Abstract
Objective
To determine the effect of torasemide, a loop diuretic with antialdosteronergic properties, compared with furosemide on cardiac sympathetic nerve activity in patients with congestive heart failure (CHF).
Methods
40 patients with non‐ischaemic CHF (left ventricular ejection fraction (LVEF) < 45%) were randomly assigned to torasemide (4–8 mg/day; n = 20) or furosemide (20–40 mg/day; n = 20). All patients were also treated with angiotensin‐converting enzyme inhibitor. The delayed heart to mediastinum count (H/M) ratio, delayed total defect score (TDS) and washout rate were determined from iodine‐123 meta‐iodobenzylguanidine measured before and 6 months after treatment. Left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV) and LVEF were also determined by echocardiography.
Results
After treatment, in patients receiving torasemide, TDS decreased from 44 (8) to 36 (8) (p < 0.001), H/M ratio increased from 1.61 (0.19) to 1.77 (0.24) (p < 0.001), and washout rate decreased from 52 (12)% to 41 (14)% (p = 0.001). In addition, LVEDV decreased from 173 (22) ml to 147 (30) ml (p < 0.001) and LVESV decreased from 117 (19) ml to 95(24) ml (p < 0.001). Although LVEF tended to increase, the change was not significant (from 31 (7)% to 34 (7)%, NS). Conversely, these parameters did not change significantly in patients receiving furosemide. Moreover, percentage change of TDS was significantly correlated with percentage change of LVEDV (r = 0.473, p < 0.05) and of LVESV (r = 0.579, p < 0.01) after torasemide treatment.
Conclusion
These findings indicate that torasemide treatment can ameliorate cardiac sympathetic nerve activity and left ventricular remodelling in patients with CHF.
Keywords: aldosterone, heart failure, loop diuretics, torasemide
Loop diuretics, such as furosemide and torasemide, are recommended for treatment of congestive heart failure (CHF) because diuretics relieve cardiac load by reducing water retention.1,2 In a report on the prospective TORIC (TORasemide In Chronic heart failure) study, torasemide was associated with lower mortality in patients with CHF than furosemide.3 Furthermore, torasemide has been reported to attenuate left ventricular remodelling in patients with CHF to a greater extent than furosemide.4,5,6 Torasemide is also reported to inhibit the renin–angiotensin–aldosterone system (RAAS) in a few pharmacological studies with experimental models.7,8
Cardiac imaging with meta‐iodobenzylguanidine (MIBG) labelled with iodine‐123, an analogue of norepinephrine, is a useful tool for detecting abnormalities of the myocardial adrenergic nervous system in patients with CHF.9,10,11 Many studies have suggested that treatment of heart failure in patients with CHF can improve cardiac sympathetic nerve activity as shown by cardiac 123I‐MIBG scintigraphy.12,13,14,15,16,17,18,19,20,21,22,23,24 Moreover, cardiac 123I‐MIBG scintigraphic findings and left ventricular function are correlated,11 and 123I‐MIBG scintigraphy has useful prognostic value in patients with CHF.10,11,12 However, there have been no reports on the effects of torasemide treatment on cardiac sympathetic nerve activity in patients with CHF compared with furosemide treatment.
This study evaluated the effect of torasemide treatment on cardiac sympathetic nerve activity in patients with non‐ischaemic CHF in comparison with furosemide treatment.
METHODS
Study patients
From December 2000 through February 2002, 48 patients were admitted to our institution with their first episode of non‐ischaemic heart failure with impaired cardiac function. All patients had symptoms and signs of CHF with New York Heart Association (NYHA) functional class III or IV. In the acute phase, all patients were given standard heart failure treatment. However, three patients died from progression of heart failure, and one patient died from lethal arrhythmia. Therefore, 44 patients had a stable symptomatic period in NYHA functional class II or III at the time of enrolment in this study. Chest radiography, standard electrocardiography, echocardiography, cardiac catheterisation (including coronary angiography and left ventriculography), and thallium‐201 and 123I‐MIBG scintigraphy were also performed for all patients. Patients were excluded if they had a history of myocardial infarction, coronary artery disease, congenital heart disease, primary hepatic failure, renal failure or active cancer. We also excluded patients older than 80 years. The study was approved by the ethics review board of our institution, and written informed consent was obtained from all patients.
Study protocol
In the acute phase, all patients were treated with intravenously administered furosemide. All patients also received angiotensin‐converting (ACE) enzyme inhibitor. Forty‐four patients were randomly assigned to (double blind, 1:1 ratio) either oral torasemide (4–8 mg/day; n = 22) or oral furosemide (20–40 mg/day; n = 22) instead of intravenous furosemide. Treatment with digitalis and vasodilators was allowed, but the use of potassium‐sparing diuretics was not permitted. We performed a series of examinations before and six months after treatment.
