and to have higher heart rates, which may contribute to this catch up phenomenon at six months.
Our study also highlights that case fatality rates at six months were higher than in the multinational GRACE (global registry of acute coronary events) population.11 One potential reason for this is that the mean age was just 66 years (that is, they were four years younger); also, coronary angiography and PCI rates in GRACE were much higher (STEMI: angiography, 55%, and PCI, 53%; NSTEMI: 42% and 40%; UA: 28% and 18%) than in EMMACE‐2 (evaluation of methods and management of acute coronary events) (ACS with clinical MI, 24% and 28%; ACS with myocardial necrosis, 21% and 11%; and ACS with UA, 11% and 5%). This suggests scope for improvement in the availability and delivery of the invasive approach to the management our patients with ACS as supported by the FRISC‐II (Fragmin and fast revascularisation during instability in coronary artery disease) and TACTICS (treat angina with Aggrastat and determine cost of therapy with invasive or conservative strategy) trials and in line with both US and European guidelines.9,12,13,14 Such an approach is supported by a recent meta‐analysis and review of the literature, which shows that a routine invasive approach reduces mortality in the setting of UA and NSTEMI, as well as primary PCI for patients with STEMI.15,16
In addition to assessing differences in the use of drugs for secondary prevention and the frequency of use of more interventional strategies, we evaluated the use of newer evidence based antithrombotic treatments in the acute management of patients with ACS.9,14,17,18 Despite the advice of UK (National Institute for Health and Clinical Excellence) and European authorities based on evidence supporting greater use of glycoprotein IIb/IIIa inhibitors in ACS,9,19 only a small proportion of patients received this treatment in hospital (7% for ACS with clinical MI, 7% for ACS with myocardial necrosis, and 1% for ACS with UA). In contrast, the use of oral clopidogrel in the short term and after discharge was better (51% and 39% for all patients with ACS), being highest among patients having ACS with myocardial necrosis (55% and 42% short term and after discharge). Nevertheless, there remains much scope for improvement also for this aspect of routine care.
The applicability of our results is limited by the BCS Working Group's proposal of a double cut off for troponin: the lowest to diagnose myocardial injury and the highest to diagnose MI. The recommendations to revise the ACC/ESC definition suggest that an analytical target of 10% CV be used as a cut off when diagnosing myocardial injury. Furthermore, as this concentration varies considerably between different assays, improvements are also needed in other aspects of assay performance. The 8.6% six month mortality seen in patients having ACS with UA emphasises the need for better discrimination between and triage of patients with ACS at this end of the spectrum.
For most troponin assays, little is known about the amount of troponin rise that corresponds to a CK or CK‐MB rise that would satisfy the WHO MI definition.2,3 Indeed in the BCS paper (which takes into consideration only one of several available TnI assays) only a personal communication is quoted to determine the cut off concentration for TnI corresponding to a CK or CK‐MB rise that would satisfy the WHO criteria.4 However, this higher cut off is of much less clinical relevance if patients having ACS with MI and ACS with myocyte necrosis are treated in a much more similar manner than is current practice.
Definite MI was previously defined as the combination of two of three characteristics: typical symptoms of infarction (that is, chest pain or discomfort), a rise in plasma or serum cardiac enzymes, and a typical ECG pattern involving the development of Q waves.2 However, a clinical event could also potentially be classified as either a probable or a possible MI. In 2000 the ACC and the ESC redefined the diagnostic criteria for MI to include the measurement of cardiac troponin as a diagnostic indicator of myocardial injury.1 This change in definition has meant that a previous diagnosis of UA may now be classified as an MI according to the ACC/ESC definition.1 The adoption of the ACC/ESC definition has been shown to double the number of MI diagnoses.20 These additional patients who were previously classified as having UA tend to be older, have greater co‐morbidities, and have less favourable outcomes than do patients with MI diagnosed according to the WHO criteria.8,20 As this definition has an impact on a wide range of other factors (for example, guidelines, treatment pathways, and epidemiological monitoring) and because there are practical challenges in the use of imperfect troponin assays, implementation has been variable and incomplete and has given rise to new complexity and confusion. This suggests the need for an interim or transition period between old and new definitions.
