Abstract
Objective
To verify whether a deficiency in the cardiac sarcoplasmic reticulum pump SERCA2a causes cardiac dysfunction in humans.
Design
Cardiac performance was measured in a serendipitous human model of primary SERCA2a deficiency, Darier's disease, an autosomal dominant skin disorder caused by mutations inactivating one copy of the ATP2A2 gene, which encodes SERCA2a.
Methods
Systolic and diastolic function and contractility were assessed by echocardiography at rest and during exercise in patients with Darier's disease with known mutations. Fourteen patients with Darier's disease were compared with 14 normal controls and six patients with dilated cardiomyopathy with stable heart failure.
Results
Resting systolic and diastolic function was normal in patients with Darier's disease and in controls. The increase in systolic function during exercise was not different between patients with Darier's disease and normal controls; neither was there a difference in contractility. As expected, patients with dilated cardiomyopathy had impaired diastolic and systolic function with depressed contractility at rest and during exercise.
Conclusion
Contrary to expectations, heterozygous disruption of SERCA2a is not associated with the impairment of cardiac performance in humans. Attempts to increase SERCA2a levels in heart failure, although showing promise in rodent studies, may not be addressing a critical causal pathway in humans.
Keywords: heart failure, sarco(endo)plasmic reticulum Ca2+ ATPase 2 (ATP2A2) gene, ATP2A2 gene, Darier's disease
The sarco(endo)plasmic reticulum Ca2+ ATPase 2 (ATP2A2) gene encodes, by alternative splicing of mRNA, both the cardiac sarcoplasmic reticulum pump (SERCA2a) and the SERCA2b calcium pump, which is expressed in all tissues.1 In the heart, the sarcoplasmic reticulum provides the bulk of the Ca2+ release responsible for initiating cardiac contraction and subsequently reuptake of Ca2+ by the SERCA2a pump produces active myofilament relaxation.2 Substantial evidence indicates that SERCA2a protein level and function are reduced by about 30–50% in the failing human heart, in parallel to both the depressed contraction and impaired relaxation.3,4,5,6,7 One of the characteristic features of sarcoplasmic reticular function is that its contribution to cardiac performance becomes more prominent at higher heart rates.8 A parallel is observed in heart failure, where cardiac function is often normal at rest but fails to increase with exercise.4,8 Given this, the reduction in SERCA2a activity is certainly plausible as a primary causal abnormality in heart failure5,6,7,9 but, alternatively, the reduction may be a compensation or an epiphenomenon.10
Support for a causal role of reduction in SERCA2a in heart failure has been provided by studies in mouse knockout models, as impairment of cardiac dP/dTmax (a measure of contractile function) is seen in mice with a single copy of the gene inactivated.11,12 Accordingly, therapeutic upregulation of SERCA2a protein level is being explored.13,14 However, the effects of primary reductions in SERCA2a on cardiac performance in humans remain unknown. Of note, attempts to identify pathogenic mutations in SERCA2a in human heart failure have been unsuccessful.15 We have recently identified a serendipitous human knockout model of SERCA2a, Darier's disease, a rare autosomal dominant skin disorder with no known cardiac associations that is caused by heterozygous mutation of the ATPA2A gene.16,17 In Darier's disease, the majority of mutations occur in the region of ATP2A2 that encodes both the SERCA2a cardiac isoform and the ubiquitous SERCA2b isoform, which is important in the skin; these mutations result in complete or partial loss of function of the mutated calcium pumps.18,19 Thus, Darier's disease provides a unique opportunity to study the effects of primary SERCA2a deficiency on cardiac performance in humans.
METHODS
We investigated whether heterozygous disruption of SERCA2a in patients with Darier's disease results in clinically measurable effects in cardiac performance, especially at higher heart rates during exercise. The Central Oxford Research Ethics Committee approved the study, and all participants gave written informed consent. Fourteen patients with Darier's disease were compared with 14 normal controls. The normal controls were recruited from members of staff at the John Radcliffe Hospital and were matched for age of the patients with Darier's disease. To reduce the incidence of coexisting cardiovascular disease, only subjects < 60 years with no history of cardiac disease or hypertension were studied. Patients with coronary artery disease were excluded. Echocardiography was performed in the resting state and systolic and diastolic performance was measured. The reproducibility estimates for echocardiographic measurements in our laboratory have been reported previously.20 Systolic function was measured by M mode fractional shortening (FS). The ratio of the Doppler transmitral early (E) to atrial (A) filling velocities (E:A ratio) and the deceleration time of the E wave (DT) were used to measure diastolic function.
