The aorta represents a complex organ system which begins in the aortic ring adjacent to the aortic root with the origin of the two major coronary arteries, and ends at the iliac bifurcation. The subdivision into seven segments seems to be clinically important—the aortic root, the ascending aorta, the ascending aortic arch, the vessel bearing arch, the aortic isthmus, and the thoracic descending and abdominal aorta. The aorta as an organ can be regarded as a biological “windkessel”, storing kinetic energy during systole which is delivered during diastole in order to maintain a relative constant mean aortic pressure. In particular, a high diastolic blood pressure is important for the coronary perfusion.
The size of the aorta decreases with distance from the aortic valve in a tapering fashion. The normal diameter of the ascending aorta has been defined as <2.1 cm/m2 and of the descending aorta as <1.6 cm/m2.1 The normal diameter of the abdominal aorta is regarded to be less than 3.0 cm. The normal range has to be corrected for age and sex, as well as daily workload.
The aortic wall consists of three layers: intima, media, and adventitia. The intima is thin, the media contains the elastic fibres and smooth muscle cells forming a spiral layer of tissue providing the strength of the aortic wall, and the adventitia provides the nutrition with the arterial and venous vasa vasorum. An inner vasa vasorum from the aortic lumen also seems to be present.2 A wall thickness of < 4 mm is regarded as normal.
AGEING
During life the size of the aorta increases. The normal expansion rate is about 1–2 mm/year. It involves all segments which, during childhood and in young adulthood, result in an increase of the luminal diameter of the entire aorta (figs 1 and 2).3 In adulthood the aortic size is related to exercise and workload. The ageing of the aorta is accompanied by a loss of compliance, and an increase of wall stiffness caused by structural changes including an increase in the collagen content and formation of intimal atherosclerosis with calcium deposits.1,4,5 As a result pulse pressure is enhanced and pulse wave velocity raised, decreasing the resulting organ perfusion, particularly the predominant diastolic myocardial perfusion.
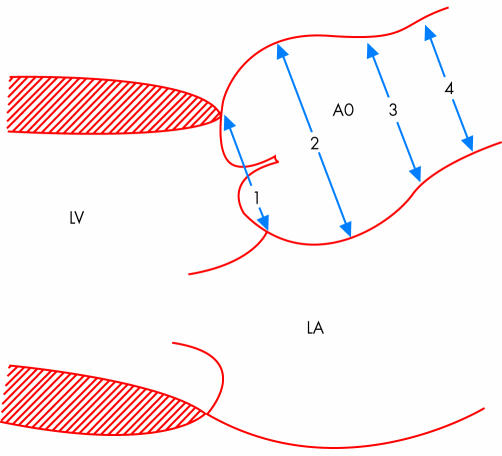
Figure 1 Schematic presentation of the longitudinal parasternal view with four regions where aortic diameters are measured for follow up analysis in Marfan's syndrome. LA, left atrium; LV, left ventricle; 1, valve annulus; 2, aortic sinuses; 3, sinotubular junction; 4, proximal ascending aorta. Reproduced from Roman et al,3 with permission from Exerpta Medica Inc.
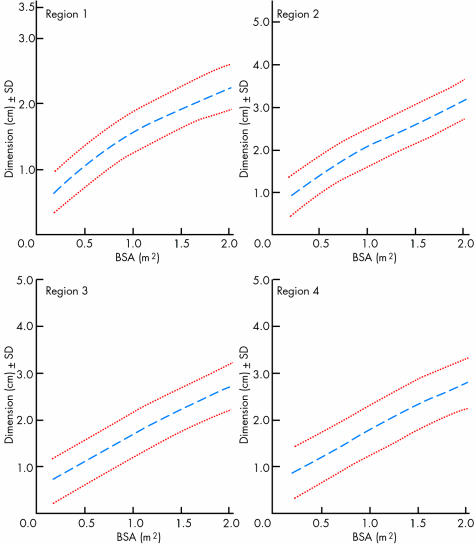
Figure 2 Aortic diameter changes related to body surface area (BSA) for the four different regions illustrated in fig 1. Useful for follow up studies and detection of abnormal enlargement of the aorta in Marfan's syndrome. Reproduced from Roman et al,3 with permission from Exerpta Medica Inc.
