Evaluation of left ventricular (LV) size and function are by far the most common reasons for performing echocardiography in the adult patient. Important diagnostic, prognostic, and treatment decisions rest upon LV morphology analysis; the widespread bedside availability, cost, and non‐invasive nature of echocardiography has meant that this technique has become the method of choice in most situations for performing this analysis. However, both M mode and two dimensional (2D) echocardiography make important geometric assumptions about the LV which leads to inaccuracies in measurements. There is also poor inter‐ and intra‐observer variability which limits the use of the technique in follow up of patients and also in scientific studies. Many echocardiographic departments perform “eyeball” analysis of global and regional LV function and provide visual estimates of ejection fraction because existing quantification methods (from M mode and 2D Echo) are both time consuming and difficult to perform. In an era when so many important and often costly decisions are made upon these data it is incumbent upon departments that accurate and reproducible echo quantification methods are utilised—especially since the “gold standard” technique of cardiac magnetic resonance (CMR) is not so widely available, is more costly, cannot be used on those with implanted pacemakers or defibrillators, and is disliked by many patients.
Three dimensional (3D) echocardiography has been available for several years using time consuming and difficult reconstruction techniques (often utilising transoesophageal studies). However, recent advances in computer processing and transducer construction techniques have meant that real time transthoracic 3D echocardiography is now available from major ultrasound system manufacturers. Software programs to analyse 3D datasets of the LV are also now readily available; this combination of new instrumentation and software has been shown to provide highly accurate (compared to CMR) analysis of LV morphology and function, such that this methodology is likely to ensure that echocardiography remains the first choice technique for non‐invasive evaluation of the LV.
REAL TIME 3D ECHO TECHNOLOGY
As previously mentioned, early 3D echocardiographic techniques relied upon the acquisition of multiple cross sectional (2D) images using freehand transthoracic or transoesophageal imaging. The spatial and temporal relationships of each image had to be registered and gating to the cardiac and respiratory cycle also performed before a time consuming reconstruction of a 3D dataset could be undertaken.
ECG and respiratory cycle gating can largely be avoided by acquiring the 3D datasets in real time and this is achieved by using a matrix array probe. This type of probe contains complex electronics and 3–4000 individual elements which permits multidirectional beam steering and allows a 3D dataset of approximately 30° × 60° to be acquired. This facilitates 3D visualisation of valve structures or part of the LV in real time. In order to capture a dataset large enough to cover the whole of the LV, the transducer is positioned over the apex and several (usually four) smaller real time datasets are acquired during briefly held respiration and electronically “stitched” together over four or five sequential cardiac cycles. In this way a pyramidal 3D dataset of 90° × 90° is obtained at a frame rate of 20–25 Hz. This is usually large enough and also fast enough to allow comprehensive analysis of the LV.
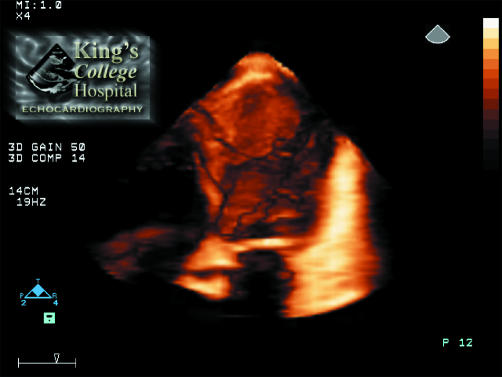
However, in order for this analysis to be performed, the 3D dataset needs to be rendered within the ultrasound system. Volume rendering is a process whereby the intracardiac structures are reconstructed within the computer memory so that the dataset can be sectioned electronically in any plane, allowing visualisation of any structure within the heart from any viewpoint. Viewing a volume rendered 3D dataset of the heart is analogous to standing outside a house and being unable to see in without taking some or part of the walls away. By sectioning or cropping away part of the dataset it is possible to see inside the heart and view the anatomical orientation and motion of intracardiac structures, including the LV myocardium. An example of a 3D echo image of the LV is shown in fig 1. In this case the anterior wall of the ventricle has been cropped away to allow visualisation of the LV morphology, revealing a large apical aneurysm containing thrombus.
