Abstract
Objective
To assess regional mechanical dyssynchrony as a determinant of the degree of functional mitral regurgitation (FMR).
Setting
Tertiary cardiology clinic.
Patients
74 consecutive patients with left ventricular (LV) dysfunction (ejection fraction < 40%, mean 32.2 (SD 7.3)%) were evaluated.
Methods
Effective regurgitant orifice (ERO) area, indices of mitral deformation (systolic valvular tenting, mitral annular contraction) and of global LV function and remodelling (ejection fraction, end systolic volume, sphericity index) and local remodelling (papillary‐fibrosa distance, regional wall motion score index), and tissue Doppler‐derived dyssynchrony index (DI) (regional DI, defined as the standard deviation of time to peak myocardial systolic contraction of eight LV segments supporting the papillary muscles attachment) were measured.
Results
All the assessed variables correlated significantly with ERO. By multivariate analysis, systolic valvular tenting was the strongest independent predictor of ERO (R2 = 0.77, p = 0.0001), with a minor influence of papillary‐fibrosa distance (R2 = 0.77, p = 0.01) and regional DI (R2 = 0.77, p = 0.03). Local LV remodelling (regional wall motion score index: R2 = 0.58, p = 0.001; papillary‐fibrosa distance: R2 = 0.58, p = 0.002) and global remodelling indices (sphericity index: R2 = 0.58, p = 0.003) were the main determinants of systolic valvular tenting, whereas regional DI did not enter into the model. Regional DI was an independent predictor of ERO (R2 = 0.56, p = 0.005) in patients with non‐ischaemic LV dysfunction but not in patients with ischaemic LV dysfunction when these groups were analysed separately.
Conclusions
The degree of FMR is associated mainly with mitral deformation indices. The regional dyssynchrony also has an independent association with ERO but with a minor influence; however, it is not a determinant of FMR in patients with ischaemic LV dysfunction.
Functional mitral regurgitation (FMR) occurs despite a structurally normal mitral valve as a consequence of left ventricular (LV) dysfunction. Systolic mitral valve tenting is well known to be the main determinant of FMR due to apical and posterior papillary muscle displacements, with dilatation contributing.1,2,3
Recently, one of the beneficial effects of cardiac resynchronisation therapy was shown to be the immediate reduction of FMR due to improved coordinated timing of mechanical activation of papillary muscle insertion sites and the remote decrease of FMR secondary to LV reverse remodelling.4,5,6,7 Although anatomical deformation of the mitral valve apparatus secondary to LV remodelling is an important mechanism of FMR, it is conceivable that mechanical dyssynchrony of the LV can contribute
We therefore undertook a prospective quantitative study of patients with LV dysfunction to evaluate the contribution of regional intraventricular dyssynchrony, the degree of deformation of the mitral valve apparatus, and of global and local LV remodelling to FMR.
PATIENTS AND METHODS
Study population
Seventy four consecutive patients with chronic LV systolic dysfunction, defined as LV ejection fraction < 40% measured by two‐dimensional echocardiography, were recruited between March and October 2004. Exclusion criteria were previous implantation of all types of pacemaker, a recent myocardial infarction (< 2 months), greater than mild aortic regurgitation, organic mitral valve disease, atrial fibrillation and clinical or echocardiographic evidence of other cardiac diseases, such as pericardial, infiltrative or congenital heart disease.
Study methods
All patients underwent standard transthoracic echocardiography, including tissue Doppler imaging, to assess global LV function, global and local LV remodelling, intraventricular dyssynchrony, degree of FMR and mitral deformation. All echocardiographic examinations were done with the Vivid 7 machine (GE, Horten, Norway) equipped with a 3MS transducer.
The degree of FMR was quantified with the use of the proximal isovelocity surface area method.8 The proximal isovelocity radius was measured from at least three frames with optimal flow convergence. The largest radius was selected for analysis. The effective regurgitant orifice (ERO) area was calculated with the standard formula.