Aortic sclerosis is subdivided into four stages:
intimal thickening
atheroma formation
protruding plaques
intimal flap and/or thrombus formation.
Aortic sclerosis risk factors are hypertension, hyperlipidaemia, diabetes mellitus, and smoking. A thickness up to 7 mm is regarded as the upper normal tolerance limit.1
The incidence of ruptured thoracic aneurysms in individuals aged 60–69 years is about 100 cases per 10 000, in those aged 70–79 years it is about 300/10 000, and in those aged 80–89 years the incidence is 550/10 000.6
DEFINITIONS
An enlargement of the aortic diameter exceeding at least 50% of the normal range represents an ectasia, which results in aneurysm formation when the ectasia exceeds tolerance limits.5
There are different forms of aneurysms:
true aneurysm means enlargement of the inner lumen caused by vessel wall expansion
false aneurysm (also called pseudoaneurysm) means lumen enlargement caused by perforation (penetration) of all parts of the vessel wall forming an outer sack in communication with the inner lumen of the aorta
circumscript (localised) aneurysm means that only segments of the entire aorta are involved
diffuse aneurysm includes an enlargement of the ascending aorta, the aortic arch, the descending thoracic (thoracic aortic aneurysms) or abdominal aorta (abdominal aortic aneurysm) or even the whole aorta
the term dissecting aneurysm should not be used.
ANEURYSM FORMATION
Three major inherited disorders are known to cause aortic diseases such as Marfan's syndrome, Ehler‐Danlos syndrome, and other familial forms of connective tissue diseases. For Marfan's syndrome more than 100 mutations have been identified at one locus on the fibrillin gene.7 The prevalence is about 1/5000.8 It accounts for 6–9% of all dissections.9 Complications are aortic aneurysms, aortic regurgitation caused by aortic ring dilatation, and aortic dissection. Other familial clusters of thoracic aortic aneurysms have also been identified in about 20% of 1600 patients.10 It has been suggested that this number may be even higher, when new imaging techniques are used for screening. Ehler‐Danlos syndrome belongs to the group of connective tissue syndromes.
The isolated aneurysm formation of the ascending aorta has been regarded as a “forme fruste” of Marfan's syndrome, but represents a specific genetically determined disease. The aortic arch is usually not involved.11 In these patients an association with the bicuspid aortic valve (BAV) is found (table 1) with a prevalence 1%.12 In a review of 21 417 cases with 161 patients suffering from aortic dissection, the prevalence of BAV was 10‐fold that of controls13 and it was found in 6–10% of all dissections.14 Patients with BAV have a ninefold higher risk of dissection than those with tricuspid aortic valves.11 A high incidence of BAV is also found in cases with aortic coarctation. In patients with BAV enlarged aortic diameters are reported.14
Table 1 Comparison of Marfan's syndrome versus bicuspid aortic valve in aetiology of aortic dissection.10.
| Incidence | Likelihood of aortic dissection | |
|---|---|---|
| Marfan's syndrome | 0.01% | 40% |
| Bicuspid aortic valve | 1–2% | 5% |
Due to the higher incidence, bicuspid aortic valve disease causes more cases of aortic dissection than Marfan's syndrome.10
Annuloaortic ectasia affects 5–10% of patients undergoing valve replacement and has been described with a familial sex linked aggregation with a probable existence of genetic heterogeneity.15,16 Five mutations have been identified.11
Abdominal aortic aneurysm formation is uncommon before the sixth decade. The process is often combined with more proximal disease.17 The prevalence in men over the age of 50 years is 5%.18,19 A familial aggregation is suggested predominantly affecting women, whereas men who are affected tend to be younger.20 A genetic mutation has been described.21
A weakening of the aortic wall can also be induced by inflammation. This can be the result of microbiological diseases or multisystem vasculitis disorders. The aortitis induced by syphilis is well known, but Staphylococcus aureus infection can also be a cause. The Kawasaki syndrome is characterised by more circumscript aneurysm formation, whereas syphilis can induce a diffuse wall thickening and aneurysm formation of the ascending aorta; penetrating ulcers can also be observed.
