Abstract
Objective
To compare the extent and distribution of focal fibrosis by gadolinium contrast‐enhanced magnetic resonance imaging (MRI; delayed hyperenhancement) in severe left ventricular (LV) hypertrophy in patients with pressure overload caused by aortic stenosis (AS) and with genetically determined hypertrophic cardiomyopathy (HCM).
Methods
44 patients with symptomatic valvular AS (n = 22) and HCM (n = 22) were studied. Cine images were acquired with fast imaging with steady‐state precession (trueFISP) on a 1.5 T scanner (Sonata, Siemens Medical Solutions). Gadolinium contrast‐enhanced MRI was performed with a segmented inversion–recovery sequence. The location, extent and enhancement pattern of hyperenhanced myocardium was analysed in a 12‐segment model.
Results
Mean LV mass was 238.6 (SD 75.3) g in AS and 205.4 (SD 80.5) g in HCM (p = 0.17). Hyperenhancement was observed in 27% of patients with AS and in 73% of patients with HCM (p < 0.01). In AS, hyperenhancement was observed in 60% of patients with a maximum diastolic wall thickness ⩾ 18 mm, whereas no patient with a maximum diastolic wall thickness < 18 mm had hyperenhancement (p < 0.05). Patients with hyperenhancement had more severe AS than patients without hyperenhancement (aortic valve area 0.80 (0.09) cm2v 0.99 (0.3) cm2, p < 0.05; maximum gradient 98 (22) mm Hg v 74 (24) mm Hg, p < 0.05). In HCM, hyperenhancement was predominant in the anteroseptal regions and patients with hyperenhancement had higher end diastolic (125.4 (36.9) ml v 98.8 (16.9) ml, p < 0.05) and end systolic volumes (38.9 (18.2) ml v 25.2 (1.7) ml, p < 0.05). The volume of hyperenhancement (percentage of total LV myocardium), where present, was lower in AS than in HCM (4.3 (1.9)% v 8.6 (7.4)%, p< 0.05). Hyperenhancement was observed in 4.5 (3.1) and 4.6 (2.7) segments in AS and HCM, respectively (p = 0.93), and the enhancement pattern was mostly patchy with multiple foci.
Conclusions
Focal scarring can be observed in severe LV hypertrophy caused by AS and HCM, and correlates with the severity of LV remodelling. However, focal scarring is significantly less prevalent in adaptive LV hypertrophy caused by AS than in genetically determined HCM.
Keywords: aortic stenosis, focal fibrosis, hypertrophic cardiomyopathy, magnetic resonance imaging
Remodelling in left ventricular (LV) hypertrophy is accompanied by several structural changes. Interstitial and replacement fibrosis are among the morphological alterations that have been observed in LV hypertrophy caused by pressure overload and in genetically determined hypertrophic cardiomyopathy (HCM).1,2,3,4,5
HCM is a common genetic cardiac disease with notable heterogeneity in clinical expression, natural history and prognosis. HCM is defined anatomically by typical histological features, namely disarray, small vessel disease and fibrosis. Clinical presentation can be in any phase of life and most patients have a favourable prognosis. A subset of 10–20% of patients with HCM have a higher risk of sudden death, and myocardial scarring and disarray have been postulated as possible arrhythmogenic substrates for sudden death.6,7
Myocardial fibrosis has also been described in patients with adaptive LV hypertrophy secondary to aortic stenosis (AS). Recently, a close structure–function correlation in patients with AS was observed during the progression from compensated hypertrophy to failure. With worsening of fibrosis and myocyte degeneration, LV end diastolic pressure increases and later ejection fraction decreases.5
Transmural and non‐transmural scarring can now be detected by gadolinium contrast‐enhanced magnetic resonance imaging (MRI) as delayed hyperenhancement, as has recently been reported for myocardial infarction.8,9 Additionally, visualisation of focal scarring in HCM by MRI has recently been described.10,11 Interestingly, virtually no data are available on the detection of scarring in adaptive LV hypertrophy. We therefore hypothesised that gadolinium contrast‐enhanced MRI can also visualise focal scarring in severe adaptive LV hypertrophy.
To test this hypothesis we assessed the presence of delayed hyperenhancement in severe adaptive LV hypertrophy due to AS by gadolinium contrast‐enhanced MRI and compared the extent and distribution with those observed in genetically determined HCM.