Subjects performed a symptom limited supine bicycle exercise test with repeat echocardiography at four minute intervals to assess changes in cardiac performance during exercise.21 Echocardiographic imaging during the supine bicycle exercise test was accomplished with a specialised bed with an integrated bicycle ergometer that provided lateral bed rotation for optimisation of echocardiographic windows (SBSS 1000, Redbud Medical Systems, Cashmere, Washington, USA). A single operator (AK) performed echocardiography and one reader (BMM) scored the images; the reader was blinded to the allocation group of the participants at the time of reading.
The protocol called for a workload of 50 W in the first stage of exercise and 25 W increments in subsequent stages. Heart rate and blood pressure were measured during the fourth minute of every stage of exercise. Oxygen consumption (Vo2) was measured by standard equipment that incorporated a computer for the calculation of Vo2 every 30 seconds during exercise. FS and the FS‐end systolic wall stress relation were used as non‐invasive measures of systolic function response and contractility during exercise, respectively.22,23 End systolic wall stress of the left ventricle was calculated by the formula σES = [(ESP) × (ESD) × (1.35)]/[(4) × (EST) × (1 + EST/ESD)], where σES is end systolic wall stress in g/cm2; ESP is end systolic blood pressure in mm Hg; ESD and EST are end systolic diameter and wall thickness, respectively, in cm; and 1.35 is the factor to convert pressure from mm Hg to g/cm2. End systolic pressure was calculated as [2 × systolic blood pressure + diastolic blood pressure]/3.23 The cardiac performance of patients with Darier's disease at rest and during exercise was also compared with that of six patients with stable heart failure caused by idiopathic dilated cardiomyopathy (New York Heart Association class II–III). The patients with heart failure acted as positive controls to determine whether the non‐invasive methods used in the study were sufficient to detect an impaired cardiac performance response to exercise.
Data were statistically analysed with the Stata package (StataCorp LP, College Station, Texas, USA). The differences in means of physiological characteristics of the patient groups were compared by the unpaired Student's t test. A repeated measures analysis of variance was used to compare the cardiac response to exercise between groups. FS was determined at rest, in at least two stages during exercise, and at one minute after cessation of exercise, such that all participants had at least four sequential measurements available for analysis. The data were arranged with four variables so that four FS measurements were recorded for each participant. FS was defined as a within‐subject factor and study group was defined as a between‐subject factor. The hypothesis regarding the between‐subject factor was tested.
Left ventricular contractile performance was assessed by the FS end systolic wall stress relation by plotting FS as a function of end systolic wall stress at baseline, during exercise, and at one minute after cessation of exercise. In the comparison of contractile performance between the groups, an analysis of covariance was used to determine whether the slopes of the regression lines obtained from the groups were significantly different. A probability value of p < 0.05 was considered significant.
RESULTS
Mean (SD) age was 46.9 (9.0) years in the Darier's group and 46.2 (11.8) years in the normal controls. There were seven men and seven women in the Darier's group, and 10 men and four women in the normal control group. The causative ATP2A2 mutations had been identified in 10 of 14 patients with Darier's disease; the mutations predict premature termination of translation in five patients, non‐conservative missense mutations in functional domains in four, and an in‐frame deletion in one (table 1).17,24 The mutations disrupt important domains of the SERCA2 molecule and result in complete or partial loss of function of the mutated calcium pumps.18,19
Table 1 ATP2A2 mutations in patients with Darier's disease17, 24.
| Patient | Mutation | Exon | Consequence | Protein domain |
|---|---|---|---|---|
| 1 | E412G | 10 | Missense | ATP binding |
| 2 | 2134delA | 15 | PTC (+43aa) | Phosphorylation |
| 3 | W551X | 13 | PTC | ATP binding |
| 4 | 2102del36 | 15 | In‐frame deletion | Phosphorylation |
| 5 | T357K | 8 | Missense | Phosphorylation |
| 6 | S920Y | 19 | Missense | M8–M9 loop |
| 8 | D702N | 15 | Missense | Phosphorylation |
| 11 | 2608delAG | 18 | PTC (+4aa) | M7–M8 loop |
| 13* | 2026insG | 14 | PTC (+3aa) | Phosphorylation |
| 14* | 2026insG | 14 | PTC (+3aa) | Phosphorylation |
*Patients 13 and 14 are related.