The risk of rupture increases with the diameter of the aorta, but also occurs in small aorta. Inflammatory cells and raised concentration of cytokines within the aneurysm wall have been observed.22 Cytokines may trigger an increased production of matrix metalloproteinase (MMP) by macrophages and smooth muscle cells.23 A strong relation between infiltration and MMP activation was found.22 Behçet disease, like other forms of vasculitis, leads more to local aneurysm formation and perforation than dissection.24 The Kawasaki syndrome is a disease with an incidence of 135/100 000 children and 8–17 cases in 100 000 children < 5 years.25 Coronary aneurysm is the main sign, but also other arterial segments can be involved. In giant cell arteritis thoracic and abdominal aneurysms are feared complications.26
Toxic substances such as cocaine and amphetamines can also lead to aortic wall thinning and aneurysm formation.
In aortic stenosis a poststenotic aneurysm formation can occur, which may even be enhanced after aortic valve prosthesis implantation.27 Previous aortic valve surgery accounts for 2–4% of patients receiving aortic root surgery.27
An important cause of aneurysm formation is related to trauma, particularly high speed accidents involving the aortic isthmus.28 About 15–20% of deaths are related to aortic trauma in these patients.
PATHOPHYSIOLOGY
Pathological/anatomical studies demonstrate typical cystic degeneration of the aortic media, mucoid material, and loss of elastic fibres. The loss of elastic fibres, deposits of mucopolysaccharide‐like material, and cystic anomalies are found in Marfan's syndrome as well as anuloaortic ectasia.29 This leads to a weakening of the wall strength and consecutive vessel dilatation. The circumferential wall stress (W) can be calculated according to the La Place law for thin wall structures: W = P × r/2 h, where P = pressure, D = diameter, r = radius, and h = wall thickness.
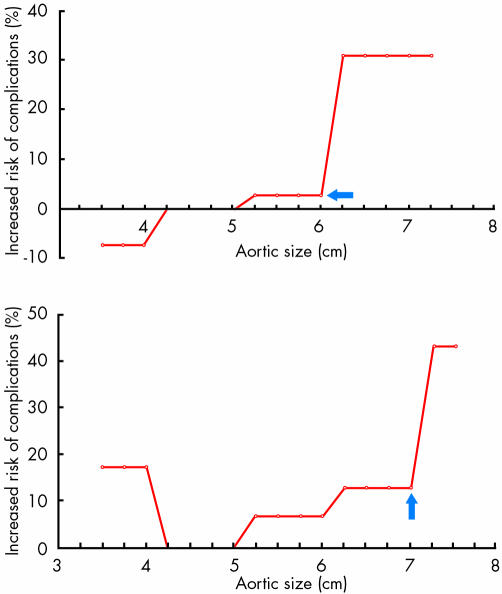
Hypertension, wall thinning, and aortic enlargement are the most important factors increasing wall stress and leading to aortic rupture or dissection.30,31 Aortic diameter is a marker of risk, but is not always enlarged. Whereas in connective tissue disease up to 40% of cases show an aortic enlargement greater than the normal range, in other forms this is found in only up to 10% of cases. The critical point of rupture (fig 3) is at 6 cm for the ascending aorta and 7 cm for the descending aorta.32 When this point is reached, up to 30% of cases with enlargement of the ascending aorta and 40% of cases with enlargement of the descending aorta have suffered rupture or dissection.32
Figure 3 Influence of aortic size on cumulative, lifetime incidence of natural complications of aortic aneurysm. The incidence of natural complications (rupture or dissection) is plotted along the y axis, and the aortic size is plotted along the x axis. The plot for the ascending aorta is shown in the upper panel and for the descending aorta in the lower panel. Note the hinge point at 6 cm and 7 cm. Reproduced from Elefteriades,10 with permission from Elsevier Science Inc.
