Abstract
Objectives
To assess survival and long term arch patency rates in a consecutive group of children after extended arch repair for coarctation of the aorta.
Methods
Review of 191 consecutive children (154 (81%) under 1 year of age) operated on between 1990 and 2002 by a single surgeon using extended arch reconstructive techniques. For assessment of survival patients were divided into three groups: 1, coarctation alone, n = 104; 2, coarctation and ventricular septal defect, n = 38; and 3, coarctation in association with complex intracardiac anomalies, n = 49. A prospective and systematic clinical and echocardiographic evaluation of the aortic arch was undertaken.
Results
Median time to follow up was 4.2 years (range 1–10.6 years). Overall actuarial survival was 92%, 88%, and 88% at two, five, and 10 years. Mortality was significantly higher in those patients with complex intracardiac anatomy. Arch obstruction recurred in seven of 165 (4.2%) patients: four of 139 (2.9%) term and three of 10 (30%) premature infants (p < 0.001).
Conclusions
Survival after extended arch reconstruction for coarctation is excellent. At long follow up recurrent arch obstruction is rare, with prematurity the only risk factor.
Keywords: coarctation, aorta, surgery
Corrective surgery for coarctation of the aorta was first described 60 years ago.1 Classic surgical techniques do not address arch hypoplasia or encroachment of ductal tissue beyond the anastomosis and consequently recurrent arch obstruction is relatively common.2,3,4,5 Recurrent arch obstruction assumes greater importance as a measure of successful outcome after coarctation repair given the excellent survival rates in the modern era. Of all current strategies, extended arch reconstruction (both extended end to end and end to side anastomoses) best addresses both the problem of ductal extension through the aorta and associated arch hypoplasia.6 Reports of extended arch repair thus far have been in selected groups of patients with relatively short follow up times.7,8,9,10,11,12,13,14
We report a systematic clinical review of over a decade of single surgeon experience with extended arch repairs in unselected children of all anatomical subgroups.
PATIENTS AND METHODS
The Yorkshire Heart Centre is a regional cardiothoracic centre serving a local population of 5.5 million people with stable referral patterns. Between January 1990 and January 2002, 191 children underwent surgical repair of coarctation of the aorta by a single cardiac surgeon (KGW) using an extended arch repair (which can be subdivided into either extended end to end or extended end to side; see below). One hundred and twelve (59%) were boys and the group was heavily biased towards infants with 154 (81%) under 1 year of age. The median age at operation was 1 month (range 1 day to 15.2 years) and median weight 3.7 kg (range 1–58 kg).
During the same period an additional 10 patients underwent subclavian flap repair (all before 1994) and four underwent patch augmentation of coarctation (all older patients). Anatomy or demographic characteristics did not differ between patients undergoing subclavian flap repair and those undergoing extended arch repair. The mixture of techniques early in the series resulted from transition from one surgical strategy to another.
Additional cardiac lesions
One hundred and forty four (75%) patients had intracardiac lesions.
For the survival analysis patients were divided into the following three groups. Group 1 consisted of 104 patients (54%) who had “isolated” coarctation without associated major intracardiac lesions but including “minor” abnormalities (for example, bicuspid aortic valves and atrial septal defects). Their median age and weight at surgery were 2 months (range 2 days to 15 years) and 4.2 kg (1–58 kg), respectively. Group 2 consisted of 38 patients (20%) with coarctation and a ventricular septal defect. Their median age and weight at surgery were 2 weeks (range 1 day to 10.8 years) and 3.2 kg (range 2.1–25 kg), respectively. Group 3 consisted of 49 patients (26%) with coarctation with associated complex intracardiac anomalies (table 1). Their median age and weight at surgery were 10 days (range 1 day to 4 years) and 3.4 kg (range 2–22 kg), respectively.