METHODS
Patients
Cardiac catheterisation was performed in all patients with AS. Coronary artery disease without evidence of previous myocardial infarction was diagnosed in three of these patients. Importantly, none of these three patients had hyperenhancement on MRI. In all other patients, significant coronary artery disease was excluded (< 50% lumen diameter reduction).
HCM was diagnosed by the presence of a non‐dilated and hypertrophied left ventricle on two‐dimensional echocardiography (wall thickness ⩾ 15 mm) in the absence of another disease that could account for the hypertrophy. Resting LV outflow tract obstruction was defined as a peak instantaneous gradient > 30 mm Hg by continuous‐wave Doppler echocardiography. Additionally, significant coronary artery disease at catheterisation was excluded within two weeks of the MRI studies in 10 patients. In a further eight patients with HCM enrolled from our outpatient department, coronary artery disease had been excluded at catheterisation in a previous hospital stay. In both subgroups, no patient had a history of ablation therapy or myocarditis. All patients gave informed consent to participate in the study.
MRI protocol
MRI studies were performed on a 1.5 T scanner (Sonata, Siemens Medical Solutions, Erlangen, Germany) with a phased array receiver coil and breath‐hold acquisitions prospectively triggered by the ECG. Cine images were acquired in multiple short‐axis and long‐axis views with fast imaging with steady‐state precession (trueFISP; slice thickness 8 mm, echo time 1.53 ms, pixel bandwidth 1.085 Hz, repetition time 3.14 ms, temporal resolution about 43 ms, matrix 256 × 202). The number of k‐space lines for each heart beat was adjusted to permit the acquisition of 20 cardiac phases covering systole and diastole in a cardiac cycle. The field of view was 340 mm on average and adapted to the size of the patient, leading to a spatial resolution of about 1.3 × 1.6 × 8 mm. LV volumes, mass and ejection fraction were calculated in the serial short‐axis slices (usually 8–14) with no gap between the slices. Additionally, several slices of the aortic orifice for calculation of the valve area were acquired in patients with AS. A gadolinium‐based contrast agent (0.1 mmol/kg) was then given intravenously, and contrast‐enhanced images were acquired in a segmented inversion–recovery sequence in the same views used for cine MRI 10–20 min after contrast administration.12
Image analysis
LV function and volumes were calculated by planimetry of the endocardial and epicardial borders from the serial short‐axis views. Ejection fraction, end diastolic volume and end systolic volume were analysed. Hyperenhanced myocardium was defined as an image intensity level > 2 SD above the mean of remote myocardium. The location, extent and enhancement pattern (subendocardial, mid‐wall, subepicardial and transmural) of hyperenhanced myocardium was analysed in a 12‐segment model based on short‐axis views (basal, medial and apical) that were divided into anteroseptal, anterolateral, inferoseptal and inferolateral segments. Volumes of hyperenhanced areas were calculated by planimetry in all short‐axis slices and the total volume of hyperenhancement was expressed as a percentage of total myocardium. In patients with AS, valve area was assessed by direct planimetry as described previously.13,14,15,16,17 All imaging studies were analysed separately by two observers (KD and BD). Only studies in which both readers agreed on the presence of hyperenhancement were analysed quantitatively for volumes and distribution of hyperenhancement by a single reader (KD).
Statistical analysis
Results are shown as mean (SD). Differences in mean values between two groups were analysed by Student's t test. The χ2 test was performed to compare frequencies between groups. A level of significance of p < 0.05 was accepted as significant.
RESULTS
Patient characteristics
Table 1 lists patient characteristics. The prevalence of angina pectoris, dyspnoea and syncope were not different in HCM and AS. Sinus rhythm was more prevalent in AS than in HCM (p < 0.05). In patients with AS mean aortic valve area assessed by planimetry was 0.94 (0.28) cm2. Fifty five per cent of the patients (n = 12) with HCM had LV outflow tract obstruction > 30 mm Hg. In HCM, 19 patients (86%) had asymmetric LV hypertrophy and three patients (14%) had apical hypertrophy.