LV volumes were obtained by the biplane Simpson's method and LV sphericity was estimated by the LV short‐ to long‐axis dimension ratio (D:L) in the end systolic apical four‐chamber view as indices of global LV remodelling. LV ejection fraction was assessed as an index of global LV function.9
The annular‐papillary distance and the wall motion score index (WMSI) at the level of the papillary muscle attachment (regional WMSI) were chosen as the local LV remodelling indices and were measured as previously reported.2 Briefly, on the long‐axis view, the annular‐papillary distance was measured as the distance between the posterior papillary muscle head and the fixed intervalvular fibrosa. WMSI was also calculated for the corresponding eight LV segments supporting the papillary muscle attachment.
End systolic and end diastolic mitral annular areas were obtained from dimensions in the apical four‐ and two‐chambers views, by the ellipsoid assumption that mitral annular area = d1 × d2 × π/4, where d is dimension. Mitral annular contraction was then calculated.1 Diameters of the mitral annulus were measured at the base of the leaflets at the time of maximum valvular opening from the apical four‐ and two‐chamber views. Systolic leaflet deformation, defined as valvular tenting area, was measured as the area enclosed between the annular plane and the mitral leaflets from the parasternal long‐axis view at mid‐systole.1,2
To evaluate LV long‐axis systolic synchronicity, tissue Doppler images were assessed in the apical views (four chamber, two chamber and long axis).10,11 By the adjustment of filter frequency, gain setting, pulse repetition frequency and colour saturation, three consecutive beats were stored and the images were digitised and analysed off line (EchoPac 6.3.6; GE). Myocardial regional systolic velocity curves were constructed from the tissue Doppler colour images in eight LV segments supporting papillary muscle attachment. In this model, the following segments were interrogated for detailed assessment of regional myocardial function: anterior, lateral, inferior and posterior segments at both basal and mid‐levels. By using the onset of QRS complex as a reference point,10 the time to peak sustained systolic contraction for each of these eight LV segments was measured. The regional dyssynchrony index (DI) was derived as the standard deviation of the eight assessed LV segments in each patient.
Reproducibility of measurements
We calculated the intraobserver variability and the interobserver variabilities for papillary‐fibrosa distance, tenting area and regional DI for 20 patients. Intraobserver coefficients were r = 0.95 for the papillary‐fibrosa distance, r = 0.94 for the tenting area (both p = 0.003) and r = 0.94 for regional DI (p = 0.002). The interobserver relation coefficients were r = 0.97 and r = 0.94 for the papillary‐fibrosa distance and the tenting area, respectively (both p = 0.005), and r = 0.94 for regional DI (p = 0.005).
Statistical analysis
Data are expressed as mean (SD) or percentages. Linear regression analysis and partial correlation tests with Pearson's method were used to assess univariate relations. ERO was predicted by stepwise, forward multiple regression analysis that included potential determinants not obviously related to each other. The null hypothesis was rejected at p < 0.05.
RESULTS
Baseline characteristics
The study assessed 74 patients (mean age 64.4 (SD 12) years; 57 men). LV dysfunction was severe but with a wide range (ejection fraction 32.2 (7.3)%, range 15–40%). The aetiology of LV dysfunction was ischaemic in 39 (53%) patients, and 29 (39%) patients had idiopathic dilated cardiomyopathy, two (2.7%) patients were hypertensive, two (2.7%) patients had post‐myocarditis, and two (2.7%) patients had toxic cardiomyopathy (alcoholic or chemotherapy induced). The degree of FMR ranged widely, and 13 patients (17.6%) had no or trace FMR. Among the other 61, ERO ranged from 1 to 67.2 mm2. New York Heart Association functional class distribution was I in three patients (4%), II in 34 (46%) and III in 37 (50%). Forty nine per cent of patients were taking β blockers, 70% diuretics, 65% angiotensin‐converting enzyme inhibitors, 46% spironolactone, 40% nitrates, 13% digoxin and 19% antiarrhythmic drugs.
