Infective endocarditis (IE) is a disease that is continually changing, with new high risk patients, new diagnostic procedures, the involvement of new microorganisms, and new therapeutic methods.1 Despite knowledge of these changes, and considerable improvements in diagnostic and therapeutic strategies, IE is still a severe disease.2 The high morbidity and mortality rate of IE is the consequence of both the destructive valvar lesions causing valve regurgitation and heart failure, and the valvar vegetations with their high embolic potential. Although the incidence of IE is relatively stable, those patients affected by the disease are older and sicker, and the co‐morbidity rate is high.3 As soon as the diagnosis of IE is suspected, the physician is faced with four specific problems:
First, the diagnosis of IE is still difficult and is frequently delayed, causing progressive and irreparable valvar damage.
Second, IE is still associated with high in‐hospital mortality, ranging from 16–25%, and high incidence of embolic events, ranging from 10–49%, potentially the source of severe complications and sequelae.4
Third, the optimal therapeutic strategy in these patients is still to be defined and may vary in the individual patient.
Fourth, some patients present with specific features or complications and need a specific management.
These four issues will be addressed here.
HOW TO DIAGNOSE INFECTIVE ENDOCARDITIS?
When to consider IE?
The knowledge of potential at‐risk patients may increase the level of suspicion of IE. Although the absolute number of new cases of IE has not changed over the last 10 years, the at‐risk population has been completely modified during this period. Rheumatic valve disease is no longer the main underlying disease in IE, and has been replaced by an increasing number of episodes of IE occurring on intracardiac devices, in intravenous drug abusers, and haemodialysis or elderly patients. Nosocomial disease is also much more frequent. Thus, we have to consider IE in all these situations.
Occurring in such a broad range of patients and circumstances, the clinical presentation of IE may also be varied, including symptoms such as fever and cardiac and non‐cardiac manifestations.
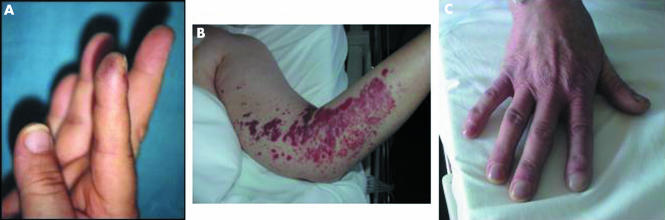
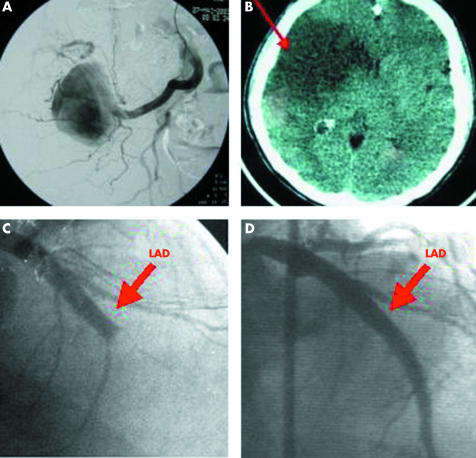
Fever is the most frequent symptom of IE, but may be absent in patients with severe debility or elderly patients, and in the case of previous antibiotic treatment. Fever is frequently intermittent in IE and may be associated with weight loss, fatigue, and anorexia. Cardiac manifestations include congestive heart failure, new heart murmur, and atrioventricular block. Severe heart failure in the context of IE is generally the consequence of severe valvar lesions. These patients require close follow up and frequently urgent surgery is mandatory. Finally, extracardiac manifestations may be the first sign of IE, including cutaneous manifestations (fig 1), splenomegaly, rheumatological symptoms, neurological manifestations, or other consequences of vegetation embolism (fig 2). In right sided and pacemaker lead endocarditis, the clinical presentation is frequently atypical and includes local and pulmonary symptoms as the first manifestations of the disease.5
Figure 1 Cutaneous manifestations in infective endocarditis. (A) Osler's node in a patient with hypertrophic obstructive cardiomyopathy and streptococcal endocarditis. (B) Severe purpuric lesions in a woman with staphylococcal pacemaker endocarditis. (C) Digital hippocratism in a patient with chronic streptococcal infective endocarditis.