Table 1 Characteristics of patients with aortic stenosis (AS) and hypertrophic cardiomyopathy (HCM).
| AS (n = 22) | HCM (n = 22) | |
|---|---|---|
| Age (year) | 64 (13) | 58 (14) |
| Men | 73% | 64% |
| Angina pectoris | 45% | 45% |
| Dyspnoea | 82% | 95% |
| Syncope | 18% | 14% |
| Sinus rhythm | 86% | 55%* |
| AVA (cm2) | 0.94 (0.28) | NA |
| MWT ⩾18 mm | 45% | 77%* |
| Echocardiographic gradient (mm Hg) | ||
| <30 | 0 | 45% |
| >30 | 100% | 55% |
| Pattern of hypertrophy | ||
| ASH | NA | 19 (86%) |
| Apical | NA | 3 (14%) |
Data are mean (SD) or number (%).
*p<0.05 v AS.
ASH, asymmetric septal hypertrophy; AVA, aortic valve area by magnetic resonance imaging planimetry; MWT, maximum diastolic wall thickness; NA, not applicable.
MRI results
Mean LV mass was 238.6 (75.3) g (range 137–388 g) in AS and 205.4 (80.5) g (range 85–321 g) in HCM (p = 0.17) (table 2). In AS, the maximum diastolic wall thickness ranged from 12–25 mm (mean 17.9 (3.9)) and in HCM from 16–33 mm (mean 21.3 (4.3), p < 0.01). Mean ejection fraction was 52.7 (17.3)% in AS and 71.1 (7.6)% in HCM (p < 0.001). Hyperenhancement was observed in 27% of patients with AS and in 73% in patients with HCM (p < 0.01).
Table 2 Magnetic resonance imaging results of patients with hypertrophic cardiomyopathy (HCM) and aortic stenosis (AS).
| AS (n = 22) | HCM (n = 22) | |
|---|---|---|
| LV mass (g) | 238.6 (75.3) | 205.4 (80.5) |
| MWT (mm) | 17.9 (3.9) | 21.3 (4.3)* |
| EF (%) | 52.7 (17.3) | 71.1 (7.6)* |
| EDV (ml) | 173.5 (73.2) | 118.2 (34.4)* |
| ESV (ml) | 89.7 (62.4) | 35.1 (16.6)* |
| HE present | 27% | 73%* |
Data are mean (SD) or number (%).
*p<0.01 v AS.
EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; HE, hyperenhancement; LV, left ventricular; MWT, maximum diastolic wall thickness.
Focal hyperenhancement
The volume of hyperenhancement (percentage of total LV myocardium) was 4.3 (1.9)% in AS and 8.6 (7.4)% in HCM (p < 0.05; table 3). Hyperenhancement was present in 4.5 (3.1) and 4.6 (2.7) segments in AS and HCM, respectively (p = 0.93).
Table 3 Characteristics of patients with aortic stenosis (AS) and hypertrophic cardiomyopathy (HCM) with (+) and without (–) focal hyperenhancement (HE).
| AS (n = 22) | HCM (n = 22) | |||
|---|---|---|---|---|
| HE− (n = 16) | HE+ (n = 6) | HE− (n = 6) | HE+ (n = 16) | |
| LV mass (g) | 221.6 (60.8) | 283.8 (96.8) | 156.7 (68.5) | 223.6 (78.8) |
| MWT (mm) | 16.3 (3.0) | 22.0 (3.1)* | 20.5 (3.2) | 21.6 (4.7) |
| EF (%) | 49.4 (17.7) | 61.7 (13.7) | 74.5 (4.0) | 69.9 (8.3) |
| EDV (ml) | 179.2 (76.3) | 158.3 (68.5) | 98.8 (16.9) | 125.4 (36.9)† |
| ESV (ml) | 96.5 (68.6) | 71.5 (40.8)‡ | 25.2 (1.7) | 38.9 (18.2)† |
| AVA (cm2) | 0.99 (0.3) | 0.80 (0.09)* | NA | NA |
| Echocardiographic gradient (mm Hg) | 74 (24) | 98 (22)* | NA | NA |
| HE volume (% LV) | NA | 4.3 (1.9) | NA | 8.6 (7.4)§ |
| Number of segments with HE | NA | 4.5 (3.1) | NA | 4.6 (2.7) |
| Location of HE | ||||
| Anteroseptal | NA | 29% | NA | 48%¶ |
| Anterolateral | NA | 15% | NA | 12% |
| Inferoseptal | NA | 15% | NA | 22% |
| Inferolateral | NA | 41% | NA | 18% |
Data are mean (SD) or number (%).