Mean QRS duration was 134 (34.4) ms and 43 (58%) patients had ECG evidence of a prolonged QRS complex (> 120 ms).
Determinants of degree of FMR
Table 1 shows the correlations of dyssynchrony indices, global and local LV remodelling, and mitral deformation indices with ERO.
Table 1 Correlation between indices of functional mitral regurgitation and effective regurgitant orifice (ERO) in 74 patients with left ventricular dysfunction.
| Index | All patients | Correlation with ERO | |
|---|---|---|---|
| R | p Value | ||
| Dyssynchrony indices | |||
| Dyssynchrony index (ms) | 43.3 (15.2) | 0.39 | 0.001 |
| QRS duration (ms) | 134 (34.4) | 0.18 | NS |
| Mitral deformation indices | |||
| Tenting area (cm2) | 1.4 (0.6) | 0.65 | 0.0001 |
| MAC (%) | 27 (12) | −0.40 | 0.001 |
| Global left ventricular remodelling | |||
| D:L | 0.62 (0.17) | 0.43 | 0.0001 |
| ESVI (ml/m2) | 91.3 (27.4) | 0.32 | 0.01 |
| Local left ventricular remodelling | |||
| WMSI | 1.4 (0.4) | 0.39 | 0.001 |
| PPM‐fibrosa distance (cm) | 3.4 (0.6) | 0.43 | 0.0001 |
D:L, left ventricular short‐ to long‐axis dimension ratio; ESVI, end systolic volume index; MAC, mitral annular contraction; PPM, posterior papillary muscle; WMSI, wall motion score index.
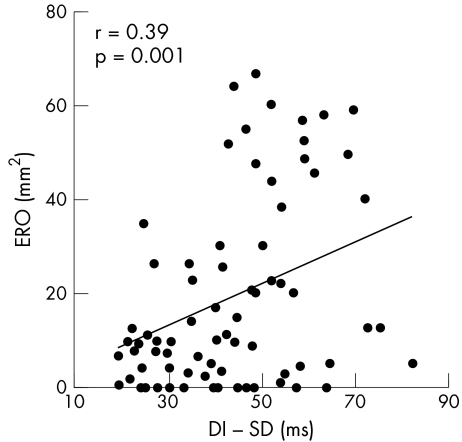
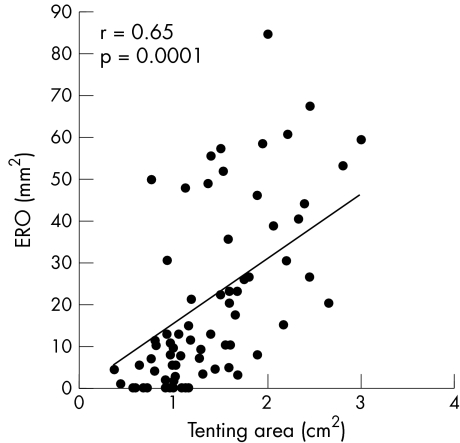
Worth noting is that ERO correlated significantly with regional DI (r = 0.39, p = 0.001) (fig 1) but not with QRS duration. The degree of FMR increased with higher mitral deformation. The strongest correlation with ERO was the systolic mitral tenting area (r = 0.65, p = 0.0001) (fig 2). Larger EROs were also associated with decreased annular contraction (r = −0.40, p = 0.001). Greater LV volumes and D:L were evident with a higher degree of FMR (r = 0.32, p = 0.01 for end systolic volume index, and r = 0.43, p = 0.0001 for D:L). For local LV remodelling, regional WMSI was associated with a larger ERO (r = 0.39,p = 0.001). Also, apical displacement of the papillary muscles, measured as the papillary‐fibrosa distance, was significantly associated with ERO (r = 0.43, p = 0.0001).