M, transmembrane domain; PTC + n, premature termination codon at n amino acid downstream of the mutation.
Echocardiographic data was of adequate quality from 13 of the 14 normal controls and from all 14 of the patients with Darier's disease. The patients with Darier's disease had normal resting cardiac function as shown by the indices of diastolic (DT and E:A ratio) and systolic function (FS) that were similar to those of the normal control group (table 2). As a group the patients with Darier's disease were significantly undertrained compared with the normal controls as shown by their tendency to tire early (significantly shorter duration of exercise, p = 0.020) and the achievement of a significantly lower peak Vo2 (p = 0.007) (table 2). These significant differences in baseline physiological characteristics between the patients with Darier's disease and normal controls were due to inadequate matching of the female members of the groups for heart size, resting FS, peak heart rate, and peak Vo2 (table 3). Therefore, men from each group who were well matched with regard to baseline physiological characteristics and exercise performance (table 4) were compared.
Table 2 Physiological characteristics of patients with Darier's disease and normal controls.
| Darier group (n = 14) | Control group (n = 14) | p Value | |
|---|---|---|---|
| Age (years) | 46.9 (2.4) | 46.2 (3.3) | 0.849 |
| LVMI (g/m2) | 116.9 (5.9) | 113.5 (7.7) | 0.728 |
| Resting FS (%) | 39.7 (1.6) | 42.2 (2.3) | 0.770 |
| Resting E:A ratio | 1.4 (0.1) | 1.3 (0.07) | 0.747 |
| Resting DT (ms) | 210.2 (12.9) | 216.2 (12.9) | 0.369 |
| Exercise duration (min) | 11.4 (1.0) | 15.0 (1.0) | 0.020* |
| HRmax (beats/min) | 128.9 (3.5) | 144.8 (5.7) | 0.023* |
| Vo2peak (ml/kg/min) | 17.7 (1.0) | 23.7 (1.9) | 0.007* |
Values are means (SEM).
Echocardiographic data were available for 13 of the control subjects.
*Significant differences.
DT, deceleration time of the E wave; E:A, ratio of early (E) to atrial (A) transmitral Doppler velocity; FS, fractional shortening; HRmax, maximum heart rate; LVMI, left ventricular mass index (left ventricular mass/body surface area); Vo2peak, peak oxygen consumption.
Table 3 Selected physiological characteristics of patients with Darier's disease and normal female controls.
| Darier group (n = 7) | Control group (n = 4) | p Value | |
|---|---|---|---|
| Age (years) | 46.1 (4.5) | 42.8 (6.8) | 0.675 |
| LVMI (g/m2) | 107.4 (8.1) | 93.5 (9.8) | 0.006* |
| Resting FS (%) | 38.6 (1.7) | 46.0 (2.0) | 0.026* |
| Resting E:A ratio | 1.5 (0.2) | 1.3 (0.3) | 0.312 |
| Resting DT (ms) | 204.3 (14.5) | 225.8 (15.7) | 0.528 |
| Exercise duration (min) | 9.1 (0.6) | 10.1 (0.7) | 0.368 |
| HRmax (beats/min) | 135.4 (5.5) | 164.0 (4.3) | 0.025* |
| Vo2peak (ml/kg/min) | 16.7 (1.2) | 25.5 (5.1) | 0.057* |
Values are means (SEM).
*Significant differences.
Table 4 Selected physiological characteristics of patients with Darier's disease and normal male controls.
| Darier group (n = 7) | Control group (n = 10) | p Value | |
|---|---|---|---|
| Age (years) | 47.7 (2.2) | 47.7 (2.3) | 0.992 |
| LVMI (g/m2) | 126.3 (7.3) | 122.3 (9.1) | 0.751 |
| Resting FS (%) | 40.7 (2.7) | 40.4 (3.1) | 0.302 |
| Resting E:A ratio | 1.2 (0.09) | 1.3 (0.09) | 0.882 |
| Resting DT (ms) | 216.1 (22.3) | 211.9 (17.6) | 0.946 |
| Exercise duration (min) | 13.6 (1.5) | 15.2 (1.0) | 0.161 |
| HRmax (beats/min) | 122.3 (2.7) | 136.2 (6.2) | 0.083 |
| Vo2peak (ml/kg/min) | 18.7 (1.6) | 21.1 (1.3) | 0.098 |
Values are means (SEM).
*Significant differences.