Table 1 Significant associated cardiac anomalies.
| Abnormality | No |
|---|---|
| Significant mitral valve abnormality | 6 (3%) |
| Shone's complex | 4 (2%) |
| TGA (± VSD) | 17 (9%) |
| TGA + dextrocardia | 2 (1%) |
| Single ventricle (DORV, DILV, etc) | 8 (4%) |
| Atrioventricular septal defect | 2 (1%) |
| Anomalous pulmonary vein drainage | 1 (0.5%) |
| Atrial isomerism + VSD | 1 (0.5%) |
| Severe subaortic stenosis | 2 (1%) |
| Cor triatriatum | 1 (0.5%) |
| Sinus venosus atrial septal defect | 1 (0.5%) |
| Ebstein's anomaly | 1 (0.5%) |
| Congenitally corrected TGA | 1 (0.5%) |
| Aortopulmonary window | 1 (0.5%) |
| Dextrocardia | 1 (0.5%) |
| Complex intracardiac anomalies (total) | 49 (26%) |
DILV, double inlet left ventricle; DORV, double outlet right ventricle; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Transverse arch hypoplasia (defined as a diameter of the aorta between the common carotid and left subclavian artery of < 50% of the ascending aorta) was identified retrospectively from either the operation note or the preoperative echocardiogram.15 Transverse arch hypoplasia was present in 79 (41%) patients (52% of infants under 1 year) and was more common in patients undergoing extended end to side repair through a median sternotomy (30 of 50 patients v 49 of 141, p < 0.002).
Operative techniques
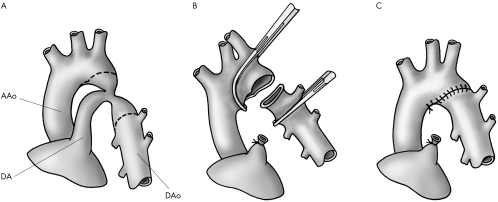
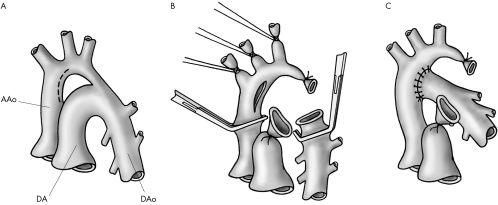
Extended arch reconstruction refers to two similar techniques (figs 1 and 2), both based on the principles of wide anastomosis with maximal ductal excision.
Figure 1 Extended end to end repair (see text for detailed description of surgical technique). (A) Basic anatomy including lines of incision. (B) Surgical procedure. (C) Final repair including lines of suture.
Figure 2 Extended end to side repair (see text for detailed description of surgical technique). (A) Basic anatomy including lines of incision. (B) Surgical procedure. (C) Final repair including lines of suture.
Extended end to end arch repair (fig 1) was used in 141 patients (73%) through a left lateral thoracotomy. The subclavian artery, descending aorta, ductus arteriosus, and aortic arch up to the innominate artery were dissected and mobilised. The aortic arch, subclavian artery, and descending aorta were clamped and the narrowed segment of the aorta was excised with particular attention paid to complete excision of all ductal tissue. The anastomosis was constructed with continuous 7‐0 polypropylene.
Extended end to side repair (fig 2) was used in 50 patients. The procedure was performed through median sternotomy with cardiopulmonary bypass and deep hypothermic circulatory arrest. The innominate, left carotid, and subclavian arteries and descending aorta were dissected and extensively mobilised. The ascending and descending aorta were clamped and the circulation was arrested. Head vessels were snared and the ductus was ligated and excised. An incision slightly larger than the diameter of the descending aorta was made on the convexity of the proximal aortic arch opposite the innominate artery. The descending aorta was moved upward and the anastomosis was created with continuous 7‐0 polypropylene.