Figure 1 Correlation between effective regurgitant orifice (ERO) and regional dyssynchrony index (DI).
Figure 2 Correlation between effective regurgitant orifice (ERO) and tenting area.
Multivariate analysis of ERO determinants (table 2) showed the systolic tenting area to be the most powerful predictor (R2 = 0.77 in all models). Moreover, apical displacement of papillary muscles (papillary‐fibrosa distance) and regional DI, but with minor influence, also contributed independently to larger EROs in all models.
Table 2 Results (p values) of multivariate analysis in four models of the overall study population.
| Model including DI | Adjusted for global LV remodelling | Adjusted for local LV remodelling | Adjusted for mitral deformation | |
|---|---|---|---|---|
| Determinants of ERO | ||||
| DI | 0.001 | 0.01 | 0.02 | 0.03 |
| D:L | 0.001 | NS | NS | |
| ESVI | NS | NS | NS | |
| WMSI | 0.02 | NS | ||
| PPM‐fibrosa distance | 0.002 | 0.01 | ||
| Tenting area | 0.0001 | |||
| MAC | NS | |||
| R2 | 0.37 | 0.52 | 0.62 | 0.77 |
| Determinants of tenting area | ||||
| DI | 0.02 | 0.04 | NS | |
| D:L | 0.0001 | 0.003 | ||
| ESVI | NS | S | ||
| WMSI | 0.001 | |||
| PPM‐fibrosa distance | 0.002 | |||
| R2 | 0.28 | 0.44 | 0.58 |
DI, dyssynchrony index; D:L, left ventricular short to long‐axis dimension ratio; ERO, effective regurgitant orifice; ESVI, end systolic volume index; LV, left ventricular; MAC, mitral annular contraction; PPM, posterior papillary muscle; WMSI, wall motion score index.
Determinants of valvular tenting
Tenting was significantly correlated with regional WMSI (r = 0.43, p = 0.0001), apical papillary muscle displacement (papillary‐fibrosa distance, r = 0.36, p = 0.002), D:L (r = 0.30, p = 0.01) and regional DI (r = 0.27, p = 0.02). Multivariate analysis showed that tenting was determined by regional WMSI, apical displacement of papillary muscles and D:L, independent of regional DI (table 2).
Subgroups analysis
We subdivided the study population into two groups: 39 patients with ischaemia and 35 without. The two groups did not differ significantly in age (65 (12.4) v 63.7 (12.5) years, NS), ERO (18 (11.4) v 20.9 (14.7) mm2, NS), annular contraction (25.6 (10.7)% v 28.3 (12.7)%, NS), ejection fraction (33 (7.3)% v 31.4 (7.3)%, NS), end systolic volume index (70.4 (28.6) v 72.3 (26.4) ml/m2, NS), regional WMSI (1.3 (0.4) v 1.5 (0.4), NS), papillary‐fibrosa distance (3.4 (0.6) v 3.5 (0.6) cm, NS), QRS duration (128.2 (34.4) v 140.4 (33.8) ms, NS) and regional DI (41.7 (14.9) v 45.7 (15.1) ms, NS). Non‐ischaemic patients had a larger systolic mitral tenting area (1.5 (0.6) v 1.1 (0.5) cm2, p = 0.003) and more global remodelling (D:L, 0.68 (015) v 0.57 (0.17), p = 0.009) than ischaemic patients.
In patients with ischaemic LV dysfunction, ERO correlated significantly with regional DI (r = 0.26, p = 0.04), systolic mitral tenting area (r = 0.69, p = 0.0001), decreased annular contraction (r = −0.32, p = 0.04), regional WMSI (r = 0.54, p = 0.0001), papillary‐fibrosa distance (r = 0.49, p = 0.001) and D:L (r = 0.38, p = 0.01). ERO did not correlate significantly with end systolic volume index and QRS duration. Multivariate analysis of ERO determinants showed systolic tenting area to be the most powerful predictor (R2 = 0.70 in all models) (table 3).