The yearly risk of complications can be calculated:
Ln λ = −21.055 + 0.0093 (age)
+0.841 (pain) + 1.282 (COPD)
+0.643 (descending aorta diameter) + 0.405 (abdominal aorta diameter).32 Probability of rupture within one year = 1−e−(365)
For both the thoracic and the abdominal aorta, aortic sclerosis was only a weak predictor of expansion.33,34 Patients with the most atherosclerotic burden had the slowest growth of the abdominal aorta.35 Smoking increased the growth rate by 15–20%, but this effect is too small to provide a guideline for screening smokers and non‐smokers.34
CLINICAL FEATURES
Aortic aneurysms are silent as long as there are no complications, because no symptoms are produced by enlargement of the aortic diameter alone, except when rapid overexpansion occurs. Pain receptors are located in the adventitia so that as long as an intact structure is present, pain is not produced.
Aortic regurgitation is a typical consequence of ascending aortic aneurysm formation caused by an aortic ring dilatation with or without valve degeneration. Also circumscript pseudoaneurysm of the aortic root can lead to aortic regurgitation when the aortic ring is destroyed, which is seen more often in mycotic aneurysm, particularly when related to endocarditis involving the aortic–mitral connective triangle. The aortic regurgitation may even be the first sign of the aneurysm formation. It may be several years before patients become symptomatic.
Aortic dissection and rupture is related to aortic diameter and expansion rate in many situations (table 2). The mean (SD) expansion rate in the ascending aorta is 1.3 (1.2) mm/year, in the descending aorta it is 3.1 (3.2) mm/year, and in the abdominal aorta it is 2.6 mm/year (1 to 6.1 mm/year). The diameter accelerates as the aneurysm enlarges.34
Table 2 Yearly complication rates as a function of aortic size10.
| Aortic size | ||||
|---|---|---|---|---|
| >3.5 cm | >4 cm | >5 cm | >6 cm | |
| Rupture | 0.0% | 0.3% | 1.7% | 3.6% |
| Dissection | 2.2% | 1.5% | 2.5% | 3.7% |
| Death | 5.9% | 4.6% | 4.8% | 10.8% |
| Any of above | 7.2% | 5.3% | 6.5% | 14.1% |
The main risk factor is smoking. The aortic diameter is, however, similar in those with and without dissection, but the risk of complication accelerates beyond a diameter of 6 cm for the ascending aorta and 7 cm for the descending aorta.32 The risk of rupture, dissection, and death is in the range of 5–6.5% below 6 cm and more than 14% above 6 cm.32
SURGICAL AND ENDOVASCULAR MANAGEMENT
Aortic dissection and/or rupture are the most severe complications of aortic aneurysm formation leading in urgent or emergency situations to high operative risk (table 3).36,37,38,39,40,41 Operative mortality has been reported to be 1.5% for elective surgery, 2.6% for emergency, and 11.7% for urgent surgery.
Table 3 Current risk of thoracic aortic surgery10.
| Mortality | Stroke | Paraplegia | |
|---|---|---|---|
| Ascending aorta/aortic arch | 2.5% | 8.3% | 0.0% |
| Descending aorta/ thoracoabdominal aorta | 8.2% | 4.1% | 2.0% |
Data for allcomers, including elective cases and most experienced surgeons.10
Thus, elective surgery has been recommended for aneurysms of the ascending aorta beyond 5.5 cm in patients with Marfan's syndrome and 6.0 cm for those without connective tissue disease.1
Reoperation was necessary in 10–20% of the patients during a follow up of 10–20 years, with a trend for more reoperations in those with valve preserving aortic root reconstruction versus composite graft replacement (16% v 5%). A predictor for reoperation was found to be an annulus of > 2.5 cm.38 Other predictors were found to be Marfan's syndrome, mitral valve prolapse, preoperative atrial fibrillation, aortic valve preserving operation, and concomitant procedures performed with a mean (SD) time to reoperation of 4.5 (5) years.39 Recurrent aortic aneurysm was found in 3.5%, mitral valve disease in 2%.