Figure 2 Peripheral manifestations of infective endocarditis (IE). (A) Large mycotic aneurysm of the iliac artery, which was successfully treated by interventional catheterisation. (B) Cerebral infarction demonstrated by computed tomographic scan in a patient with staphylococcal IE. (C) Embolic obstruction of the left anterior descending (arrow) coronary artery in a 45 year old patient with Streptococcus bovis endocarditis. (D) Same patient after successful emergency percutaneous transluminal coronary angioplasty.
Finally, IE has to be suspected both in very acute situations including cardiogenic or septic shock (fulminant endocarditis) representing life threatening situations, as well as in the case of more insidious presentations—for example, prolonged unexplained fever—in which case the diagnosis of IE is the main challenge. In all these situations, echocardiography and blood cultures have to be performed.
How to diagnose IE?
IE may be suspected when non‐specific laboratory abnormalities are present, including anaemia, leucocytosis, elevated C reactive protein, and sedimentation rate. However, the diagnosis of IE is mainly based on two tests—blood culture and echocardiography.
Blood culture is the best method for identification of the microorganisms causing IE. Blood cultures are positive in about 90% of cases, but may be negative in cases of intracellular or fastidious pathogens or after previous antibiotic treatment. Performing blood cultures before any antibiotic treatment is thus mandatory when IE is suspected.
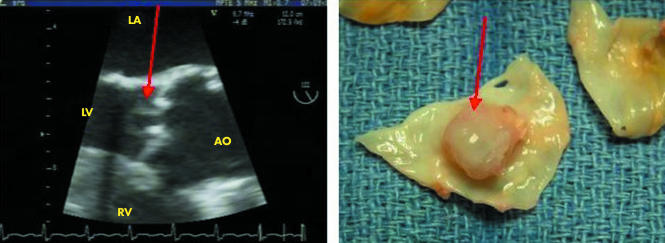
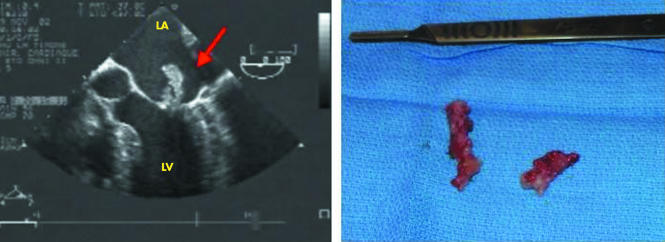
Echocardiography has to be performed in all cases of suspected IE. It combines the advantages of identifying vegetations, abscesses, and new prosthetic dehiscence, which are the hallmarks of IE, assessing the severity of valve damage, detecting cardiac complications, and predicting prognosis and embolic risk.6 Transthoracic echocardiography (TTE) must be performed first, and has a sensitivity of about 75% for the diagnosis of vegetations. Transoesophageal echocardiography (TOE) is mandatory in cases of doubtful transthoracic examination, in prosthetic and pacemaker IE, and when an abscess is suspected. TOE enhances the sensitivity of TTE to about 85–90% for the diagnosis of vegetations, and the additive value of TOE is even more important for the diagnosis of abscesses and, more generally, perivalvar extension, including false aneurysms, perforations, and fistulas. Figures 3 and 4 illustrate the excellent correlation between echocardiographic and anatomic findings.
Figure 3 Echocardiographic/anatomic correlations. Mycotic aneurysm of an aortic leaflet (arrow). AO, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
Figure 4 Echocardiographic/anatomic correlations. Large vegetations on the two mitral leaflets (arrow).
However, both echocardiography and blood cultures may be falsely negative in some patients. To reconcile such differing clinical pictures and underline the value of both blood cultures and echocardiography, Durack et al7 proposed new criteria for IE (table 1). The value of these criteria has been largely confirmed by several studies,8 giving a mean sensitivity of about 80% for the diagnosis of IE. However, we must remember that Duke criteria were developed to help epidemiological and clinical research studies and not for their application to clinical practice. In addition, despite successive attempts at refinement, the diagnostic criteria have some limitations, and are of very limited value in some subgroups.