*p<0.05 v AS HE−; †p<0.05 v HCM HE−; ‡p<0.05 v HCM HE+; §p<0.05 v AS HE+; ¶p<0.05 v anterolateral, inferoseptal and inferolateral in HCM.
EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; LV, left ventricular; MWT, maximum diastolic wall thickness; NA, not applicable.
In AS, LV mass of patients with hyperenhancement tended to be higher than in patients without hyperenhancement (p = 0.19). Maximum diastolic wall thickness was significantly higher in patients with than in patients without hyperenhancement (p < 0.01). Specifically, 60% of all patients with a maximum wall thickness ⩾ 18 mm had hyperenhancement, whereas no patient with a maximum wall thickness < 18 mm had hyperenhancement (p < 0.05). Aortic valve area was smaller (p < 0.05) and the maximum gradient was higher (p < 0.05) in patients with hyperenhancement. In AS, hyperenhancement was equal in all segments. The enhancement pattern was mostly patchy and did not correspond to coronary artery disease. Additionally, significant coronary artery disease had been excluded in all patients with hyperenhancement at catheterisation. Interestingly, in one patient the subendocardium was predominantly involved, but coronary angiography was completely normal in this patient.
In HCM, mostly anteroseptal segments were affected and hyperenhancement was predominant in the most hypertrophied regions. The enhancement pattern was patchy with multiple foci and predominantly the mid‐wall of the left ventricle was involved. In HCM, LV mass of patients with hyperenhancement tended to be higher than in patients without hyperenhancement (p = 0.08). Patients without hyperenhancement had significantly lower end diastolic and end systolic volumes (both p < 0.05) (figs 1–3).
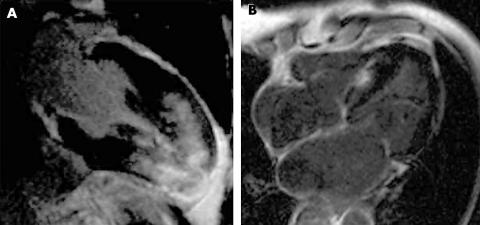
Figure 1 (A) Extensive hyperenhancement in a 28‐year‐old patient with hypertrophic cardiomyopathy (HCM). (B) Patchy septal hyperenhancement in a 64‐year‐old patient with HCM.
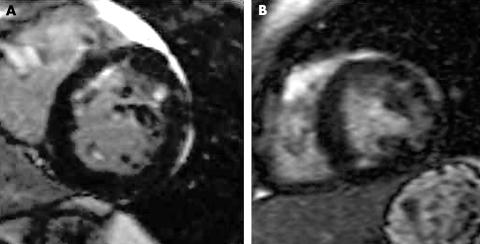
Figure 2 (A) Patchy anteroseptal and anterolateral hyperenhancement in a 46‐year‐old patient with hypertrophic cardiomyopathy (HCM). (B) Patchy lateral and inferior hyperenhancement in a 33‐year‐old patient with HCM.
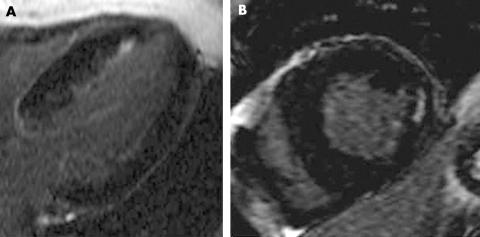
Figure 3 (A) Diffuse patchy and subendocardial septal hyperenhancement in a 61‐year‐old patient with severe aortic valve stenosis. (B) Patchy lateral mid‐wall hyperenhancement in a 29‐year‐old patient with severe bicuspid aortic stenosis.
DISCUSSION
Our data suggest that focal scarring is a characteristic of severe LV hypertrophy and partially independent of aetiology. Focal scarring was more common in genetically determined HCM than in adaptive LV hypertrophy caused by pressure overload in patients with AS. In patients with AS, scarring was associated with wall thickness with a cut off at 18 mm maximum diastolic wall thickness. The predominant locations of scarring in HCM were anteroseptal segments and the most hypertrophied regions. In both AS and HCM, the contrast enhancement pattern was predominantly patchy with multiple foci and mainly involved the mid‐ventricular wall.