Long term problems can arise from anticoagulation. Thrombembolism is reported in up to 0.42/100 patient‐years.36 Valve thrombosis was observed in 1% and life threatening haemorrhage in 2% of 203 patients with a mean period of 5.4 (4.9) years to the event. Endocarditis was found in 1% of cases only, but usually within one year after surgery. Main predictors of late death are female sex, increased age, non‐treatment with β blockers, mitral regurgitation of +3–4 at presentation, mitral ring calcification, postoperative dysrhythmia, and postoperative use of inotropes. The overall 20 year survival rate reaches 50%.39
For the aortic arch, surgical intervention is most likely to be the method of choice, which is nowadays increasingly combined with graft stent implantation in order to seal the distal aortic arch to the descending aorta. Special systems have been designed, so that the implantation can be performed in an antegrade strategy.41,42
For the thoracic descending or thoracoabdominal aortic aneurysm, current surgical strategy has been developed during the last 15 years in order to prevent ischaemic complications. The technique for spinal cord protection can reduce the rate of paraplegia from about 15% to less than 5%. Also the rate of renal failure (serum creatinine elevation > 50% above baseline) could be reduced from about 60% to 20%.43
Aortic dimension and dissection: key points
Aortic dimensions increase with age and the wall thickens leading to aortic sclerosis, the most common aortic disease graded into four categories
The larger the diameter, the greater the expansion rate
Aneurysm formation is common in Marfan's syndrome and accounts for 6–9% of dissections; familial clusters of thoracic aortic aneurysm account for 20%
Bicuspid aortic valve patients are at increased risk and account for 6–10% of all dissections
Inflammatory diseases have a potential risk for aortic aneurysm formation, such as Behçet's disease, Kawasaki syndrome, and syphilis
Trauma, as well as aortic surgery of any kind, can lead to aneurysm formation and is responsible for 2–44% of patients undergoing aortic surgery
Critical points of rupture have been established—6 cm for the ascending aorta and 7 cm for the descending aorta
Surgery is recommended well before these levels are reached—in Marfan's syndrome when the diameter exceeds 5.5 cm
Current management strategy includes surgery for type A dissection and medical treatment for type B dissection, with endovascular treatment on the horizon
In one report the five year survival rate of 1773 patients reached nearly 75%, compared to only 20% in another report.41,44 Over a 10 year period, the extent of the disease had no influence on patients' prognosis, which was in the range of 50%.41
Nowadays, for circumscript, localised aneurysm formation, either true or false aneurysms, and for chronic or in some situations for acute aortic dissection, percutaneous stent graft implantation has become an alternative option.45,46,47,48,49 Despite the limited number of patients treated and the limited follow up time, this technique could even be used in emergency situations with high procedural and clinical success rates.50 The prerequisite is close cooperation of cardiologists, radiologists, anaesthetists, and cardiovascular surgeons, because the optimal strategy has to be chosen. The success rate today reaches 95%.50,51 The positioning of the 10 or 14 cm long stent graft—which for some types is not covered for the first part of the graft but covered with Teflon or Dacron for the distal segments—has been regarded as crucial. Positioning in the thoracic aorta can be supported using the transoesophageal approach, whereas for the abdominal aorta use of the intravascular ultrasound probe located in the inferior vena cava is suggested. Lowering the blood pressure by up to 50 mm Hg or the administration of adenosine to lower the heart rate is essential before deploying the stent graft, otherwise a sudden blood pressure rise or shifting of the stent graft can occur with dislodgement. The ratio of stent graft size to aortic diameter should be of the order of 1.1–1.15 with healthy areas before and after the aneurysm neck. Higher diameters have to be avoided in order to limit the vessel wall stretch which may result in antegrade or retrograde dissection or perforation. Angiography after implantation will reveal immediately wall apposition of the stent. If endoleaks are found, additional balloon inflations may be necessary to achieve good strut apposition to the wall. It may also be necessary to implant a second stent.
After stenting, patients can usually be extubated rapidly and after some days discharged. In some patients an inflammation is seen, described as graft disease, leading to some chest discomfort which responds well to corticosteroid treatment.