Table 3 Results (p values) of multivariate analysis in four models of patients with ischaemia.
| Determinants of ERO | Model including DI | Adjusted for global LV remodelling | Adjusted for local LV remodelling | Adjusted for mitral deformation |
|---|---|---|---|---|
| DI | 0.04 | 0.04 | NS | NS |
| D:L | 0.02 | NS | NS | |
| WMSI | 0.001 | 0.008 | ||
| PPM‐fibrosa distance | 0.02 | 0.03 | ||
| Tenting area | 0.0001 | |||
| MAC | NS | |||
| R2 | 0.18 | 0.34 | 0.50 | 0.70 |
DI, dyssynchrony index; D:L, left ventricular short to long‐axis dimension ratio; ERO, effective regurgitant orifice; LV, left ventricular; MAC, mitral annular contraction; PPM, posterior papillary muscle; WMSI, wall motion score index.
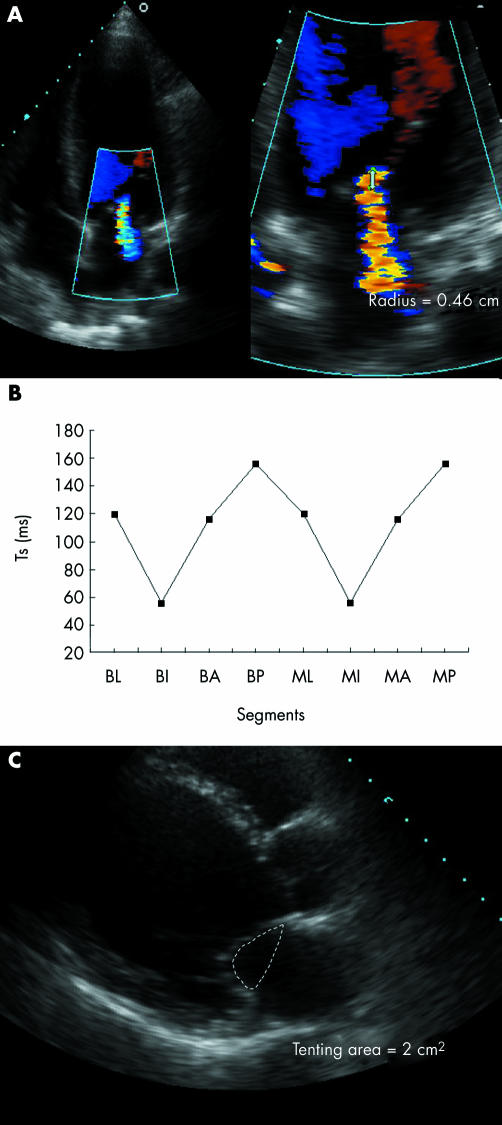
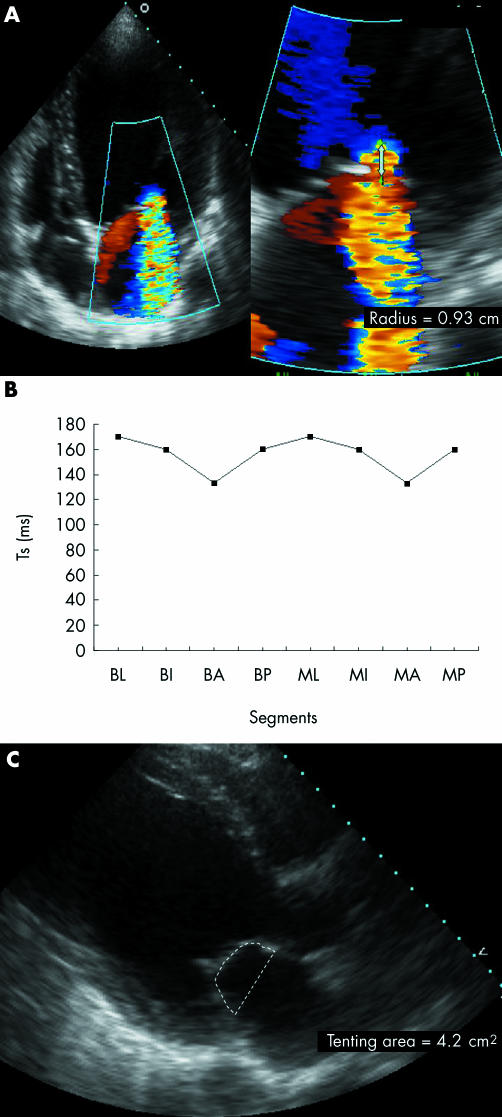
Moreover, regional WMSI and papillary‐fibrosa distance, but with minor influence, also contributed independently to larger EROs in all models, whereas regional DI did not enter into the model (figs 3 and 4).