In this study we have investigated the implications of the new BCS definition of MI in a contemporary UK cohort. We have identified that patients having ACS with myocardial necrosis, despite having less cardiac muscle damage, have the same mortality at six months as patients having ACS with clinical MI. In the context of previous definitions of MI, ACS with clinical MI corresponds to the WHO definition of MI2 and ACS with myocardial necrosis corresponds to UA. Together these two categories fulfil a revised ACC/ESC definition of MI.1,5,6,7 However, until an identical range of treatments is routinely given to patients within the different methods of categorisation (ACC/ESC definition MI; BCS definition MI; WHO definition MI) it is impossible to assess the mortality associated with each of these various definitions.
In conclusion, we have looked in detail at the ease of application and clinical usefulness of the newly proposed BCS definition of MI. We have assessed differences in treatment received and the associated short and long term mortality for a real world UK cohort. Our findings support the value of a revised ACC/ESC definition of MI (use of 10% CV as diagnostic cut off), as the additional cohort of patients identified by the new diagnosis of MI have a prognosis identical to those covered by the old definition. The relatively limited use of interventional strategies and of short term and secondary prevention drugs further suggests that, in particular, patients with myocardial necrosis may benefit both from early identification and from being treated more aggressively.
The new BCS recommendations regarding ACS classification were found to be very practical by simultaneously allowing identification of patients previously categorised as having either MI or UA and covering the new extended definition of MI.1 This is of great help during the transition from one definition to the other, as many clinical guidelines, care pathways, clinical trials, and epidemiology studies have yet to fully respond to a change of definition. Furthermore, the identification of a category of patients having ACS with UA, on clinical but not biochemical grounds, brings additional focus on the inadequacies of current diagnostic strategies. Additional research into the concomitant use of alternative diagnostic assays and the development of better troponin assays are to be strongly encouraged, as the six month mortality (8.6%) for this form of ACS is also high.
ACKNOWLEDGEMENTS
We thank all the staff in the biochemistry departments, coronary care units, and audit and medical records departments from all the 11 hospitals in West Yorkshire, as well as the Office of National Statistics. We also thank the Myocardial Infarction National Audit Project (MINAP) for their help and support.
Abbreviations
ACC - American College of Cardiology
ACE - angiotensin converting enzyme
ACS - acute coronary syndrome
BCS - British Cardiac Society
CK - creatine kinase
CV - coefficient of variation
EMMACE‐2 - evaluation of methods and management of acute coronary events
ESC - European Society of Cardiology
FRISC‐II - Fragmin and fast revascularisation during instability in coronary artery disease
GRACE - global registry of acute coronary events
MI - myocardial infarction
NSTEMI - non‐ST elevation myocardial infarction
PCI - percutaneous coronary intervention
STEMI - ST elevation myocardial infarction
TACTICS - treat angina with Aggrastat and determine cost of therapy with invasive or conservative strategy
UA - unstable angina
WHO - World Health Organization
Appendix
STRUCTURE OF THE EMMACE‐2 STUDY GROUP
Principal investigator: Alistair S Hall
Co‐supervisors: Michael B Robinson (public health) and Julian H Barth (clinical biochemistry)
Clinical coordinators: Rajiv Das and Niamh Kilcullen
Nurse coordinator: Christine Morrell
Research assistants: Mark Rowe, Sally Hall, Yvonne McGill, John Arnell, Tammie Drake, Sarah Burkett, Judith Beevers, Bev Durkin, Carol Taylor, and Martin Price
Statistical analysis: Alistair S Hall, Rajiv Das, and Niamh Killcullen
Database management: Peter Tooze
Key investigators: M Appleby, W Baig, SG Ball, PD Batin, KE Berkin, JM Blaxill, T Bloomer, WP Brooksby, JC Cowan, R Crook, C Dickinson, S Grant, H Larkin, RV Lewis, S Lindsey, AF Mackintosh, J Mclenachan, S McGerrary , LCA Morley, GW Morrison, CB Pepper, EJ Perrins, MM Pye, G Reynolds, RJ Sapsford, NP Silverton, JH Smyllie, RN Stevenson, LB Tan, CJP Welsh, GJ Williams, JI Wilson, and AV Zezulka
Footnotes
Funding: This work funded by educational grants from Astra Zeneca and Beckman Coulter Ltd. RD and NK held British Heart Foundation Junior Research Fellowships.