Hybrid techniques—the combination of stent graft placement and open visceral bypass grafting—have also been described.52,53,54 For the abdominal aorta even fenestrated grafts, additional visceral grafts, and directly anchoring the distal landing site have also been described.55,56,57,58
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Erbel R, Alfonso F, Boileau C.et al Task force on aortic dissection of the European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J 2001221642–1681.Task force report of a committee consisting of molecular biologists, cardiologists, radiologist, and surgeons with endorsement of the American College of Cardiology and review by the ESC board with paediatric cardiologists [DOI] [PubMed] [Google Scholar]
- 2.Gössl M, Rosol M, Malyar N M.et al Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol 2003272526–537.Basic research about vasa vasorum of the arterial wall. [DOI] [PubMed] [Google Scholar]
- 3.Roman M J, Devereux R B, Kramer‐Fox R.et al Two‐dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 198964507–512.Echocardiographically assessed normal values of the ascending aorta, which can be used as reference during childhood [DOI] [PubMed] [Google Scholar]
- 4.Aronberg D J, Glazer H S, Madsen K.et al Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr 19848247–250. [PubMed] [Google Scholar]
- 5.Hager A, Kaemmerer H, Rapp‐Bernhardt U.et al Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg 20021231060–1066. [DOI] [PubMed] [Google Scholar]
- 6.Johansson G, Markstrom U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg 199521985–988. [DOI] [PubMed] [Google Scholar]
- 7.Collod G, Babron M C, Jondeau G.et al A second locus for Marfan syndrome maps to chromosome 3p24.2–p25. Nat Genet 19948264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyeritz R. Genetics and cardiovascular disease. In: Braunwald E, ed. Heart disease. 7th ed. Philadelphia: WB Saunders, 20041867–1909.
- 9.Fenoglio J, McAllister H, DeCastro C.et al Congenital bicuspid aortic valve after age 20. Am J Cardiol 197739164–169. [DOI] [PubMed] [Google Scholar]
- 10.Elefteriades J A. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 200274S1877–S1880.Important surgical view of the relation between aortic dimensions and risk of dissection [DOI] [PubMed] [Google Scholar]
- 11.Burks J M, Illes R W, Keating E C.et al Ascending aortic aneurysm and dissection in young adults with bicuspid aortic valve: Implications for echocardiographic surveillance. Clin Cardiol 199821439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson E W, Edwards W D. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol 198453849–855. [DOI] [PubMed] [Google Scholar]
- 13.Roberts W. Aortic dissection: anatomy, consequences, and causes. Am Heart J 1981101195–214.Early and important report on aortic pathology and diseases. [DOI] [PubMed] [Google Scholar]
- 14.Isselbacher E M. Diseases of the aorta. In: Braunwald E, ed. Heart disease. 7th ed. Philadelphia: WB Saunders, 20041403–1435.
- 15.Griepp R B, Ergin M A, Galla J D.et al Natural history of descending thoracic and thoracoabdominal aneurysms. Ann Thorac Surg 1999671927–1930.Model for calculation of risk of aortic dissection. [DOI] [PubMed] [Google Scholar]
- 16.Ellis P R, Cooley D A, De Bakey M E. Clinical consideration and surgical treatment of annuloaortic ectasie. J Thorac Cardiovasc Surg 196142363–370. [PubMed] [Google Scholar]
- 17.Coady M A, Davies R R, Roberts M.et al Familial patterns of thoracic aortic aneurysms. Arch Surg 1999134361–367. [DOI] [PubMed] [Google Scholar]
- 18.Muluk S C, Gertler J P, Brewster D C.et al Presentation and patterns of aortic aneurysms in young patients. J Vasc Surg 199420880–888. [DOI] [PubMed] [Google Scholar]