Table 1 Duke criteria for infective endocarditis (IE)7.
| Major criteria |
| Blood cultures positive for IE |
| Typical microorganisms consistent with IE: |
| Viridans streptococci, Streptococcus bovis, HACEK group, Staphylococcus aureus; or |
| Community acquired enterococci, in the absence of a primary focus; |
| or |
| Microorganisms consistent with IE from persistently positive blood cultures; |
| or |
| Single positive blood culture for Coxiella burnetii or phase I IgG antibody titre >1:800 |
| Evidence of endocardial involvement |
| Echocardiogram positive for IE: |
| Vegetation |
| Abscess |
| New partial dehiscence of prosthetic valve |
| New valvar regurgitation |
| Minor criteria |
| Predisposition, predisposing heart condition, injection drug use |
| Fever, temperature >38°C |
| Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial haemorrhages, Janeway's lesions |
| Immunologic phenomena: glomerulonephritis, Osler's nodes, Roth's spots, rheumatoid factor |
| Microbiological evidence: positive blood culture but does not meet a major criterion |
Diagnosis of IE is definite in the presence of: two major criteria, or one major and three minor criteria, or five minor criteria.
Diagnosis of IE is possible in the presence of: one major and one minor criteria, or three minor criteria.
HACEK, Heamophilus, Actinobacillus, Cardiobacterium, Eikenella, Kingella
Sometimes difficult?
In clinical practice, the diagnosis of IE remains difficult in three main situations:
in the case of normal or doubtful echocardiography
in IE affecting intracardiac devices
in blood culture negative IE (BCNIE).
Negative echocardiography findings may be observed in about 15% of cases of IE. The most frequent explanations for negative echocardiography are very small or absent vegetations and difficulties in identifying vegetations in the presence of pre‐existent severe lesions (mitral valve prolapse, degenerative lesions, prosthetic valves). Conversely, false diagnosis of IE may occur in other situations—for example, it may be difficult to differentiate between vegetations and thrombi, cusp prolapse, cardiac tumours, myxomatous changes, Lambl's excrescences, strands, or non‐infective vegetations (marantic endocarditis). Similarly, diagnosis of a perivalvar abscess may be difficult, even with the use of TOE, when echocardiography is performed very early in the course of the disease, or in the immediate postoperative period following aortic root replacement or Bentall procedure. In these latter situations, a thickening of the aortic wall may be observed in the absence of IE, mimicking abscess formation. More importantly, a normal echocardiogram does not completely rule out IE, even if TOE is performed in expert hands, and a repeat examination has to be performed where there is a high level of clinical suspicion.
Infective endocarditis affecting intracardiac devices is a growing disease. Pacemaker endocarditis is a difficult diagnosis, especially in its chronic forms, in which local symptoms, pneumonia, or even spondylitis may be the first symptoms of the disease.5 In both pacemaker lead IE and prosthetic valve IE (PVIE), the diagnosis is frequently delayed, and both echocardiography and blood cultures are more frequently negative than in IE affecting native valves. For these reasons, Duke criteria have a low sensitivity and cannot reasonably be applied in these two populations, even when proposed modifications are applied.
Blood culture negative IE is the third situation in which the diagnosis of IE is particularly difficult. BCNIE is observed in about 10% of cases and may be explained in the majority of patients by prior antibiotic treatment, underlying the need to perform blood cultures in high risk patients before any antibiotic treatment, when they present with fever. In a recent series,9 63 cases of BCNIE were studied, in which previous antibiotic treatment had been administered in half of the patients. In another significant group of patients with BCNIE, blood cultures are negative because of difficulty in isolating certain microorganisms, including Coxiella burnetii, Bartonella species, Tropheryma whipplei, Brucella species, Chlamydia species, Aspergillus species, and Legionella species. In another more recent series,10 among 348 cases of BCNIE, 167 (48%) were associated with C burnetii, 99 (28%) with Bartonella species, and 5 (1%) with rare, fastidious agents of endocarditis (T whipplei, Abiotropha elegans, Mycoplasma hominis, Legionella pneumophila). Among 73 cases without aetiology, 58 received antibiotic treatment before blood cultures.