Non‐invasive assessment and patient follow up
In addition to reviewing routine follow up clinic notes and echocardiograms, we recalled patients for evaluation in a specific study clinic held between 1999 and 2003. Ninety six per cent of patients attended the clinic. Six of 171 (3.5%) patients were lost to follow up. For the remaining 165 patients a single paediatric cardiologist (JT) followed a protocol of clinical and echocardiographic assessment. All patients were assessed at least 12 months after surgery. Firstly, femoral pulses were assessed as follows: grade 3, normal; grade 2, slightly reduced compared with brachial pulse; grade 1, clearly reduced femoral pulsation; grade 0, absent. Secondly, a mean of three upper and lower limb blood pressures were measured with an automated oscillometric blood pressure machine (Critikon, General Electric, Fairfield, Connecticut, USA) with a blood pressure cuff appropriate to each patient. Age and sex appropriate centiles were derived from published normal data.16 Thirdly, a full two dimensional echocardiogram was recorded with a Sonos ultrasound machine (Hewlett Packard, Minnesota, USA). Ascending and descending aortic velocities were taken from the suprasternal notch with a dedicated 1.9 MHz continuous wave Doppler transducer. Peak systolic, time to half peak systolic, and time to half peak diastolic velocities were calculated from the descending aortic traces.17
Table 2 Cause of death.
| In‐hospital mortality (<30 days) | |
| Failure to recover from operation | 6 |
| Sepsis | 1 |
| Ventricular failure | 1 |
| Blocked ET tube and arrest | 1 |
| Total | 9 |
| Late mortality (>30 days) | |
| Pulmonary hypertension | 3 |
| Parents declined further surgery) | 2 |
| Sepsis | 2 |
| Sudden unexplained death at home | 1 |
| Ventricular failure | 1 |
| Chronic lung disease | 1 |
| Hydrocephalus (trisomy 9 mosaic) | 1 |
| Total | 11 |
ET, endotracheal.
Residual or recurrent arch obstruction
Recoarctation was defined as (a) a 20 mm Hg difference between mean arm and leg blood pressures; (b) grade 0 or 1 femoral pulses; (c) a descending aortic Doppler gradient of > 25 mm Hg with a deceleration half time > 100 ms; or (d) previous reintervention to the repair site based on clinical judgement at that time.
Statistical analysis
Data were statistically analysed with GB Stat version 6.5 (Dynamic Microsystems, Silver Spring, Maryland, USA).
Outcome variables were survival and recurrent arch obstruction. The Fisher exact test was used to compare univariate categorical variables. Actuarial survival and re/residual coarctation were estimated by the Kaplan‐Meier method with the Cox proportional hazards model to establish variables independently associated with outcome. A probability value of p < 0.05 was considered significant.
RESULTS
Follow up
Median time to follow up was 4.2 years (range 1–10.6 years).
Mortality
Nine patients died within 30 days of the original operation. Early mortality was two of 104 (1.9%), two of 38 (5.3%), and five of 49 (10.2%) for groups 1, 2, and 3, respectively, with a significant difference between groups 1 and 3 (p < 0.04).
Eleven of the 182 survivors died late (> 30 days) at a median of 6.9 months (range 1.3–50.8 months) after surgery. Late mortality was 2.9%, 5.6%, and 13.6% for groups 1, 2, and 3, respectively (significant difference between groups 1 and 3, p < 0.03). Actuarial survival for the whole cohort at two, five, and 10 years was 92%, 88%, and 88%, respectively (fig 3).
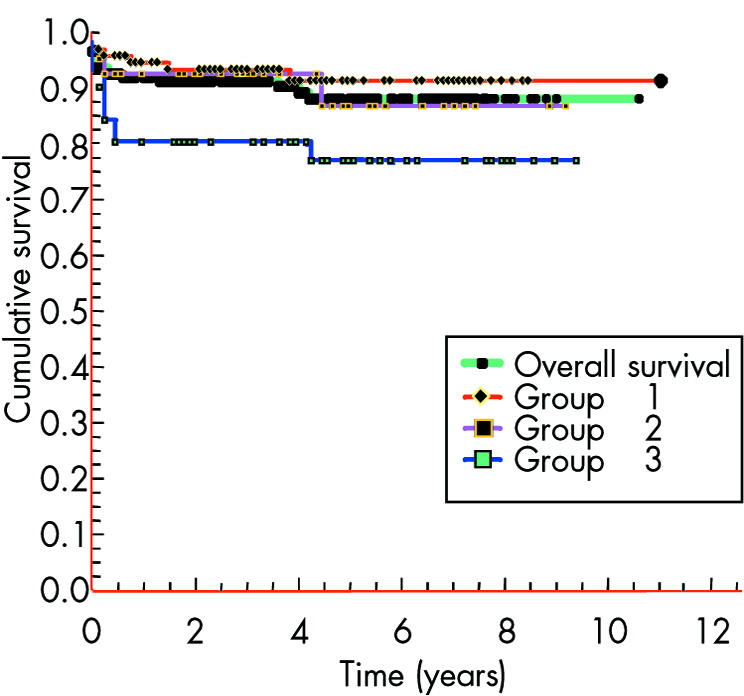
Figure 3 Kaplan‐Meier survival curves.