- 19.Lederle F A, Johnson G R, Wilson S E.et al for the Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. The aneurysm detection and management study screening program validation cohort and final results. Arch Intern Med 20001601425–1430. [DOI] [PubMed] [Google Scholar]
- 20.Multicentre Aneurysm Screening Study Group The multicentre aneurysm screening study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 20023601531–1539. [DOI] [PubMed] [Google Scholar]
- 21.Verloes A, Sakalihasan N, Koulischer L.et al Aneurysms of the abdominal aorta: familial and genetic aspects in three hundred thirteen pedigrees. J Vasc Surg 199521646–655. [DOI] [PubMed] [Google Scholar]
- 22.Pope F M, Narcisi P, Nicholls A C.et al COL3A1 mutations cause variable clinical phenotypes including acrogeria and vascular rupture. Br J Dermatol 1996135163–181. [PubMed] [Google Scholar]
- 23.McMillan W D, Pearce W H. Inflammation and cytokine signalling in aneurysms. Ann Vasc Surg 199711540–545. [DOI] [PubMed] [Google Scholar]
- 24.Lindholt J S, Vammen S, Fasting H.et al The plasma level of matrix metalloproteinase 9 may predict to natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg 200020281–285. [DOI] [PubMed] [Google Scholar]
- 25.Tsui K L, Lee K W, Chan W K.et al Behçet's aortitis and aortic regurgitation: a report of two cases. J Am Soc Echocardiogr 20041783–86. [DOI] [PubMed] [Google Scholar]
- 26.Burns J C, Glode M P. Kawasaki syndrome. Lancet 2004364533–544. [DOI] [PubMed] [Google Scholar]
- 27.Calvo‐Romero J M. Giant cell arteritis. Postgrad Med J 200379511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrie G M, Earle N, DeBakey M E. Long‐term in fate of the aortic root and aortic valve after ascending aneurysm surgery. Ann Surg 1993217711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmley L F, Mattingly T W, Manion W C.et al Nonpenetrating traumatic injury of the aorta. Circulation 1958171086–1101. [DOI] [PubMed] [Google Scholar]
- 30.Kakko S, Raisanen T, Tamminen M.et al Candidate locus analysis of familial ascending aortic aneurysms and dissection confirms the linkage to the chromosone 5q13‐14 in Finnish families. J Thorac Cardiovasc Surg 2003126106–113. [DOI] [PubMed] [Google Scholar]
- 31.Coady M A, Rizzo J A, Hammond G L.et al What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997113476–491. [DOI] [PubMed] [Google Scholar]
- 32.Hahn R, Romas M, Mogtader A.et al Association of aortic dilation with regurgitant, stenotic, and functionally normal bicuspid aortic valves. J Am Coll Cardiol 199219283–288. [DOI] [PubMed] [Google Scholar]
- 33.Bubb M R. Replacement of the aortic root in Marfan's syndrome. N Engl J Med 19993411473–1474.New aspects of surgery for Marfan's syndrome are reported. [DOI] [PubMed] [Google Scholar]
- 34.Agmon Y, Khandheria B K, Meissner I.et al Is aortic dilatation an atherosclerosis‐related process? J Am Coll Cardiol 2003421076–1083. [DOI] [PubMed] [Google Scholar]
- 35.Brady A, Thompson S, Fowkes F.et al Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 200411016–21. [DOI] [PubMed] [Google Scholar]
- 36.Gott V L, Gillinov A M, Pyeritz R E.et al Aortic root replacement: risk factor analysis of a seventeen‐year experience with 270 patients. J Thorac Cardiovasc Surg 1995109536–544. [DOI] [PubMed] [Google Scholar]
- 37.Karck M, Kallenbach K, Hagl C.et al Aortic root surgery in Marfan's syndrome: comparison of aortic valve sparing reimplantation versus composite grafting. J Thorac Cardiovasc Surg 2004127391–398. [DOI] [PubMed] [Google Scholar]
- 38.Burkhart H M, Zehr K J, Schaff H V.et al Valve‐preserving aortic root reconstruction: a comparison of techniques. J Heart Valve Dis 20031262–67. [PubMed] [Google Scholar]
- 39.Zehr K J, Orszulak T A, Mullany C J.et al Surgery for aneurysms of the aortic root. Circulation 20041101364–1371. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira N C, David T E, Ivanov J.et al Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2003125789–796. [DOI] [PubMed] [Google Scholar]