Several methods of identification have been proposed to enhance the sensitivity of detection of these microorganisms, including serology, isolation from another site, microscopy of the excised valve, polymerase chain reaction (PCR) identification of the excised valve,11 or even histology of the valve.12 Serology is nearly always systematically performed when IE is suspected. Serology is particularly useful for the diagnosis of Q fever. Recently, positive serology for Q fever was added as a major Duke criterion and this modification has been shown to improve the sensitivity of Duke criteria.13 PCR of the excised valve must be performed in all cases of BCNIE and has been shown to be a very useful adjunct to blood cultures and serology for the diagnosis of IE. However, PCR results may remain positive several months after IE is resolved, and must be interpreted with caution. Finally, histologic examination of the infected valve may also be useful in difficult cases, and histologic criteria have been recently proposed.12
As in patients with pacemaker lead IE or PVIE, Duke criteria are difficult to apply in patients with negative blood culture. Despite several proposed modifications to the Duke criteria, it is clear that they cannot be effective in all situations. No published criteria can replace clinical judgement in the diagnosis of IE.
HOW TO ASSESS THE RISK OF EMBOLISM AND DEATH IN INFECTIVE ENDOCARDITIS
Who is at risk of embolism?
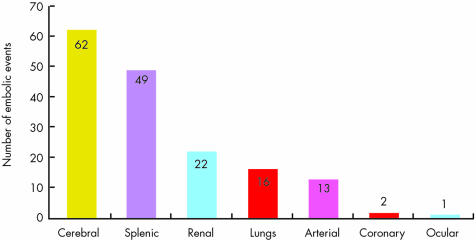
Embolic events are a frequent and life threatening complication of IE.4 They are related to the migration of cardiac valvar vegetations into the major arterial beds, including brain, lungs, spleen, and coronary arteries (fig 5). Embolism is associated with an increased morbidity and mortality in IE. Thus, an accurate prediction of the embolic risk is a desirable goal. Embolism occurs in 20–40% of IE, but its incidence decreases to 9–21% after initiation of antibiotic treatment. Embolism may be asymptomatic in about 20% of patients with IE and must be diagnosed by systematic non‐invasive imaging.
Figure 5 Number of embolic events by site of embolisation in our series of 365 patients with 131 (34%) embolic events (some patients had more than one site of embolisation).
Echocardiography plays a key role in predicting embolic events. Several factors have been associated with an increased risk of embolism, including the size and mobility of vegetations, the localisation of the vegetation on the mitral valve, the increasing or decreasing size of the vegetation under antibiotic treatment, some microorganisms (staphylococci, Streptococcus bovis, Candida species), and biological markers. However, although still controversial, the size and mobility of the vegetations are the most potent independent predictors of new embolic events in patients with IE. In a recent series of 384 patients,14 an embolic event was observed in 131 (34.1%) patients; among them 28 (7.3%) occurred after the initiation of antibiotic treatment. By multivariable analysis, factors associated with a new embolic event were vegetation > 10 mm in length (odds ratio (OR) 9) and severe vegetation mobility (OR 2.4). Thus, patients with vegetations longer than 10 mm are at higher risk of embolism, and this risk is particularly high in patients with very large (> 15 mm) and mobile vegetations. The risk of embolism has been found to be higher in mitral valve IE compared with aortic valve IE in some studies, but this trend was not confirmed by recent series. Finally, the risk of embolism is highest during the first days following the initiation of antibiotic treatment and decreases after two weeks. For this reason, if surgery is decided upon because of a large vegetation to reduce the risk of new embolism, it must be performed early during the first week of antibiotic treatment, because the risk of embolism is greatest during this period.
Who is at risk of death?