Figure 1 Full volume 3D echo image of the left ventricle (LV) in a four chamber equivalent view. The anterior wall of the LV has been cropped (sectioned) away to reveal a large apical aneurysm containing thrombus. The size, shape, and morphology of the thrombus can be appreciated. In the moving real time images its mobility and overall LV function can be appreciated.
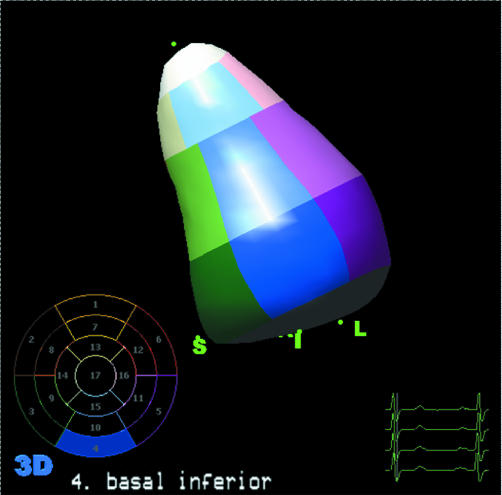
The starting point for any analysis of LV morphology or function is to use the 3D dataset to calculate volumes of the ventricle and/or myocardium at multiple points during the cardiac cycle. Several online or offline software packages are available to do this. They usually work by sectioning the voxel based dataset of the LV into several separate 2D planes and a semi‐automated endocardial border detection process is performed on each plane. Anatomical landmarks such as the mitral annulus and apex are identified by the user and the software creates a mathematical model or “cast” of the LV which allows time–volume calculations to be performed for the entire cardiac cycle (fig 2). The dataset acquisition takes approximately 4–5 seconds and, providing no correction to the detected endocardial boundaries needs to be performed, the analysis to this stage can be performed in under a minute.
Figure 2 A mathematical model or cast of the LV which is obtained using semi‐automated endocardial border tracking from the 3D dataset. Following identification of a few anatomical landmarks the cast is automatically segmented into the standard 16 or 17 segments. The volume of each segment (relative to the LV centre of gravity) or the volume of the whole cavity can be calculated for each frame in the cardiac cycle (see fig 3).
Three dimensional echocardiography can suffer with poor image quality in the same way that 2D echo can. Fortunately, ultrasound contrast agents can also be used with 3D echo when image quality is suboptimal and this means that analysable 3D datasets can be obtained in virtually all patients.
MEASUREMENT OF LV VOLUME AND EJECTION FRACTION
M Mode calculations of LV volumes assume that the LV is a prolate ellipse and that by measuring a single minor axis dimension and cubing it, volume can be calculated. Despite the fact that there are numerous flaws in this assumption and that errors in measurement become very large when cubed, it is surprising how often this calculation is still utilised in clinical practice. Furthermore, it is still widely available in echo reporting and analysis software. Two dimensional echo calculation of LV volumes using the method of discs (Simpson's rule) makes significantly less geometric assumptions, especially when utilised in a biplane format. However, there is still the assumption that the ventricle can be represented by a series of stacked discs with varying diameters. In patients with regional wall motion abnormalities or LV aneurysms, etc, this assumption may fail. Three dimensional echocardiography makes no assumptions about the shape of the LV—it calculates it as it is. In addition, the endocardial position is measured at many hundreds of points over the LV surface, therefore the calculation of volume is more accurate and reproducible than when only one or two 2D echo planes are used.
There are now many published studies which have shown high concordance between 3D echo calculations of LV volume and ejection fraction compared to the “gold standard” of CMR.1,2,3,4,5 Most of these studies have also shown significant increases in accuracy and reproducibility over conventional 2D echocardiography methods.
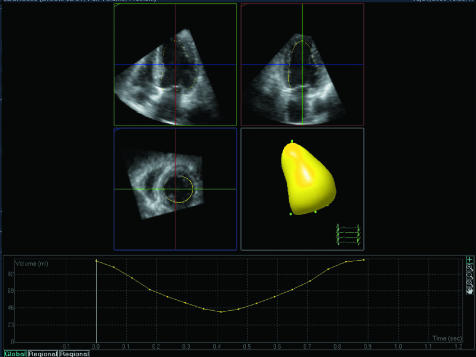
Figure 2 shows an example of a mathematically derived model or cast of the LV. In real time the cast moves to simulate LV contraction and relaxation. It can be rotated on the computer screen using a mouse so that regional function can be visually appreciated. In addition, the 16 or 17 American Society of Echocardiography defined segments are identified on the cast. The volume of the cast (LV) is calculated for every frame in the 3D dataset and plotted as a graph of volume against time, as shown. End diastolic volume, end systolic volume, and ejection fraction are automatically derived from this graph and displayed, as shown in fig 3.