123I‐MIBG imaging
The method of 123I‐MIBG imaging has been described previously.16,17,18,19,20,21,22,23,24 The 123I‐MIBG was obtained from a commercial source (Daiichi Radioisotope Laboratories, Tokyo, Japan). Patients were injected intravenously with 123I‐MIBG (111 MBq) while in a supine position. At 15 min and at 4 h after injection, static data were acquired in the anterior view with a single‐head gamma camera (Millennium MPR, GE Medical Systems, Waukesha, Wisconsin, USA) equipped with a low‐energy, general‐purpose, parallel‐hole collimator. Static images on a 128 × 128 matrix were collected for 5 min with a 20% window centred on 159 keV, corresponding to the 123I photopeak. After the static planar images were acquired, single‐photon emission computed tomography (SPECT) images were obtained. The camera was rotated over 180° from the 45° right anterior oblique position to the 45° left posterior oblique position in 32 views with an acquisition time of 40 s/view. Scans were acquired in a 64 × 64 matrix by a filtered back‐projection method for reconstruction.
The heart to mediastinum count (H/M) ratio was determined from the anterior planar delayed 123I‐MIBG image. The washout rate (WR) was calculated with the following formula: {([H] − [M])early − ([H] − [M])delayed}/([H] − [M])early × 100 (%), where [H] is mean count/pixel in the left ventricle and [M] is mean count/pixel in the upper mediastinum. In this study, time decay was not corrected for the calculation of WR. In our laboratory, the normal range for the delayed H/M ratio is 2.00 to 2.80, and the normal WR range is 22–32%.
The delayed myocardial SPECT images of each patient were divided into 17 segments as recommended by the American Heart Association.25 Regional tracer uptake was assessed semiquantitatively with a five‐point scoring system (0, normal uptake; 1, mildly reduced uptake; 2, moderately reduced uptake; 3, significantly reduced uptake; 4, no uptake). The total defect score (TDS) was calculated as the sum of all defect scores.
Interobserver variability was determined in a blinded fashion by two independent observers with no knowledge of patient clinical status or drug treatment. The interobserver correlation was highly significant (r = 0.90, p < 0.001).
Echocardiography
Echocardiography was performed according to standard methods in a blinded manner. Two experienced, independent echocardiographers who had no knowledge about the study took all of the measurements. Left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV) and left ventricular ejection fraction (LVEF) were calculated by the modified Simpson's method.26 Interobserver and intraobserver variations in these measurements were small (LVEDV: r = 0.90 (p < 0.001) and r = 0.94 (p < 0.0001), respectively; LVESV: r = 0.90 (p < 0.001) and r = 0.93 (p < 0.0001), respectively; LVEF: r = 0.90 (p < 0.001) and r = 0.93 (p < 0.0001), respectively).
Plasma brain natriuretic peptide concentration
Blood samples were collected in test tubes containing EDTA after the patient had rested in a supine position for at least 30 min. Plasma was separated by centrifugation and frozen at −84°C until measurement. Then the plasma concentration of brain natriuretic peptide (BNP) was measured with a specific immunoradiometric assay for human BNP with a commercial kit (Shionogi, Osaka, Japan) as previously reported.21,23,24,27
Statistical analysis
Data were statistically analysed with Statview (Abacus Concepts, Berkeley, California, USA) for Macintosh (Apple Computer, Inc, Cupertino, California, USA). Numerical results are expressed as mean (SD). Baseline categorical data of the two groups were compared by the χ2 test. The differences between continuous variables were evaluated by an unpaired t test. Changes in NYHA functional class were assessed with the Wilcoxon matched pairs signed rank test. For patients who underwent repeated assessments, changes from baseline were evaluated within each treatment group by a paired t test and between the torasemide and furosemide groups by two‐way analysis of variance. Linear regression analysis was used to determine the relationship between continuous variables. In all analyses, p < 0.05 was considered significant.
RESULTS
Clinical characteristics
Among patients receiving torasemide, one patient had a cerebral haemorrhage and one was excluded because of the onset of unstable angina. Among patients receiving furosemide, one died of CHF during the follow‐up period and one was excluded because of the onset of cerebral infarction. Therefore, 40 of the 44 patients (29 men and 11 women, mean age 68 (7) years, range 46–79 years) enrolled in the trial completed the entire protocol. The mean dose of torasemide was 6.8 (1.9) mg/day and the mean dose of furosemide was 33.0 (9.8) mg/day. The causes of heart failure were idiopathic dilated cardiomyopathy (n = 21), valvular disease (n = 9) and hypertensive heart disease (n = 10).