Mortality is still high in IE, although it has declined in recent years. Mortality in IE may be associated with factors related to the patient himself or to factors related to the disease, the former being potentially preventable. Thus, the identification of factors associated with increased mortality is a crucial challenge, as it will allow the identification of high risk subgroups in which an aggressive strategy will be potentially useful.
Several markers have previously been identified in past studies, including age, occurrence of complications, staphylococcal infection, serum creatinine concentration, co‐morbidity, and prosthetic valve IE. The most comprehensive study has recently been published by Hasbun et al.15 They studied six month mortality in a series of 513 patients with complicated IE. They found that co‐morbidity, abnormal mental status, moderate to severe congestive heart failure, staphylococcal infection, and medical treatment were independent predictors of mortality. Large vegetations (> 15 mm) were also associated with a worse prognosis in a recent series.14 Echocardiography appears to be predictive of the risk of both embolism and death in IE.
WHAT IS THE OPTIMAL THERAPEUTIC STRATEGY IN INFECTIVE ENDOCARDITIS?
Optimal treatment of IE is based on the combination of prolonged and adapted antibiotic treatment with surgical excision of all the infected tissues in about 50% of patients. Anticoagulant treatment and aspirin are not indicated in infective endocarditis (unless another indication exists) and are contraindicated in IE with severe cerebral complications or mycotic aneurysms.
Although surgical treatment has been shown to have a beneficial effect on outcome in IE,16 the type and optimal timing of surgery are still debated and may vary among patients and centres.
Conservative surgery is more and more frequently performed in mitral valve IE. In a recent series of 78 patients operated on between 1990 and 1999 for IE,17 63 (81%) benefited from conservative surgery with good short term and long term results. In aortic IE, homograft surgery is frequently used, and has been shown to be particularly useful in patients with perivalvar involvement.
In IE early surgery must be performed in three circumstances:
In the case of severe congestive heart failure related to acute mitral or aortic regurgitation. In a recent series of 513 IE patients,16 230 (40%) were operated on. The benefit of surgery was particularly high in patients with moderate to severe heart failure.
In the case of persistent or particularly severe infection. Current guidelines18 recommend surgery when fever and bacteraemia are evident for more than 7–10 days despite adequate antibiotic treatment. Abscess formation, perivalvar involvement, and fungal IE are also considered indications for early surgery.
“Embolic” indications for surgery are more controversial. Surgery is usually performed in cases of recurrent emboli despite appropriate antibiotic treatment. Because of increased risk of embolism in patients with vegetations > 10 mm in length, surgery must also be performed in the presence of a large vegetation (> 10 mm) following one or more clinical or even asymptomatic embolic events, or when the presence of the large vegetation is associated with known other predictors of a complicated course (heart failure, persistent infection under treatment, abscess, prosthetic valve). Surgery may also be considered in the presence of very large (> 15 mm) and mobile vegetations, even in the absence of previous embolism or other prognostic markers. The recently published Euro Heart Survey revealed that the size of the vegetation was one of the reasons for surgery in 54% of cases of native valve IE, and in 25% of PVIE.2
Infective endocarditis: key points
The mortality in IE remains high (10–20% in‐hospital mortality)
The at‐risk patients are changing, with more nosocomial infections, haemodialysis patients, elderly patients, intravenous drug abusers, and intracardiac device infections
Echocardiography and blood culture remain the two main tests for the diagnosis of IE, but both may be negative in some patients
New diagnostic techniques, including polymerase chain reaction (PCR) and histology of the excised valves, may be useful in these patients
Echocardiography plays a key role in the management of IE: for diagnosis, detection of complications, follow up, and prognostic assessment of patients
Finally, the decision of whether or not to operate early in infective endocarditis is always difficult and remains specific for the individual patient. The benefit of surgery must be weighed against the operative risk and take into account the clinical status of the patient and the co‐morbidities.
MAY THE MANAGEMENT OF PATIENTS WITH IE BE DIFFERENT IN SOME SUBGROUPS?
IE affecting intracardiac devices
A quite different and more aggressive therapeutic strategy is needed in patients with intracardiac devices.