Figure 3 Three dimensional apical full volume dataset which has been segmented into 2D four chamber, two chamber, and short axis slices. Semi‐automated endocardial tracking (yellow line) can be seen, checked, and edited using these views. From this a mathematical model or cast of the LV is created using all 3D data points. At the bottom, calculated LV volume (from the cast) is plotted against time during one cardiac cycle. End diastolic and end systolic volumes plus ejection fraction and sphericity index are derived from these data.
LV SHAPE
It is well known that the shape of the LV is an additional useful parameter to assess in patients with LV dysfunction. As function deteriorates and LV size increases, the ventricle assumes a more globular rather than elliptical shape. Two dimensional echocardiography has previously been used to derive a 2D sphericity index which relates to the ratio of the cross sectional area of the LV (from an apical four chamber view) to a circle with a diameter equivalent to LV major end diastolic long axis. As the ventricle becomes more circular, the ratio approaches unity.
Clearly a sphericity index which is derived from 3D LV volumes rather than 2D area will reflect ventricular shape more accurately. A 3D derived sphericity index has been described6 and is calculated by dividing the LV end diastolic volume (calculated from a 3D dataset) by the volume of a sphere, the diameter of which is the LV major end diastolic long axis. This sphericity index has been shown to be an earlier and more accurate predictor of remodelling in patients following acute myocardial infarction than other clinical, electrocardiographic, or echocardiographic variables.6 Offline 3D analysis software now permits rapid calculation of a 3D sphericity index using 3D LV volume data, derived as previously described.
LV MASS
Calculation of LV mass from either M mode or 2D echocardiography makes the same inherent assumptions and suffers from the same inaccuracies as previously described for volume calculations.7,8 It is surprising that, given the poor reproducibility of these conventional echo methods for LV mass calculations, they are still widely used in both routine clinical and research follow up of patients undergoing antihypertensive treatment, where regression of mass is being studied.
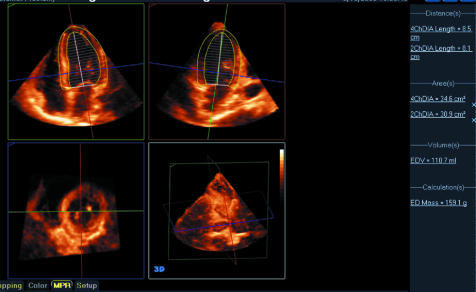
Using the same full volume 3D dataset of the LV and user interaction it is possible to identify epicardial boundaries of the LV myocardium. This is used by analysis software to calculate an epicardial cast of the ventricle. The volume of this cast can be subtracted from an endocardial cast (created from the same dataset) to give the volume of the LV myocardium. By multiplying this by the specific gravity of myocardium, LV mass is derived. This has been demonstrated to be a rapid and highly accurate calculation when compared to CMR.9 Furthermore it has been shown to have significantly better agreement with CMR than 2D echo methods. An example of LV mass calculation from a 3D dataset is shown in fig 4.
Figure 4 Example of LV mass calculation where apical four and two chamber sections have been created from a full volume dataset of the LV. In a semi‐automated process the endocardial and epicardial/right ventricular septal borders of the LV myocardium is identified and a biplane Simpson's rule calculation applied to derive both LV and myocardial volumes. The latter is multiplied by the specific gravity of heart muscle to obtain the displayed mass of 159 g.
The poor reproducibility of conventional echocardiographic methods for calculation of LV mass has led some to speculate that, despite increased procedural cost, CMR requires significantly less patients in LV hypertrophy regression studies and therefore the overall cost of using CMR is lower than echocardiography. However, now that 3D echo techniques have been shown to have high accuracy and reproducibility (compared to CMR) it is likely that this method will be the technique of choice in the future for these types of studies.