Figure 3 (A) Apical four‐chamber view showing colour flow Doppler and proximal flow convergence region in a patient with a small effective regurgitant orifice, (B) major regional variation in time to peak sustained systolic contraction (Ts) between the left ventricular segments supporting the papillary muscles, and (C) small tenting area. A, anterior; B, basal; I, inferior; L, lateral; M, mid; P, posterior.
Figure 4 (A) Apical four‐chamber view showing colour flow Doppler and proximal flow convergence region in a patient with a large effective regurgitant orifice, (B) small regional variation in time to peak sustained systolic contraction (Ts) between the left ventricular segments supporting the papillary muscles, and (C) large tenting area. A, anterior; B, basal; I, inferior; L, lateral; M, mid; P, posterior.
In patients with non‐ischaemic LV dysfunction, ERO correlated significantly with regional DI (r = 0.52, p = 0.001), systolic mitral tenting area (r = 0.67, p = 0.0001), decreased annular contraction (r = −48, p = 0.003), papillary‐fibrosa distance (r = 0.61, p = 0.0001), end systolic volume index (r = 0.43, p = 0.01) and D:L (r = 0.50, p = 0.003). ERO did not correlated significantly with regional WMSI and QRS duration. Multivariate analysis of ERO determinants showed the systolic tenting area to be the most powerful predictor (R2 = 0.56 in all models) (table 4). Moreover, regional DI, papillary‐fibrosa distance and D:L, but with minor influence, also contributed independently to larger EROs in all models.
Table 4 Results (p values) of multivariate analysis in four models of patients without ischaemia.
| Determinants of ERO | Model including DI | Adjusted for global LV remodelling | Adjusted for local LV remodelling | Adjusted for mitral deformation |
|---|---|---|---|---|
| DI | 0.001 | 0.004 | 0.04 | 0.005 |
| D:L | 0.004 | 0.02 | 0.02 | |
| ESVI | NS | NS | NS | |
| PPM‐fibrosa distance | 0.01 | 0.003 | ||
| Tenting area | 0.0001 | |||
| MAC | NS | |||
| R2 | 0.36 | 0.53 | 0.53 | 0.56 |
DI, dyssynchrony index; D:L, left ventricular short to long‐axis dimension ratio; ERO, effective regurgitant orifice; ESVI, end systolic volume index; LV, left ventricular; MAC, mitral annular contraction; PPM, posterior papillary muscle.
DISCUSSION
This study shows that, when all known potential variables are considered, systolic mitral valvular tenting is the major determinant of ERO in patients with FMR, with a smaller contribution from factors of local LV remodelling such as the apical displacement of the papillary muscles and regional mechanical dyssynchrony. Systolic mitral valvular tenting is directly determined by local and global LV remodelling, whereas regional dyssynchrony has no or a minimal additional independent association with the degree of tenting. Moreover, regional dyssynchrony is not an independent predictor of the degree of FMR in patients with ischaemic LV dysfunction.