Patients with prosthetic valve IE are more difficult to treat by antibiotic therapy alone, and surgical treatment is more frequently needed than for native valves. Surgery is generally considered in early PVIE and must be recommended in PVIE complicated by valve dysfunction, abscess formation, conduction abnormalities, and large vegetations, particularly if staphylococci are the infecting agents. However, even with the use of an aggressive surgical strategy, PVIE is still associated with high in‐hospital and long term mortality.19
Pacemaker lead IE (PMLIE) is a life threatening form of IE, with high morbidity and mortality. In almost all cases of PMLIE, surgical or percutaneous removal of all the implanted material is necessary, together with prolonged antibiotic treatment.
Neurological complications
Cerebral complications represent the second cause of death in IE, after congestive heart failure. The incidence of cerebral complications varies from 25–56% and mortality ranges from 21–83% in these patients. Cerebral complications may result from two main mechanisms.
The migration of cardiac valvar vegetations into the cerebral arteries, causing cerebral infarction. Cerebral arteries and the spleen are the most frequent sites of embolisation in left sided IE, cerebral embolism being observed in 20% of patients with IE in our experience. Cerebral embolism may present as a stroke of varying severity associated with fever, or may be asymptomatic and detected only by computed tomography (CT) scan.
Cerebral hemorrhage may complicate cerebral infarction or be the consequence of the rupture of a mycotic aneurysm. The reported incidence of cerebral mycotic aneurysm is 1.2–5%. It may present with headaches, meningitis, or severe cerebral haemorrhage. Mycotic aneurysms can be detected either by CT scan, or magnetic resonance angiography, but the gold standard remains conventional cerebral angiography. Mycotic aneurysms may heal with antibiotic treatment in 50% of cases.
The main problem in these patients is the optimal timing of valve surgery, when needed. The best therapeutic strategy in patients with cerebral complications is still debated. Some authors recommend delaying cardiac surgery for at least two weeks after cerebral infarction and for at least one month after cerebral hemorrhage, arguing that the risk of neurological deterioration or death is very high when surgery is performed during the first two weeks after a stroke.20 Conversely, other authors reported a very low rate of neurological deterioration in these patients when surgery was performed very soon after an embolic infarction.21 Although recent guidelines18 recommend early surgery (< 72 hours) in the case of focal deficit without haemorrhage on CT scan, surgery must be delayed in the presence of more severe neurological symptoms or cerebral hemorrhage, unless surgery is formally indicated because of severe congestive heart failure.
Embolic events in IE: key points
Embolism occurs in 20–40% of IE cases, but its incidence decreases to 9–21% after initiation of antibiotic treatment
The risk of embolism is especially high during the first two weeks following the initiation of antibiotic treatment
Embolism may be asymptomatic in about 20% of patients with IE and must be diagnosed by systematic non‐invasive imaging
The brain and spleen are the most frequent sites of embolism in IE
Patients with large vegetations (> 10 mm) have a higher risk of embolism. Very large (> 15 mm) and mobile vegetations are associated with an increased mortality
CONCLUSION
IE is undergoing a period of change, with the coexistence of patients with the classic form of infection with a predominance of streptococcal infection, and the emergence of new at‐risk patients with a growing incidence of nosocomial endocarditis and infections affecting intracardiac devices. The fact that IE has several manifestations limits the usefulness of any diagnostic criteria and also limits the efficacy of the usual prophylactic methods. The last decade has seen considerable improvements concerning diagnostic procedures, surgical techniques, and identification of patients at high risk of embolism and death. The challenge for the next 10 years will be to reduce the morbidity and mortality of IE, which remain unacceptably high. An aggressive therapeutic strategy in those patients most at risk, combined with a multidisciplinary approach, will be necessary if we are to achieve this goal.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Myonakis E, Calderwood S B. Infective endocarditis in adults. N Engl J Med 20013451318–1330.Excellent review on infective endocarditis. [DOI] [PubMed] [Google Scholar]