REGIONAL LV FUNCTION AND DYSSYNCHRONY ANALYSIS
While accurate non‐invasive calculation of global LV function is important, in the context of patients with heart failure and potential LV dyssynchrony, analysis of regional function in the time domain is of more importance. Several echocardiographic techniques including tissue Doppler have been shown to detect intraventricular dyssynchrony, and these methods have been used in the selection of patients for cardiac resynchronisation therapy (CRT).
Real time 3D echocardiography is showing considerable promise in this direction as an accurate and reproducible tool for detecting and quantifying LV intraventricular dyssynchrony. It also appears to be helpful in predicting patients who will respond to CRT and those that will achieve reverse remodelling following treatment.10 In order to do this, an LV cast is derived from a full volume 3D dataset, as previously described. Some anatomical landmarks are identified on the cast and then it is automatically divided into the standard 16 or 17 segments described by the American Society of Echocardiography. The centre of gravity of each cast can also be calculated and the volume of each segment relative to the centre of gravity measured. Each of these segmental volumes has a pyramidal shape and the volume of each pyramid is calculated and plotted for each cast/dataset throughout the cardiac cycle.
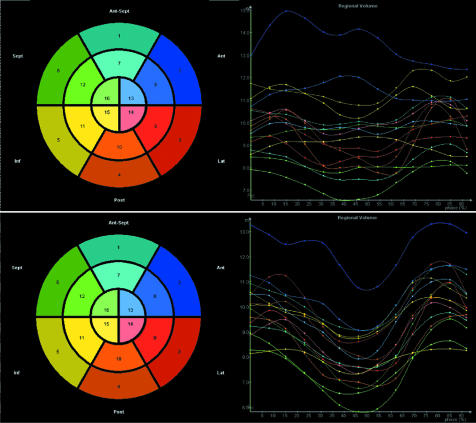
In this way we achieve a series of plots representing the change in volume for each segment throughout the cycle, as shown in fig 5. In a ventricle with synchronous contraction of all segments, we would expect each segment to achieve its minimum volume at almost the same point in the cardiac cycle, whereas in a dyssynchronous ventricle there will be a dispersion in the timing of the point of minimum volume for each of the 16 or 17 segments. The degree of dispersion can be calculated by measuring the standard deviation of the time to achieve minimum volume and then correcting that for the R‐R interval. This allows derivation of a systolic dyssynchrony index which can be used to quantify the degree of LV dyssynchrony from a comparison of all segments, whereas B mode imaging techniques such as tissue Doppler only allow simultaneous comparison of segments within the scan plane. We have shown that there is modest correlation between some tissue Doppler measures of LV dyssynchrony and the 3D systolic dyssynchrony index in patients with both good and poor LV function. Tissue Doppler methods are currently considered the “gold standard” for evaluating dyssynchrony and it remains to be determined if 3D based methods are superior.
Figure 5 LV regional volume curves plotted for one cardiac cycle are seen at the top in a patient with a biventricular pacemaker turned into sense mode. At the bottom the same regional volume curves are seen once pacing has been activated. With pacing turned off it can be seen that each of the LV regions or segments achieve their minimum volume at a different point in the cardiac cycle, indicating significant intraventricular dyssynchrony. However, when biventricular pacing is activated, the regional curves are much more aligned indicating more synchronous contraction of all segments.
Intuitively, one would expect that CRT is likely to be of more benefit in patients with evidence of dyssynchrony, whereas patients with a low dyssynchrony index and therefore relatively synchronous LV contraction may not achieve much benefit from CRT.
The systolic dyssynchrony index in patients with heart failure appears to be independent of the aetiology of the LV dysfunction; it demonstrates an inverse logarithmic correlation with the ejection fraction so that, in general, patients with a higher dyssynchrony index have a lower ejection fraction. This is not surprising. However, of more interest is the fact that the relation between the dyssynchrony index and ejection fraction is preserved, irrespective of QRS duration. This means that there is an important cohort of heart failure patients with low ejection fraction, narrow QRS, and 3D echo evidence of dyssynchrony. These may represent a potentially new patient population for CRT who are currently denied this treatment because they have normal QRS duration. Other echocardiographic techniques to evaluate LV dyssynchrony have also identified the fact that mechanical dyssynchrony can occur in patients with normal QRS. It remains to be seen which echocardiographic technique will be more effective in identifying these potential new CRT responders.