Focal scarring in AS
Focal scarring was observed in 27% of patients with AS. To our knowledge the current study is the first to report this phenomenon in severe adaptive LV hypertrophy. We observed a clear correlation between the presence of focal scarring and the severity or concentric LV remodelling as well as the severity of AS. Specifically, focal scarring was never observed below a maximum diastolic wall thickness of 18 mm, but in 60% of patients with a maximum diastolic wall thickness above 18 mm. The contrast enhancement pattern was patchy with multiple foci and mainly involved the mid‐ventricular wall and did not correspond to any epicardial coronary artery distribution. Additionally, significant epicardial coronary artery disease was excluded in all patients. This observation suggests that focal scarring occurs in severe adaptive LV hypertrophy above this critical wall thickness and the exhaustion of cellular adaptation results in myocyte degeneration and replacement fibrosis.5 In patients with AS, myocardial fibrosis has been described in histopathological studies,3 and a close structure–function correlation has been found in the progression from compensated hypertrophy to failure.5 It has to be noted, however, that an overall increase of diffuse fibrosis cannot be visualised by gadolinium contrast‐enhanced MRI. Gadolinium contrast‐enhanced MRI is only sensitive to regional differences in gadolinium concentration, because the technique depends on the ability to suppress signals of presumably normal myocardium.9 Thus, focal fibrosis hyperenhances and diffuse interstitial fibrosis does not. Focal hyperenhancement in LV hypertrophy caused by pressure overload therefore most likely represents replacement scarring, where myocyte hypertrophy and reactive fibrosis are followed by myocyte degeneration.
Focal scarring in HCM
In LV hypertrophy caused by HCM, we observed hyperenhancement in 73% of patients. Hyperenhancement was located predominantly anteroseptally, whereas anterolateral, inferolateral and inferoseptal segments were equally affected. Mostly the middle third of the ventricular wall was involved and the hyperenhancement pattern did not correspond to any epicardial coronary artery distribution. As in AS, coronary angiography excluded significant epicardial coronary artery disease in all patients. Thus, scarring due to epicardial coronary artery disease is unlikely. These findings are consistent with several pathological studies, where scarring has been described as a common finding in HCM,1,2,18,19 and our data on the prevalence of focal scarring detected by contrast‐enhanced MRI are consistent with the findings of very recently published studies.10,11 Of note, we observed a correlation between the presence of focal scarring and the severity of LV remodelling; patients with HCM with hyperenhancement had significantly larger ventricles and a strong trend towards more severe LV hypertrophy than patients without hyperenhancement. This observation may indicate that replacement scarring in HCM partially depends on mechanogenic stimuli, although the underlying disease itself is genetically determined.
Clinical relevance
More patients with AS are now evaluated by MRI because it has been shown that aortic valve area can be assessed accurately by planimetry.13,14,15,16 As a practical consequence, we propose that contrast‐enhanced MRI be added in the evaluation of these patients, as presence of hyperenhancement may indicate adverse remodelling on the basis of a self‐perpetuating process of myocyte degeneration and replacement fibrosis. In HCM, myocardial scarring and disarray have been postulated as possible arrhythmogenic substrates for sudden death. Especially young patients who die suddenly seem to have severe disarray with only mild hypertrophy and fibrosis. In contrast, fibrosis is probably the substrate for premature death from heart failure and a risk factor for primary ventricular arrhythmia in older patients.18,19 Interestingly, in a recent study the extent of hyperenhancement assessed by gadolinium contrast‐enhanced MRI was linked with progressive disease and markers of clinical risk for sudden death in patients with HCM.11,20 These patients may therefore be candidates for contrast‐enhanced MRI for risk stratification. As recent studies have also linked distinct troponin T mutations to the pattern of hypertrophy,18,21 contrast‐enhanced MRI may also permit better definition of the disease phenotype and therefore provide new insights into the pathophysiology and disease progression of HCM. Whether hyperenhancement is linked to ventricular arrhythmia in patients with AS requires further studies.