- 2.Tornos P, Iung B, Permanyer‐Miralda G.et al Infective endocarditis in Europe: lessons from the Euro Heart Survey. Heart 200591571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreillon P. Infective endocarditis. Lancet 2004363139–149.Recent review on risk factors, pathogenesis, and management of infective endocarditis. [DOI] [PubMed] [Google Scholar]
- 4.Habib G. Embolic risk in subacute bacterial endocarditis. Role of transesophageal echocardiography. Curr Cardiol Rep 20035129–136. [DOI] [PubMed] [Google Scholar]
- 5.Klug D, Lacroix D, Savoye C.et al Systemic infection related to endocarditis on pacemaker leads. Clinical presentation and management. Circulation 1997952098–2107. [DOI] [PubMed] [Google Scholar]
- 6.Kemp W E, Citrin B, Byrd B F. Echocardiography in infective endocarditis. South Med J 199992744–754.Excellent review about the role of echocardiography in IE. [PubMed] [Google Scholar]
- 7.Durack D T, Lukes A S, Bright D K. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 199496200–209.Reference series allowing definition of actual criteria for endocarditis. It included for the first time echocardiography as a major criterion. [DOI] [PubMed] [Google Scholar]
- 8.Habib G, Derumeaux G, Avierinos J F.et al Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol 1999332023–2029. [DOI] [PubMed] [Google Scholar]
- 9.Lamas C C, Eykin S J. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart 200389258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houpikian P, Raoult D. Blood culture‐negative infective endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine 200584163–173.The largest series to date concerning BCNIE. [DOI] [PubMed] [Google Scholar]
- 11.Greub G, Lepidi H, Rovery C.et al Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am J Med 2005118203–208. [DOI] [PubMed] [Google Scholar]
- 12.Lepidi H, Durack D T, Raoult D. Diagnostic methods, current best practices and guidelines for histologic evaluation in infective endocarditis. Infect Dis Clin North Am 200216339–361. [DOI] [PubMed] [Google Scholar]
- 13.Fournier P E, Casalta J P, Habib G.et al Modification of the diagnostic criteria proposed by the Duke endocarditis service to permit improved diagnosis of Q fever endocarditis. Am J Med 1996100629–633. [DOI] [PubMed] [Google Scholar]
- 14.Thuny F, Di Salvo G, Belliard O.et al Risk of embolism and death in infective endocarditis: prognostic value of echocardiography. A prospective multicenter study. Circulation 2005112744–754. [DOI] [PubMed] [Google Scholar]
- 15.Hasbun R, Vikram H R, Barakat L A.et al Complicated left‐sided native valve endocarditis in adults: risk classification for mortality. JAMA 20032891933–1940.Largest single centre study defining the prognostic factors in IE. [DOI] [PubMed] [Google Scholar]
- 16.Vikram H R, Buenconsejo J, Hasbun R.et al Impact of valve surgery on 6‐month mortality in adults with complicated, left‐sided native valve endocarditis. A propensity analysis. JAMA 20032903207–3214. [DOI] [PubMed] [Google Scholar]
- 17.Iung B, Rousseau‐Paziaud J, Cormier B.et al Contemporary results of mitral valve repair for infective endocarditis. J Am Coll Cardiol 200443386–392. [DOI] [PubMed] [Google Scholar]
- 18.Horstkotte D, Follath F, Gutschik E.et al The task force on infective endocarditis of the European Society of Cardiology. Guidelines on prevention, diagnosis and treatment of infective endocarditis. Eur Heart J 200425267–276.Current European recommendations concerning the management of infective endocarditis with complete review. [DOI] [PubMed] [Google Scholar]
- 19.Habib G, Tribouilloy C, Thuny F.et al Prosthetic valve endocarditis: who needs surgery? A multicentre study of 104 cases. Heart 200591954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eishi K, Kawazoe K, Kuriyama Y.et al Surgical management of infective endocarditis associated with cerebral complications. Multicenter retrospective study in Japan. J Thorac Cardiovasc Surg 19951101745–1755. [DOI] [PubMed] [Google Scholar]
- 21.Piper C, Wierner M, Schulte H D.et al Stroke is not a contraindication for urgent valve replacement in acute infective endocarditis. J Heart Valve Dis 200110703–711. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.