- 41.Chavan A, Karck M, Hagl C.et al Hybrid endograft for one‐step treatment of multisegment disease of the thoracic aorta. J Vasc Interv Radiol 200516823–829. [DOI] [PubMed] [Google Scholar]
- 42.Herold U, Kanler M, Aleksic I.et al One stage repair in complex aortic disease: surgery combined with open distal stent grafting requires a new stent graft design. J Thorasc Cardiovasc Surg 200553(suppl 1)S53–S56. [Google Scholar]
- 43.Köksoy C, LeMaire S A, Curling P E.et al Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 200273730–738. [DOI] [PubMed] [Google Scholar]
- 44.Crawford E S, DeNatale R W. Thoracoabdominal aortic aneurysm: observations regarding the natural course of disease. J Vasc Surg 19863578–582. [DOI] [PubMed] [Google Scholar]
- 45.Eggebrecht H, Baumgart D, Schmermund A.et al Endovascular stent‐graft repair for penetrating atherosclerotic ulcer of the descending aorta. Am J Cardiol 2003911150–1153.Endovascular treatment of penetrating aortic ulcers with graft stents is reported with good clinical results. [DOI] [PubMed] [Google Scholar]
- 46.Herold U, Piotrowski J, Baumgart D.et al Endoluminal stent graft repair for acute and chronic type B aortic dissection and atherosclerotic aneurysm of the thoracic aorta. Eur J Cardiothorac Surg 200222891–897. [DOI] [PubMed] [Google Scholar]
- 47.Nesser J H, Eggebrecht H, Baumgart D.et al Emergency stent‐graft placement for impending rupture of the descending thoracic aorta. J Endovasc Ther 20029(suppl 2)II72–II78.Even in urgent situations where surgeons have refused to operate on patients, stent graft treatment can be performed successfully. [PubMed] [Google Scholar]
- 48.Ince H, Nienaber C A. Endovascular stent‐graft prosthesis in aortic aneurysm. Z Kardiol 20019067–72. [DOI] [PubMed] [Google Scholar]
- 49.Eggebrecht H, Baumgart D, Herold U.et al Multiple penetrating atherosclerotic ulcers of the abdominal aorta: treatment by endovascular stent graft placement. Heart 200185526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggebrecht H, Nienaber C A, Neuhauser M.et al Endovascular stent‐graft placement in aortic dissection: a meta‐analysis. Eur Heart J 2005Epub ahead of print. [DOI] [PubMed]
- 51.Nienaber C A, Eagle K A. Aortic dissection: new frontiers in diagnosis and management; Part II: therapeutic management and follow‐up. Circulation 2003108772–778. [DOI] [PubMed] [Google Scholar]
- 52.Morales J P, Taylor P R, Sabharwalet al Med‐term results of endoluminal repair for type B aortic dissection. Heart. (in press)
- 53.Eggebrecht H, Herold U, Kuhnt O.et al Endovascular stent graft treatment of aortic dissection: determinants of postinterventional outcome. Eur Heart J 200526489–497. [DOI] [PubMed] [Google Scholar]
- 54.Quinones‐Baldrich W J, Panetta T F, Vescera C L.et al Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg 199930555–560. [DOI] [PubMed] [Google Scholar]
- 55.Wantabe Y, Ishimaru S, Kawaguchi S.et al Successful endografting with simultaneous visceral artery bypass grafting for severely calcified thoracoabdominal aortic aneurysm. J Vasc Surg 200235397–399. [DOI] [PubMed] [Google Scholar]
- 56.Orend K H, Kotsis T, Scharrer‐Pamler R.et al Endovascular repair of aortic rupture due to trauma and aneurysm. Eur J Vasc Endovasc Surg 20022361–67. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence‐Brown M, Sieunarine K, van Schie G.et al Hybrid open‐endoluminal technique for repair of thoracoabdominal aneurysm involving the celiac axis. J Endovasc Ther 20007513–519. [DOI] [PubMed] [Google Scholar]
- 58.Kinney E V, Kaebnick H W, Mitchell R A.et al Repair of mycotic paravisceral aneurysm with a fenestrated stent‐graft. J Endovasc Surg 20007192–197. [DOI] [PubMed] [Google Scholar]