In conclusion, gadolinium contrast‐enhanced MRI permits visualisation of focal scarring in HCM and LV hypertrophy caused by pressure overload. Focal scarring is a common finding in HCM and is less common in LV hypertrophy caused by pressure overload. Detection of focal scarring in LV hypertrophy caused by pressure overload may predict an advanced adverse remodelling process.
Acknowledgements
The expert technical support of Ms Marion Merdian, Ardiana Suli and Elisabeth Krinner is gratefully acknowledged.
Abbreviations
AS - aortic stenosis
HCM - hypertrophic cardiomyopathy
LV - left ventricular
MRI - magnetic resonance imaging
Footnotes
Competing interests: None declared.
References
- 1.Lamke G T, Allen R D, Edwards W D.et al Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy: a study of 204 cases (1996–2000). Cardiovasc Pathol 200312149–158. [DOI] [PubMed] [Google Scholar]
- 2.Varnava A M, Elliott P M, Sharma S.et al Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 200084476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krayenbuehl H P, Hess O M, Monrad E S.et al Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 198979744–755. [DOI] [PubMed] [Google Scholar]
- 4.Cohn J N, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 200035569–582. [DOI] [PubMed] [Google Scholar]
- 5.Hein S, Arnon E, Kostin S.et al Progression from compensated hypertrophy to failure in the pressure‐overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003107984–991. [DOI] [PubMed] [Google Scholar]
- 6.Maron B J, Gardin J M, Flack J M.et al Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 199592785–789. [DOI] [PubMed] [Google Scholar]
- 7.Maron B J, McKenna W J, Danielson G K.et al American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents, and European Society of Cardiology Committee for Practice Guidelines. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003241965–1991. [DOI] [PubMed] [Google Scholar]
- 8.Wagner A, Mahrholdt H, Holly T A.et al Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003361374–379. [DOI] [PubMed] [Google Scholar]
- 9.Wu E, Judd R M, Vargas J D.et al Visualisation of presence, location, and transmural extent of healed Q‐wave and non‐Q‐wave myocardial infarction. Lancet 200135721–28. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury L, Mahrholdt H, Wagner A.et al Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002402156–2164. [DOI] [PubMed] [Google Scholar]
- 11.Moon J C, McKenna W J, McCrohon J A.et al Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003411561–1567. [DOI] [PubMed] [Google Scholar]
- 12.Simonetti O P, Kim R J, Fieno D S.et al An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001218215–223. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich M G, Schulz‐Menger J, Poetsch T.et al Quantification of valvular aortic stenosis by magnetic resonance imaging. Am Heart J 2002144329–334. [DOI] [PubMed] [Google Scholar]
- 14.John A S, Dill T, Brandt R R.et al Magnetic resonance to assess the aortic valve area in aortic stenosis: how does it compare to current diagnostic standards? J Am Coll Cardiol 200342519–526. [DOI] [PubMed] [Google Scholar]
- 15.Kupfahl C, Honold M, Meinhardt G.et al Evaluation of aortic stenosis by cardiovascular magnetic resonance imaging: comparison with established routine clinical techniques. Heart 200490893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debl K, Djavidani B, Seitz J.et al Planimetry of aortic valve area in aortic stenosis by magnetic resonance imaging. Invest Radiol 200540631–636. [DOI] [PubMed] [Google Scholar]
- 17.Djavidani B, Debl K, Lenhart M.et al Planimetry of mitral valve stenosis by magnetic resonance imaging. J Am Coll Cardiol 2005452048–2053. [DOI] [PubMed] [Google Scholar]
- 18.Varnava A M, Elliott P M, Baboonian C.et al Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation 20011041380–1384. [DOI] [PubMed] [Google Scholar]
- 19.Varnava A M, Elliott P M, Mahoon N.et al Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol 200188275–279. [DOI] [PubMed] [Google Scholar]
- 20.Kim R J, Judd R M. Gadolinium‐enhanced magnetic resonance imaging in hypertrophic cardiomyopathy. J Am Coll Cardiol 2003411568–1572. [DOI] [PubMed] [Google Scholar]
- 21.Moolman J C, Corfield V A, Posen B.et al Sudden death due to troponin T mutations. J Am Coll Cardiol 199729549–555. [DOI] [PubMed] [Google Scholar]