The majority of patients who died had complex congenital heart disease (73%) and had undergone procedures in addition to coarctation repair.
Of the three late deaths occurring in the group with coarctation alone (group 1) two patients had chromosomal abnormalities and died of non‐cardiac causes (hydrocephalus and chronic lung disease) and the third patient died 3.6 years after surgery of unexplained pulmonary hypertension having had earlier reintervention for recoarctation.
Neurological injury
Paraplegia occurred in one patient after significant intraoperative bleeding from the anastomotic site. A second patient had transient lower limb weakness but recovered fully and walks normally. Both of these patients underwent extended end to end repair through a lateral thoracotomy. A third patient had a small segmental cerebral infarct on computed tomography after coarctation repair, arterial switch, and ventricular septal defect closure.
Reintervention to the coarctation site
Seven of 165 (4.2%, 95% confidence interval 1.9 to 12.1) surviving patients required reintervention (median age and weight at surgery were 2 weeks (range 1 week to 2.2 months) and 2.5 kg (range 1–4.3 kg), respectively) for residual or recurrent arch obstruction a median of 5 months (range 2–9 months) after surgery. All of these patients had diminished (grade 0 or 1) femoral pulsation and systemic hypertension in addition to echocardiographic abnormalities (see below). Three of seven were premature infants and three of seven had aortic arch hypoplasia. Six of seven patients with recurrent arch obstruction had discrete stenosis suitable for balloon angioplasty and one (premature) infant had a long segment stenosis in which angioplasty was not attempted. In this case a repeat operation was performed.
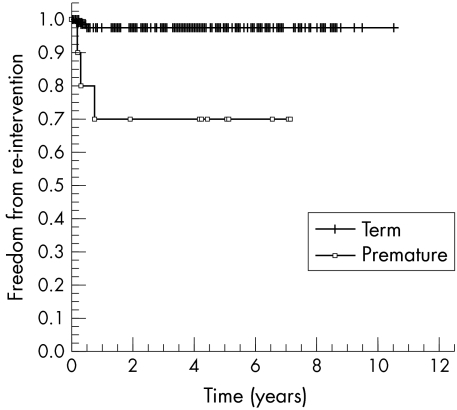
Freedom from reintervention for recurrent arch obstruction (all patients) was 96% at one, five, and 10 years, respectively, with no significant difference between the three anatomical groups.
Prematurity was the only significant risk factor (p < 0.001) for reintervention (fig 4). All seven patients with recurrent arch obstruction had undergone extended end to end repair thorough a lateral thoracotomy.
Figure 4 Freedom from reintervention (Kaplan‐Meier) in term and premature infants.
Clinical and echocardiographic data
Blood pressure
Lower limb systolic blood pressure was significantly higher than upper limb systolic pressure (p < 0.001) (table 3). Mean (SD) indexed centile for blood pressure based on mean upper limb systolic readings was 75 (20). Three patients were given antihypertensive treatment (all with no angiographic evidence of recoarctation) and 35 of 166 (21%) patients were above the 90th centile for blood pressure. In 34% of these patients (all under 5 years of age) acquiring calm blood pressure readings was noted as difficult at the time of assessment.