Haemodynamic characteristics and cardiac drug treatment did not differ significantly between the two groups on entry into the study. Before treatment, TDS, H/M ratio, WR, plasma BNP concentration, LVEDV, LVESV, LVEF and NYHA functional class were similar in both groups (table 1). None of patients changed baseline cardiac drugs during the follow‐up period. The mean dose of enalapril was 7.3 (3.1) mg/day in the torasemide group versus 7.4 (3.2) mg/day in the furosemide group (NS). The mean dose of perindopril was 3.1 (0.9) mg/day in the torasemide group versus 3.0 (1.0) mg/day in the furosemide group (NS). The mean dose of carvedilol was 14 (6) mg/day in the torasemide group versus 13 (7) mg/day in the furosemide group (NS). The dose of digitalis was only 0.25 mg/day in both groups.
Table 1 Baseline characteristics of patients with congestive heart failure treated with torasemide or furosemide.
| Torasemide (n = 20) | Furosemide (n = 20) | |
|---|---|---|
| Age (years) | 68 (6) | 68 (9) |
| Men/women | 15/5 | 14/6 |
| Height (cm) | 160 (10) | 162 (9) |
| Weight (kg) | 58 (9) | 59 (10) |
| SBP (mm Hg) | 133 (15) | 131 (16) |
| DBP (mm Hg) | 73 (8) | 72 (9) |
| NYHA functional class | ||
| II | 7 | 8 |
| III | 13 | 12 |
| Cause of CHF | ||
| DCM | 10 (50%) | 11 (55%) |
| Valvular disease | 5 (25%) | 4 (20%) |
| HHD | 5 (25%) | 5 (25%) |
| Iodine‐123 MIBG | ||
| Total defect score | 44 (8) | 42 (11) |
| H/M | 1.61 (0.19) | 1.68 (0.18) |
| Washout rate (%) | 52 (12) | 50 (8) |
| Echocardiography | ||
| LVEDV (ml) | 173 (22) | 174 (24) |
| LVESV (ml) | 117 (19) | 120 (15) |
| LVEF (%) | 31 (7) | 31 (7) |
| Plasma BNP (pg/ml) | 244 (133) | 239 (147) |
| Drug treatment | ||
| ACE inhibitor | 20 (100%) | 20 (100%) |
| β blocker | 9 (45%) | 10 (50%) |
| Nitrate | 4 (20%) | 4 (20%) |
| Calcium antagonist | 6 (30%) | 5 (25%) |
| Digitalis | 2 (10%) | 2 (10%) |
Values are mean (SD) or number (%).
All differences between groups are non‐significant.
ACE, angiotensin‐converting enzyme; BNP, brain natriuretic peptide; CHF, congestive heart failure; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; HHD, hypertensive heart disease; H/M, ratio of heart to mediastinum count; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; MIBG, meta‐iodobenzylguanidine; NYHA, New York Heart Association; SBP, systolic blood pressure.
The body weight of both groups tended to decrease after six months compared with the baseline (in patients receiving torasemide, from 58 (9) to 56 (10); in patients receiving furosemide, from 59 (10) to 56 (10)). However, these changes were not significant in either group, and weight was similar in the groups after treatment.
Comparison of cardiac 123I‐MIBG scintigraphic findings
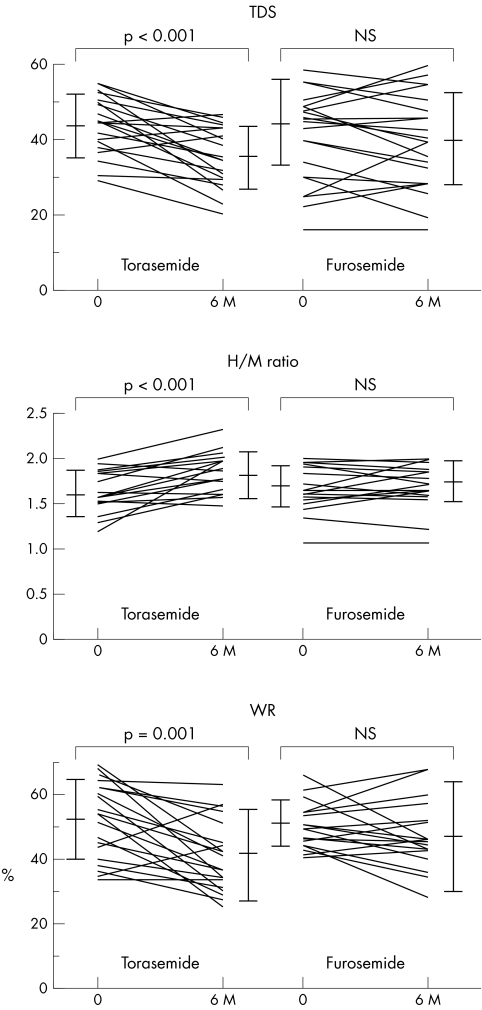
Table 2 and fig 1 summarise TDS, H/M ratio and WR. In patients receiving torasemide, TDS decreased significantly after six months compared with the baseline value (p < 0.001). In contrast, in patients receiving furosemide, baseline TDS was not significantly different from TDS after six months of treatment. In the segmental analysis of both groups, TDS tended to improve due to uptake of the inferior wall, although the improvement was not significant. In patients receiving torasemide, the H/M ratio increased significantly and WR decreased significantly after six months compared with the baseline values (H/M ratio, p < 0.001; WR, p = 0.001). In contrast, in patients receiving furosemide, the H/M ratio and WR did not differ significantly between baseline and after six months of treatment.