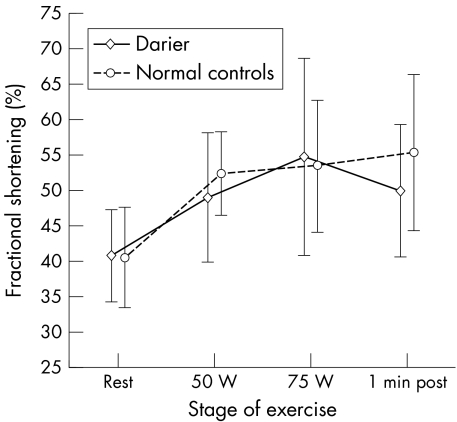
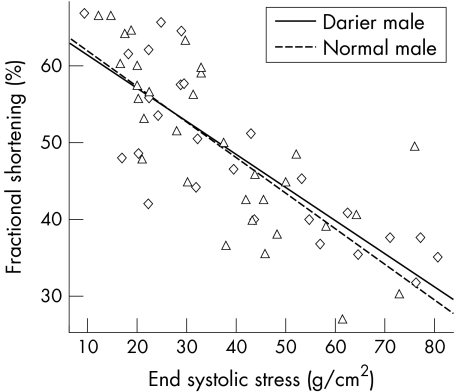
A repeated measures analysis of variance showed no differences in FS between patients with Darier's disease and normal controls during exercise (p = 0.689) (fig 1). However, the increase in FS over the stages of exercise was significant in both groups (p < 0.0001). There was no interaction between male study group and stage of exercise, suggesting that the increase in systolic function was of similar magnitude (p = 0.308). FS was significantly related to end systolic stress among patients with Darier's disease and normal controls (p < 0.0001) (fig 2). However, there was no interaction between study group and end systolic stress (p > 0.05), indicating that cardiac contractility during exercise did not differ significantly between the two groups.
Figure 1 Left ventricular systolic function at different stages of exercise in men with Darier's disease and normal male controls was determined by echocardiography at rest and in response to exercise with increasing workloads (50 W, 75 W) and one minute after cessation of exercise. Mean (SEM).
Figure 2 Contractility in men with Darier's disease and normal male control measured by the slope of the systolic shortening–end systolic stress relation. ◊, Data points for male patients with Darier's disease; Δ, data points for normal male controls.
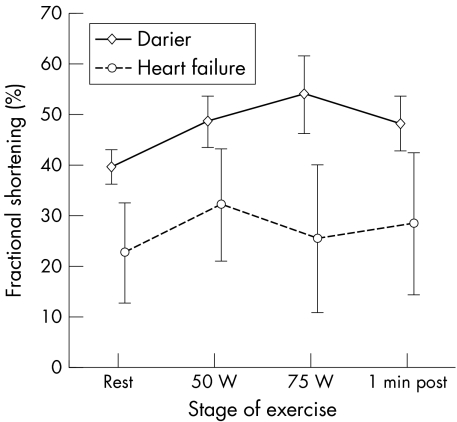
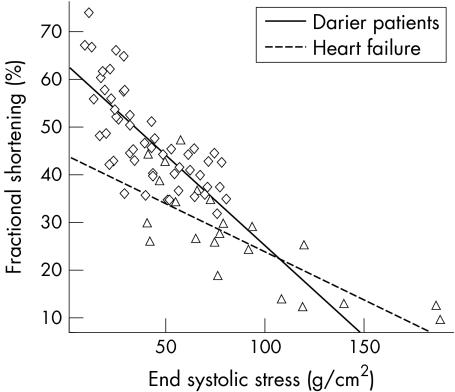
As the participants in this study had no cardiac symptoms we used a non‐invasive method to measure contractility, which may potentially have lacked sensitivity for the detection of abnormalities in cardiac performance. Therefore, we studied cardiac performance in patients with heart failure who acted as positive controls. Four women and two men with stable mild to moderate heart failure (New York Heart Association class II–III) caused by idiopathic dilated cardiomyopathy with a mean (SD) age of 62.0 (9.2) years were studied. Resting systolic and diastolic function was, as expected, significantly impaired in the patients with heart failure (FS 22.7 (9.4)%; E:A 1.03 (0.45); and DT 161.5 (36.2)). A repeated measures analysis of variance showed a significant difference in the FS in different stages of exercise between patients with Darier's disease and those with heart failure (p = 0.0002) (fig 3). FS also increased significantly over the stages of exercise in both groups (p < 0.0001). However, there was a significant interaction between study group and stage of exercise (p = 0.01)—that is, the patients with Darier's disease had a different FS response pattern to exercise from that of the patients with heart failure. FS was found to be significantly related to end systolic stress among patients with Darier's disease and those with heart failure (p < 0.0001) (fig 4). However, there was also a significant interaction between study group and the FS–end systolic stress relation (p = 0.01), confirming that patients with heart failure had impaired cardiac contractility compared with the patients with Darier's disease. This confirms that the methods used in our study would be able to detect clinically relevant impairment in cardiac performance in patients with Darier's disease, if it were present.