Table 3 Follow up data.
| Echocardiography | |
| Ascending aortic velocity (m/s) | 1.9 (0.57) |
| Descending aortic velocity (m/s)) | 2.2 (0.51) |
| Echocardiographic gradient (mm Hg) | 6.1 (7.8) |
| Time to half peak diastolic velocity (m/s) | 70 (14) |
| Femoral pulses (grade) | 4 (3–4) |
| Blood pressure | |
| Upper limb systolic (mm Hg) | 109 (14) |
| Upper limb diastolic (mm Hg) | 58 (10) |
| Lower limb systolic (mm Hg) | 123 (17.5) |
| Lower limb diastolic (mm Hg) | 68 (12.5) |
| Upper limb systolic centile (mm Hg) | 75 (20) |
| Upper to lower limb gradient (mm Hg) | −15 (17.6) |
Data are mean (SD) or median (range).
Doppler echocardiography
Table 3 shows echocardiographic data. Ten patients had descending systolic aortic Doppler velocities marginally in excess of 3 m/s with no other clinical signs of recurrent arch obstruction (normotensive, grade 2/3 femoral pulses, no diastolic prolongation of descending aortic Doppler trace). In four of these 10 patients cardiac catheter or magnetic resonance imaging showed complete luminal patency. No magnetic resonance imaging or catheter data are available for the other six patients.
Retrospective review of echocardiographic data for patients who had required reintervention for recurrent or residual arch obstruction showed time to half peak diastolic velocity to be in excess of 100 ms for all seven patients (median 130 ms, range 115–185 ms). The mean (SD) time to half peak diastolic velocity for the rest of the group was 70 (14) ms.
DISCUSSION
Surgery for coarctation of the aorta has changed considerably since the original description in 1945.1 Although there is no consensus on optimal management strategy, extended arch repairs are increasingly used.7,8,9,10,11,12,13,14 This approach allows the surgeon to resect ductal tissue fully and to address tubular hypoplasia by using a large anastomosis incorporating only autologous tissue. Several results of extended arch repair techniques have been reported,7,8,9,10,11,12,13,14 the majority in small numbers of patients and generally focusing on patients with isolated coarctation.7,8,9,10,11,12,13
Deaths are now rare after arch repair alone—in this regard our study is comparable with other recent data (95% survival at three years).7,8,9,10,11,12,13,14 In this study survival rates of patients with associated complex congenital heart disease are similar to those described by Wood et al13 but superior to those reported by Conte et al,12 both of whom used palliative pulmonary artery banding instead of a one stage repair.
Survival after isolated aortic arch repair is rarely a problem in the modern era and the focus has shifted to minimising the risk of recurrent arch obstruction. Reports of surgical repair with techniques that do not fully address duct encroachment and tubular hypoplasia (subclavian flap augmentation and end to end anastomosis) described recurrent arch obstruction rates of up to 41%.2,3,4,5 In our study actuarial freedom from recurrent or residual arch obstruction was 96% for the whole patient cohort at up to over 10 years of follow up. These medium term data add to those of others showing excellent early arch patency rates after extended arch repairs.7,8,9,10,11,12,13,14 With recurrent or residual arch obstruction being a relatively rare event after extended arch repairs this study lacks the statistical power to identify multiple risk factors for arch obstruction. There are conflicting reports regarding the relative risk of low birth weight and prematurity in the genesis of recurrent or residual arch obstruction.18,19 In this study weight at operation was not a risk factor for recurrent or residual arch obstruction but premature infants were significantly at risk with only 70% remaining free from residual or recurrent arch obstruction at a year after surgery. An extended end to end repair through a lateral thoracotomy was used in all of these infants and recoarctation was presumably related to both the size of the aorta and inadequate ductal tissue resection (ductal tissue is difficult to differentiate from normal aortic endothelium in these patients).