At the other end of the spectrum we can identify a group of patients who fit current criteria for CRT in that they have low ejection fraction and broad QRS; however, these patients do not have much evidence of LV dyssynchrony and their systolic dyssynchrony index is low. This group of patients may represent the 20–30% of subjects who do not respond to CRT.
3D echocardiography for evaluating the left ventricle: key points
Left ventricular (LV) morphology and function most common echocardiography request
M mode and 2D echocardiography make incorrect geometric assumptions about the LV
Inaccurate and poor reproducibility of M mode/2D analysis
3D echocardiography makes no geometric assumptions
3D sees the LV “as it is”
3D measures endocardial position at >700 points
3D echocardiography has excellent correlation with cardiac magnetic resonance (CMR) for volume, mass, and ejection fraction
3D reproducibility comparable with CMR
Using current offline software it can take approximately five minutes for an experienced operator to perform a dyssynchrony analysis. This makes the technique very suitable for identifying suitable patients pre‐CRT and evaluating the results of the procedure. However a five minute analysis time is too long for practical use during an optimisation procedure, where repeated measurements are required following pacemaker adjustments. Future modifications of the analysis software will facilitate rapid online calculation of the dyssynchrony index which can be measured following each pacemaker parameter change. Obviously the aim of optimisation in this way would be to achieve the lowest possible dyssynchrony index to minimise intraventricular dyssynchrony.
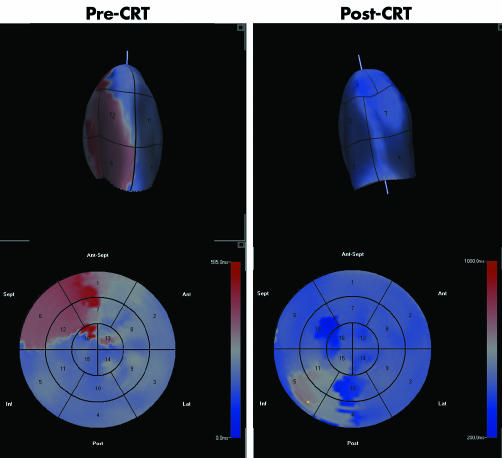
Parametric “bulls eye” displays of the timing of LV contraction are also available. This methodology examines regional LV contraction at approximately 700–800 points over the endocardial surface (from the 3D dataset) rather than in just 16 or 17 segments. Colour coding is used to identify which regions are contracting last and this could potentially be used by electrophysiologists to select the optimal position for the LV electrode. Examples of parametric 3D images pre‐ and post‐CRT are shown in fig 6. Parametric images can be fused with an angiographic display of the coronary sinus anatomy to assist in this process.
Figure 6 Parametric LV cast (top) and “bulls eye” display (bottom) in a patient pre‐ (left) and post‐ (right) cardiac resynchronisation therapy (CRT). Time to minimum volume is significantly delayed in the septal segment curves and on the parametric image (red colour) pre‐CRT. Following CRT with right ventricular pacing first, most segments achieve minimum volume at the same time in the cardiac cycle and the parametric image displays a more homogeneous blue colour.
The use of real time 3D echo for the evaluation of patients being considered for CRT is still in its infancy. However, early data suggest that this is a powerful tool for detecting, quantifying, and displaying LV dyssynchrony. Three dimensional LV dyssynchrony analysis may help to select patients who will or will not benefit from CRT, including those with narrow QRS (table 1). It has the potential to be used to guide pacing electrode positioning in the electrophysiology laboratory and it could be used to guide optimisation of pacemaker parameters. However, perhaps the most endearing feature of this technique in this context is that it is intuitive and provides a graphic display which is appealing to electrophysiologists and other cardiologists who refer patients for CRT evaluation.