Mechanical insights
FMR is the result of incomplete closure of normal leaflets without organic mitral lesions.12,13 Mitral valvular tenting directly determines the ERO of FMR and it is characterised by insufficient systolic leaflet body displacement towards the annulus,1 with coaptation limited to the leaflet tips.14 Annular alterations have an adjunct role.3 Local LV remodelling, inducing excessive papillary muscle displacement, is the main determinant of tenting and FMR. Moreover, evidence that the immediate reduction of FMR after cardiac resynchronisation therapy is independent of modifications of the mitral apparatus and LV remodelling underscores the potential role of dyssynchrony as an adjunctive independent mechanism in determining the degree of FMR.4,5,15 Starting from valvular deformations leading to FMR, however, the determinants of tenting are multiple and complex and can have a different impact on FMR in subsets of patients with LV dysfunction. Indeed, as this study shows, in patients with ischaemic LV dysfunction, mitral tenting and local LV remodelling, but not regional dyssynchrony, are independent predictors of the degree of FMR. These findings suggest that local remodelling of the region of the LV supporting the papillary muscles is necessary for the development of FMR, whereas regional dyssynchrony may only be contributing. This local alteration is the effect of direct ischaemic lesions.
In patients with non‐ischaemic LV dysfunction, although mitral tenting is the main determinant of the degree of FMR, regional dyssynchrony aggravates FMR independently of LV geometry. LV dyssynchrony potentially contributes to FMR by several mechanisms. Firstly, the mechanical dyssynchrony between LV segments supporting the papillary muscles produces uncoordinated regional LV mechanical activation in these segments, resulting in geometric changes in mitral leaflet attachments and implying tethering of the mitral leaflets.5 Secondly, a positive pressure gradient develops between the left atrium and left ventricle due to improper timing of atrioventricular relaxation and contraction cycles, which can create diastolic FMR during incomplete mitral valve closure.16,17 Lastly, LV dyssynchrony decreases LV contraction efficiency and closing forces, thereby impairing mitral valve tenting.4 This last mechanism seems to be the main mechanism by which LV dyssynchrony increases FMR in patients with LV dysfunction. Indeed, biventricular pacing reduces FMR severity both at rest and during exercise.15 During exercise, in patients with a cardiac resynchronisation device turned on, the increase in FMR is related to changes in mitral valve deformation as estimated by the systolic tenting area, whereas in patients with a cardiac resynchronisation device turned off, the exercise‐induced increase of FMR is determined by an inadequate increase in mitral closing force as estimated by LV dp/dt.15 This evidence clearly shows that the effect of LV dyssynchrony is mediated by a decrease of dp/dt, which impairs effective mitral valve closure.
Potential clinical implications
Besides drug‐induced afterload reduction, surgical correction has been shown to be effective in correcting FMR in end stage cardiomyopathy and nowadays is considered the best therapeutic option. Several trials and isolated reports have shown, however, the beneficial effects of cardiac resynchronisation therapy in reducing FMR in patients with LV dysfunction.4,5,6,7
Cardiac resynchronisation therapy can reduce the degree of FMR by several potential mechanisms that have as their common final pathway an increased leaflet coaptation surface with reduced mitral valvular tenting. In other words, cardiac resynchronisation therapy appears to coordinate the tethering forces on the papillary muscles and to improve global systolic function,4,5 effectively counteracting the increased tethering forces that impair mitral valve competence and acutely decrease systolic tenting area. In the later phase, reverse LV remodelling has an additive role.6,7 Additionally, atrioventricular sequential pacing reduces the diastolic component of FMR.18
Study limitations
The degree of MR was estimated quantitatively but FMR is a dynamic condition that depends on preload, afterload and drug status. Although echocardiography provided merely a brief snapshot of the severity of FMR at the time of the study, all patients received optimal medical treatment and no drug treatment was altered before the study.