No residual or recurrent arch obstruction was found in the 50 patients undergoing extended end to side repair through a median sternotomy under cardiopulmonary bypass. This approach was generally selected because of the need to address other intracardiac defects through a median sternotomy on cardiopulmonary bypass, although the prevalence of transverse arch hypoplasia was increased in these patients. An extended end to side repair allows greater mobilisation and exposure of the aortic arch and therefore a more aggressive resection with a wider anastomosis. Although the recoarctation rate was not significantly different between this group and those patients undergoing repair with the extended end to end approach (performed through a lateral thoracotomy), the complete absence of recoarctation after the more radical extended end to side arch repair supports the theory that the extent of resection and the size of the anastomosis are crucial in dealing with anatomical issues that may lead to recurrent or residual arch obstruction.14
Current clinical and echocardiographic criteria for the diagnosis of recurrent or residual arch obstruction have limitations. The oldest and most widespread definition for recoarctation is a 20–25 mm Hg peak systolic upper to lower limb blood pressure gradient.20 Assessment of the repaired arch by this technique has two weaknesses. The distance between the two measuring sites is relatively long, and this is of particular relevance, as the arterial pressure wave changes as it progresses along the arterial bed.21 This leads to consistently higher blood pressure readings in the lower limb than in the upper making this approach potentially inaccurate.21 In addition accurate blood pressure measurements are usually difficult in small children; in this study, despite efforts to carefully obtain blood pressure measurements, a large proportion are likely to be inaccurate because of poor subject cooperation.
Echocardiography undoubtedly improves sensitivity in the diagnosis of residual or recurrent arch obstruction but for an accurate estimation of the degree of obstruction across the repair site an assessment of flow velocities just proximal to the stenosis is essential.22 In practice, given the geometry of the transverse arch, this measurement is usually very difficult to obtain.22 Nearly all reported series rely on gradients derived from Doppler interrogation across the site of repair alone, which inevitably leads to an overestimation of the true gradient.22 In addition work by De Mey et al,21 who used simulated models of the aortic arch after coarctation repair, showed that Doppler velocities increased as a manifestation of the change in compliance of the repaired vessel wall in the absence of stenosis.23,24 Therefore, the large area of scarring related to extensive dissection in extended arch repairs may result in higher systolic descending aortic velocities in the absence of stenosis than in other forms of arch repair potentially due to changes in compliance over a long segment.
It is clear, therefore, that individual criteria all have limitations. In this study all clinical and echocardiographic factors, not least clear two dimensional visualisation of the aortic arch, were taken into account when assessing the repaired arch. We are convinced that no patient with important recurrent or residual arch obstruction was missed. It is interesting to note that in all patients requiring reintervention to the aortic arch the time to half peak diastolic velocity was > 100 m/s, whereas this measurement was < 100 m/s for the rest of the group (mean 70 m/s). This was true also for the small number of our patients (10) who had systolic descending aortic velocities in excess of 3 m/s but no other evidence of recurrent arch obstruction (including cardiac catheter (one) or magnetic resonance imaging (three) showing a completely unobstructed aorta). Carvalho et al17 found that diastolic parameters improved the sensitivity and negative predictive value of echocardiography compared with single measurements of systolic velocity in patients with unrepaired coarctation. This parameter may possibly improve accuracy in the diagnosis of recurrent or residual arch obstruction after extended arch repair.
Conclusion
Survival after extended arch repair is excellent. At long follow up times recurrent or residual arch obstruction is rare with prematurity the only significant risk factor.
Footnotes
Competing interests: None
References
- 1.Crafoord C, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Cardiovasc Surg 194514347–352. [Google Scholar]