Table 1 Three dimensional echocardiography for left ventricular (LV) dyssynchrony analysis.
| Allows comparison of timing of all LV segments |
| Regional volumes provide composite of all vectors of motion |
| Excellent spatial resolution |
| Quick acquisition and analysis |
| Systolic dyssynchrony index is simple, intuitive, reproducible, and predictive of cardiac resynchronisation therapy success |
| Graphical “parametric” display of dyssynchronous segments—guide to LV lead placement |
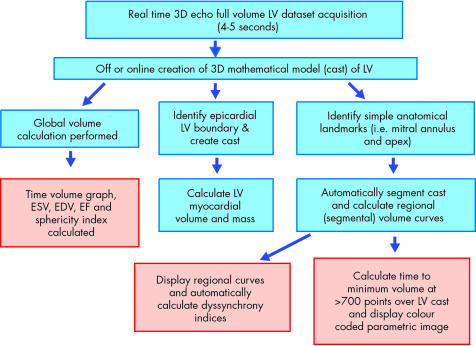
As previously described and illustrated in fig 7, the acquisition of a full volume dataset from a 3D scan takes a few seconds and may be performed at the bedside. Subsequent creation of a mathematical model of the LV allows standard parameters of global function and morphology to be calculated. In addition, more sophisticated measures of regional function and LV synchronicity may also be derived from the same dataset.
Figure 7 Stages of 3D image acquisition and LV analysis.
CONCLUSION
Advances in echocardiographic instrument technology and computer processing power have brought 3D echocardiography from being a time consuming research tool to being a powerful clinically applicable technique that can provide answers to the most commonly asked question from any cardiac imaging methodology—what is the LV function?
Three dimensional echocardiographic imaging will soon become a standard imaging modality on all new echo systems. In the future, it will seem as unacceptable to perform an echocardiographic study without using 3D to analyse global and regional LV function as it is to perform a conventional 2D echo study without use of Doppler.
References
- 1.Jenkins C, Bricknell K, Hanekom L.et al Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time three‐dimensional echocardiography. J Am Coll Cardiol 200444878–886.An important paper demonstrating good reproducibility and accuracy of 3D versus CMR in assessing LV volumes. [DOI] [PubMed] [Google Scholar]
- 2.Zeidan Z, Erbel R, Barkhausen J.et al Analysis of global systolic and diastolic left ventricular performance using volume‐time curves by real‐time three‐dimensional echocardiography. J Am Soc Echocardiogr 20031629–37. [DOI] [PubMed] [Google Scholar]
- 3.Kühl H P, Schreckenberg M, Rulands D.et al High‐resolution transthoracic real‐time three‐dimensional echocardiography: quantitation of cardiac volumes and function using semi‐automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004432083–2090. [DOI] [PubMed] [Google Scholar]
- 4.Bu L, Munns S, Zhang H.et al Rapid full volume data acquisition by real‐time 3‐dimensional echocardiography for assessment of left ventricular indexes in children: a validation study compared with magnetic resonance imaging. J Am Soc Echocard 200518299–305. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez‐Chico J L, Zamorano J L, Perez de Isla L.et al Comparison of left ventricular volumes and ejection fractions measured by three‐dimensional echocardiography versus by two‐dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. Am J Cardiol 200595809–813. [DOI] [PubMed] [Google Scholar]
- 6.Mannearts H F J, Van Der Heide J A, Kamp O.et al Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J 200428680.This is the first paper to describe the implementation of LV shape analysis using a 3D sphericity index, and it is a key reference. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener J S, Livengood S V, Meyer P S.et al Should echocardiography be used to assess effects of antihypertensive therapy? Test‐retest reliability of echocardiography for measurement of left ventricular mass and function. J Am Coll Cardiol 199525424–430.This paper identifies why we need to do better than current methods for echocardiographic evaluation of LV mass. [DOI] [PubMed] [Google Scholar]
- 8.Myerson S G, Montgomery H E, World M J.et al Left ventricular mass: reliability of M‐mode and 2‐dimensional echocardiographic formulas. Hypertension 200240673–678. [DOI] [PubMed] [Google Scholar]
- 9.Mor‐Avi V, Sugeng L, Weinart L.et al Fast measurement of left ventricular mass with real‐time three‐dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 20041101814–1818.Demonstrates that the reproducibility and accuracy of 3D mass measurements is significantly better than those obtained from 2D. [DOI] [PubMed] [Google Scholar]
- 10.Kapetenakis S, Kearney M T, Siva A.et al Real‐time three‐dimensional echocardiography. A novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005112992–1000.Although from our own unit, this is actually the first published paper to describe and validate a dyssynchrony index that can be used for patient selection for CRT. [DOI] [PubMed] [Google Scholar]