No competing interests.
Ethical approval: The study was approved by the Eastern Multi‐Research Ethics Committee and the Local Research Ethics Committees within West Yorkshire.
References
- 1.Joint European Society of Cardiology/American College of Cardiology Committee Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. Eur Heart J 2000211502–1513. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Working Group on the establishment of ischemic heart disease registers. Report of the Fifth Working Group, Copenhagen. WHO Reg Publ Eur Ser. 1972;821(suppl 5).
- 3.Joint International Society and Federation of Cardiology/World Health Organization Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 197959607–609. [DOI] [PubMed] [Google Scholar]
- 4.Fox K A, Birkhead J, Wilcox R.et al British Cardiac Society Working Group on the definition of myocardial infarction. Heart 200490603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newby L K, Alpert J S, Ohman E M.et al Changing the diagnosis of acute myocardial infarction: implications for practice and clinical investigations. Am Heart J 2002144957–980. [DOI] [PubMed] [Google Scholar]
- 6.White H D. Things ain't what they used to be: impact of a new definition of myocardial infarction [editorial]. Am Heart J 2002144933–937. [DOI] [PubMed] [Google Scholar]
- 7.Apple F S, Wu A H B, Jaffe A S. European Society of cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J 2002144981–986. [DOI] [PubMed] [Google Scholar]
- 8.Meier M A, Al‐Badr W H, Cooper J V.et al The new definition of myocardial infarction: diagnostic and prognostic implications in patients with acute coronary syndromes. Arch Intern Med 20021621585–1589. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand M E, Simoons M L, Fox K A A.et al Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Task Force of the European Society of Cardiology. Eur Heart J 2002231809–1840. [DOI] [PubMed] [Google Scholar]
- 10.Antman E M, Tanasijevic M J, Thompson B.et al Cardiac‐specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 19963351342–1349. [DOI] [PubMed] [Google Scholar]
- 11.Fox K A A, Goodman S G, Klein W.et al Management of acute coronary syndromes. Variations in practice and outcome: findings from the global registry of acute coronary events (GRACE) study, Eur Heart J 2002231177–1189. [DOI] [PubMed] [Google Scholar]
- 12.FRISC II Investigators Invasive compared with non‐invasive treatment in unstable coronary‐artery disease: FRISC II prospective randomised multicentre study. Fragmin and fast revascularisation during instability in coronary artery disease investigators. Lancet 1999354708–715. [PubMed] [Google Scholar]
- 13.Cannon C P, Weintraub W S, Demopoulos L A.et al Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban, N Engl J Med 20013441879–1887. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Antman E M, Beasley J W.et al ACC/AHA guidelines for the management of patients with unstable angina non‐ST segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). J Am Coll Cardiol 200036970–1062. [DOI] [PubMed] [Google Scholar]
- 15.Bavry A A, Kumbhani D J, Quiroz R.et al Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non‐ST‐segment elevation acute coronary syndromes: a meta‐analysis and review of the literature. Am J Cardiol 200493830–835. [DOI] [PubMed] [Google Scholar]
- 16.Keeley E C, Boura J A, Grines C L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 200336113–20. [DOI] [PubMed] [Google Scholar]
- 17.Morrow D A, Antman E M, Snapinn S.et al An integrated clinical approach to predicting the benefit of tirofiban in non‐ST elevation acute coronary syndromes: application of the TIMI risk score for UA/NSTEMI in PRISM‐PLUS. Eur Heart J 200223223–229. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Zhao F, Mehta S R.et al Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST segment elevation. N Engl J Med 2001345494–502. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Clinical Excellence Guidance on the use of glycoprotein IIb/IIIa inhibitors in the treatment of acute coronary syndromes. Technology Appraisal Guidance No 47. London: NICE, 2002
- 20.Pell J P, Simpson E, Rodger J C.et al Impact of changing diagnostic criteria on incidence, management, and outcome of acute myocardial infarction: retrospective cohort study. BMJ 2003326134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]