Conclusions
A larger ERO of FMR is associated mainly with excess mitral valvular tenting, which is determined by the degree of local LV remodelling. Regional dyssynchrony is also independently associated with ERO but it has a minor influence and is not a determinant of mitral valvular tenting. Lastly, regional dyssynchrony is an independent predictor of the degree of FMR in patients with non‐ischaemic but not in patients with ischaemic LV dysfunction.
Abbreviations
DI - dyssynchrony index
D:L - short‐ to long‐axis dimension ratio
ERO - effective regurgitant orifice
FRM - functional mitral regurgitation
LV - left ventricular
WMSI - wall motion score index
References
- 1.Yiu S F, Enriquez Sarano M, Tribouilloy C.et al Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: a quantitative clinical study. Circulation 20001021400–1406. [DOI] [PubMed] [Google Scholar]
- 2.Agricola E, Oppizzi M, Maisano F.et al Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 20045326–334. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji Y, Kumanohoso T, Yoshifuku S.et al Isolated annular dilation does not usually cause important functional mitral regurgitation: comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol 2002391651–1656. [DOI] [PubMed] [Google Scholar]
- 4.Breithardt O A, Stellbrink C, Herbots L.et al Cardiac resynchronization therapy can reverse abnormal myocardial strain distribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol 200342486–494. [DOI] [PubMed] [Google Scholar]
- 5.Kanzaki H, Bazaz R, Schwartzman D.et al A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol 2004441619–1625. [DOI] [PubMed] [Google Scholar]
- 6.Linde C, Leclercq C, Rex S.et al Long‐term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 200240111–118. [DOI] [PubMed] [Google Scholar]
- 7.St John Sutton M G, Plappert T, Abraham W T, Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group et al Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 20031071985–1990. [DOI] [PubMed] [Google Scholar]
- 8.Enriquez‐Sarano M, Seward J B, Bailey K R.et al Effective regurgitant orifice area: a noninvasive Doppler development of an old hemodynamic concept. J Am Coll Cardiol 199423443–451. [DOI] [PubMed] [Google Scholar]
- 9.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 10.Yu C M, Fung W H, Lin H.et al Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 200291684–688. [DOI] [PubMed] [Google Scholar]
- 11.Knebel F, Reibis R K, Bondke H J.et al Tissue Doppler echocardiography and biventricular pacing in heart failure: patient selection, procedural guidance, follow‐up, quantification of success. Cardiovasc Ultrasound 2004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godley R W, Wann L S, Rogers E W.et al Incomplete mitral leaflet closure in patients with papillary muscle dysfunction. Circulation 198163565–571. [DOI] [PubMed] [Google Scholar]
- 13.Boltwood C M, Tei C, Wong M.et al Quantitative echocardiography of the mitral complex in dilated cardiomyopathy: the mechanism of functional mitral regurgitation. Circulation 198368498–508. [DOI] [PubMed] [Google Scholar]
- 14.Perloff J K, Roberts W C. The mitral apparatus: functional anatomy of mitral regurgitation. Circulation 197246227–239. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Mélon P, Sakalihasan N.et al Effect of cardiac resynchronization therapy on functional mitral regurgitation in heart failure. Am J Cardiol 2004941462–1465. [DOI] [PubMed] [Google Scholar]
- 16.Grines C L, Bashore T M, Boudoulas H.et al Functional abnormalities in isolated left bundle branch block: the effect of interventricular asynchrony. Circulation 198979845–853. [DOI] [PubMed] [Google Scholar]
- 17.Appleton C P, Basnight M A, Gonzalez M S. Diastolic mitral regurgitation with atrioventricular conduction abnormalities: relation of mitral flow velocity to transmitral pressure gradients in conscious dogs. J Am Coll Cardiol 199118843–849. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura R A, Hayes D L, Holmes D R., Jret al Mechanism of hemodynamic improvement by dual‐chamber pacing for severe left ventricular dysfunction: an acute Doppler and catheterization hemodynamic study. J Am Coll Cardiol 199525281–288. [DOI] [PubMed] [Google Scholar]