Figure 3 Left ventricular systolic function response at different stages of exercise in patients with Darier's disease and in patients with heart failure was determined by echocardiography at rest and in response to exercise with increasing workloads (50 W, 75 W) and one minute after cessation of exercise. Mean (SEM).
Figure 4 Contractility in patients with Darier's disease and patients with heart failure measured by the slope of the systolic shortening–end systolic stress relation. ◊, Data points for patients with Darier's disease; Δ, data points for patients with heart failure.
DISCUSSION
Given the postulated role of SERCA2 in heart failure, it is surprising that no overt cardiac phenotype is present in Darier's disease.16 Our data show that in Darier's disease all aspects of cardiac contractile performance appear normal and in particular systolic function and contractility increase normally during exercise. These findings contrast with those of studies in mice in which one copy of the ATPA2A gene has been ablated, which do have impaired cardiac contractility.11,12 Thus, the findings underscore the data obtained from the use of SERCA2a inhibitors that suggest wide interspecies variation in ability to compensate for sarcoplasmic reticulum dysfunction.25 Our data suggest that otherwise healthy human myocardium can compensate for a primary reduction in SERCA2 level or activity. Compensation probably occurs through decreased expression and increased phosphorylation of the inhibitory protein phospholamban and increased expression of the sarcolemmal Na+‐Ca2+ exchanger. Upregulation of the normal allele or compensation by SERCA1 or SERCA3 also conceivably plays a part. Mice heterozygous for a SERCA2 null allele, in which SERCA2 protein levels are reduced by about 35%, do have a number of these adaptations,12 but presumably these are insufficient to completely rescue myocardial contractility because of the dependence on SERCA2 in the mouse. Similarly, discrepant phenotypes arising from mutations in phospholamban also highlight differences in the responses to perturbations in calcium handling in the heart between mouse and human.26
Our study has certain limitations inherent to clinical investigations. Firstly, we were unable to directly assess heart tissue in patients with Darier's disease, which would have allowed for direct measurement of SERCA2a activity and potential compensatory changes. Nevertheless, previous analyses of mutant SERCA2b isoforms are sufficient to predict a complete loss of function of one allele, such that the primary loss of cardiac SERCA2 activity in heterozygotes would be comparable with that described in heart failure.18,19 Secondly, we were restricted to non‐invasive studies in these study subjects; we cannot exclude the presence of subtle abnormalities that may be detected by invasive tests of dP/dTmax or direct measurement of the cellular function in Darier's disease.
Although we could not directly examine the cellular and molecular effects of SERCA2 deficiency in the myocardium in the patients with Darier's disease, we believe that our observations have important implications for the role of SERCA2a in the aetiology and treatment of heart failure. The normal cardiac performance in human patients with Darier's disease suggests that the observed reduction in SERCA2a activity may not have a primary role in patients with heart failure. It is possible that studies in rodent models of treatments that seek to enhance SERCA2a activity in the treatment of heart failure may not be reliably extrapolated to human heart failure.
ACKNOWLEDGEMENTS
We thank the people who participated. BMM was a Nuffield Oxford Medical Fellow during the conduct of this study. This work was supported by grants from the Wellcome Trust and the British Heart Foundation.