- 2.Williams W G, Shindo G, Trusler G A.et al Results of repair of coarctation of the aorta during infancy. J Thorac Cardiovasc Surg 198079603–608. [PubMed] [Google Scholar]
- 3.Sanchez G R, Balsara R K, Dunn J M.et al Recurrent obstruction after subclavian flap repair of coarctation of the aorta in infants. J Thorac Cardiovasc Surg 198691738–746. [PubMed] [Google Scholar]
- 4.Ziemer G, Jonas R A, Perry S B.et al Surgery for coarctation of the aorta in the neonate. Circulation 198674(su1) pp 25–1) pp 31. [PubMed] [Google Scholar]
- 5.Kappetein A P, Zwinderman A H, Bogers A J.et al More than thirty‐five years of coarctation repair: an unexpected high relapse rate. J Thorac Cardiovasc Surg 199410787–95. [PubMed] [Google Scholar]
- 6.Russell G A, Berry P J, Watterson K.et al Patterns of ductal tissue in coarctation of the aorta in the first three months of life. J Thorac Cardiovasc Surg 1991102596–601. [PubMed] [Google Scholar]
- 7.Backer C L, Mavroudis C, Zias E A.et al Repair of coarctation with resection and extended end‐to‐end anastomosis. Ann Thorac Surg 1998661365–1370. [DOI] [PubMed] [Google Scholar]
- 8.Van Son J A, Mohr F W, Hess H.et al Early repair of coarctation of the aorta. Ann Thorac Cardiovasc Surg 19995237–244. [PubMed] [Google Scholar]
- 9.Vouhe P R, Trinquet F, Lecompte Y.et al Aortic coarctation with hypoplastic aortic arch: results of extended end‐to‐end aortic arch anastomosis. J Thorac Cardiovasc Surg 198896557–563. [PubMed] [Google Scholar]
- 10.Rajasinghe H A, Reddy V M, Van Son J A.et al Coarctation repair using end‐to‐side anastomosis of descending aorta to proximal aortic arch. Ann Thorac Surg 199661840–844. [DOI] [PubMed] [Google Scholar]
- 11.Lansman S, Shapiro A J, Schiller M S.et al Extended aortic arch anastomosis for repair of coarctation of the aorta in infancy. Circulation 198674(su1) pp 37–1) pp 41. [PubMed] [Google Scholar]
- 12.Conte S, Lacour‐Gayet F, Serraf A.et al Surgical management of neonatal coarctation. J Thorac Cardiovasc Surg 1995109663–674. [DOI] [PubMed] [Google Scholar]
- 13.Wood A E, Javadpour H, Duff D.et al Is extended arch aortoplasty the operation of choice for infant aortic coarctation? Results of 15 years' experience in 181 patients. Ann Thorac Surg 2004771353–1357. [DOI] [PubMed] [Google Scholar]
- 14.Younoszai A K, Reddy V M, Hanley F L.et al Intermediate term follow‐up of the end‐to‐side aortic anastomosis for coarctation of the aorta. Ann Thorac Surg 2002741631–1634. [DOI] [PubMed] [Google Scholar]
- 15.Moulaert A J, Bruins C C, Oppenheimer‐Dekker A. Anomalies of the aortic arch and ventricular septal defect. Circulation 197695265–274. [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute Report of the second task force on blood pressure control in children–1987. Task force on blood pressure control in children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 1987791–25. [PubMed] [Google Scholar]
- 17.Carvalho J S, Redington A N, Shinebourne E A.et al Continuous wave Doppler echocardiography and coarctation of the aorta: gradients and flow patterns in the assessment of severity. Br Heart J 199064133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacha E, Almodovar M, Wessel D L.et al Surgery for coarctation of the aorta in infants weighing less than 2 kg. Ann Thorac Surg 20013573–579. [DOI] [PubMed] [Google Scholar]
- 19.McElhinney D B, Yang S G, Hogarty A N.et al Recurrent arch obstruction after repair of isolated coarctation of the aorta in neonates and young infants: is low weight a risk factor? J Thorac Cardiovasc Surg 2001122883–890. [DOI] [PubMed] [Google Scholar]
- 20.Kirklin J W, Barratt‐Boyes B G. Coarctation of the aorta and aortic arch interruption. In: Kirklin JW, Barratt‐Boyes BG, eds. Cardiac surgery. 2nd ed. New York: Churchill Livingstone, 19931263–1325.
- 21.De Mey S, Segers P, Coomans I.et al Limitations of Doppler echocardiography for the post‐operative evaluation of aortic coarctation. J Biomech 200134951–960. [DOI] [PubMed] [Google Scholar]
- 22.Chan K C, Dickinson D F, Wharton G A.et al Continuous wave Doppler echocardiography after surgical repair of coarctation of the aorta. Br Heart J 199268192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhaaren H, De Mey S, Coomans I.et al Fixed region of nondistensibility after coarctation repair: in vitro validation of its influence on Doppler peak velocities. J Am Soc Echocardiogr 200114580–587. [DOI] [PubMed] [Google Scholar]
- 24.Aldousany A W, DiSessa T G, Alpert B S.et al Significance of the Doppler‐derived gradient across a residual aortic coarctation. Pediatr Cardiol 1990118–14. [DOI] [PubMed] [Google Scholar]