Abbreviations
DT - deceleration time of the E wave
FS - fractional shortening
Vo2 - oxygen consumption
References
- 1.Missiaen L, Wuytack F, Raeymaekers L.et al Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther 199150191–232. [DOI] [PubMed] [Google Scholar]
- 2.Frank K F, Bolck B, Erdmann E.et al Sarcoplasmic reticulum Ca2+‐ATPase modulates cardiac contraction and relaxation. Cardiovasc Res 20035720–27. [DOI] [PubMed] [Google Scholar]
- 3.Arai M, Hirosuke M, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 199474555–564. [DOI] [PubMed] [Google Scholar]
- 4.Hasenfuss G, Reinecke H, Studer R.et al Relation between myocardial function and expression of sarcoplasmic reticulum calcium ATPase in failing and non‐failing human myocardium. Circ Res 199475434–442. [DOI] [PubMed] [Google Scholar]
- 5.Pieske B, Kretschmann B, Meyer M.et al Alterations in intracellular calcium handling associated with the inverse force‐frequency relation in human dilated cardiomyopathy. Circulation 1995921169–1178. [DOI] [PubMed] [Google Scholar]
- 6.Hasenfuss G. Alterations of calcium‐regulatory proteins in heart failure. Cardiovasc Res 199837279–289. [DOI] [PubMed] [Google Scholar]
- 7.Davies C H, Harding S E, Poole‐Wilson P A. Cellular mechanisms of contractile dysfunction in human heart failure. Eur Heart J 199617189–198. [DOI] [PubMed] [Google Scholar]
- 8.Davies C H, Davia K, Bennett J G.et al Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation 1995922540–2549. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt U, Hajjar R J, Helm P A.et al Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 1998301929–1937. [DOI] [PubMed] [Google Scholar]
- 10.Barry W H. Molecular inotropy: a future approach to the treatment of heart failure? Circulation 19991002303–2304. [DOI] [PubMed] [Google Scholar]
- 11.Periasamy M, Reed T D, Liu L H.et al Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+‐ATPase isoform 2 (SERCA2) gene. J Biol Chem 19992742556–2562. [DOI] [PubMed] [Google Scholar]
- 12.Ji Y, Lalli M J, Babu G J.et al Disruption of a single copy of the SERCA2 gene results in altered Ca(2+) homeostasis and cardiomyocyte function. J Biol Chem 200027538073–38080. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto M I, del Monte F, Schmidt U.et al Adenoviral gene transfer of SERCA2a improves left‐ventricular function in aortic‐banded rats in transition to heart failure. Proc Natl Acad Sci USA 200097793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Monte F, Harding S E, Schmidt U.et al Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 19991002308–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt A G, Haghighi K, Frank B.et al Polymorphic SERCA2a variants do not account for inter‐individual differences in phospholamban‐SERCA2a interactions in human heart failure. J Mol Cell Cardiol 200335867–870. [DOI] [PubMed] [Google Scholar]
- 16.Tavadia S, Tait R C, McDonagh T A.et al Platelet and cardiac function in Darier's disease. Clin Exp Dermatol 200126696–699. [DOI] [PubMed] [Google Scholar]
- 17.Sakuntabhai A, Ruiz‐Perez V, Carter S.et al Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 199921271–277. [DOI] [PubMed] [Google Scholar]
- 18.Ahn W, Lee M G, Kim K H.et al Multiple effects of SERCA2b mutations associated with Darier's disease. J Biol Chem 200327820795–20801. [DOI] [PubMed] [Google Scholar]
- 19.Dode L, Andersen J P, Leslie N.et al Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+‐ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady‐state and transient kinetic analyses. J Biol Chem 200327847877–47889. [DOI] [PubMed] [Google Scholar]
- 20.Mayosi B M, Keavney B, Kardos A.et al Electrocardiographic measures of left ventricular hypertrophy show greater heritability than echocardiographic left ventricular mass. Eur Heart J 2002231963–1971. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong W F. Stress echocardiography: introduction, history, and methods. Prog Cardiovasc Dis 199739499–522. [DOI] [PubMed] [Google Scholar]
- 22.Borow K M, Green L H, Grossman W.et al Left ventricular end‐systolic stress‐shortening and stress‐length relations in human: normal values and sensitivity to inotropic state. Am J Cardiol 1982501301–1308. [DOI] [PubMed] [Google Scholar]
- 23.Suman O E, Hasten D, Turner M J.et al Enhanced inotropic response to dobutamine in strength‐trained subjects with left ventricular hypertrophy. J Appl Physiol 200088534–539. [DOI] [PubMed] [Google Scholar]
- 24.Sakuntabhai A, Burge S, Monk S.et al Spectrum of novel ATP2A2 mutations in patients with Darier's disease. Hum Mol Genet 199981611–1619. [DOI] [PubMed] [Google Scholar]
- 25.Davia K, Davies C H, Harding S E. Effects of inhibition of sarcoplasmic reticulum calcium uptake on contraction in myocytes isolated from failing human ventricle. Cardiovasc Res 19973388–97. [DOI] [PubMed] [Google Scholar]
- 26.Haghighi K, Kolokathis F, Pater L.et al Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 2003111869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]