Table 2 Changes in iodine‐123 meta‐iodobenzylguanidine factors in patients treated with torasemide or furosemide.
| Torasemide | Furosemide | |||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| TDS | 44(8) | 36(8)* | 42(11) | 40(12) |
| H/M | 1.61(0.19) | 1.77(0.24)* | 1.68(0.18) | 1.71(0.19) |
| WR (%) | 52(12) | 41(14)† | 50(8) | 47(17) |
Values are mean (SD).
*p<0.001 v baseline; †p = 0.001 v baseline.
H/M, ratio of heart to mediastinum; TDS, total defect score; WR, washout rate.
Figure 1 Comparison of cardiac iodine‐123 meta‐iodobenzylguanidine scintigraphic findings during treatment in the two groups. 6M, after six months of treatment; H/M, ratio of heart to mediastinum count; TDS, total defect score; WR, washout rate.
Comparison of echocardiographic findings
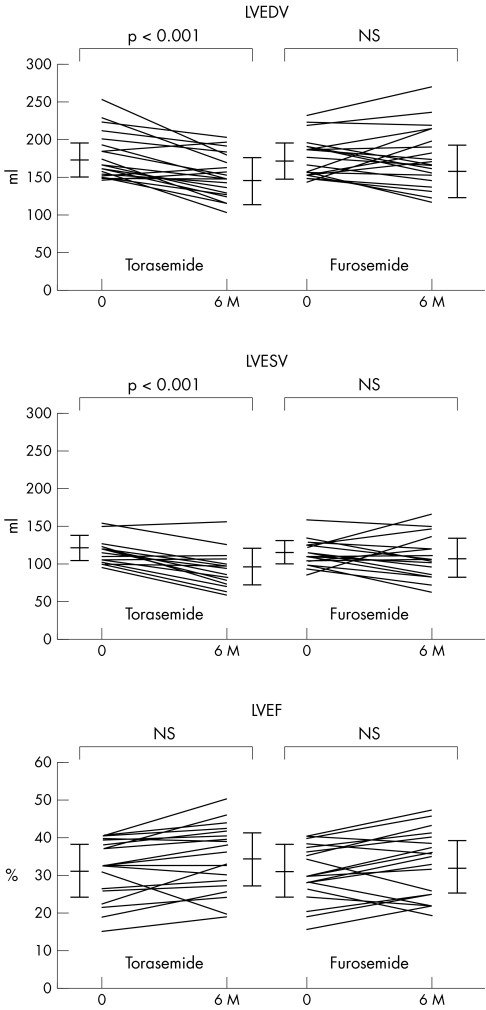
Table 3 and fig 2 summarise the changes in LVEDV, LVESV and LVEF. In patients receiving torasemide, LVEDV and LVESV decreased significantly after six months compared with their baseline values (both p < 0.001). In contrast, in patients receiving furosemide, LVEDV and LVESV at baseline and after six months of treatment were not significantly different. Patients receiving torasemide tended to have greater LVEF after six months than at baseline, but this difference was not significant. In patients receiving furosemide, the LVEF at baseline and after six months of treatment were not significantly different.
Table 3 Changes of echocardiographic, biochemical and functional factors in patients treated with torasemide or furosemide.
| Torasemide | Furosemide | |||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| Echocardiography | ||||
| LVEDV (ml) | 173 (22) | 147 (30)** | 174 (24) | 165 (34) |
| LVESV (ml) | 117 (19) | 95 (24)** | 120 (15) | 109 (33) |
| LVEF (%) | 31 (7) | 34 (7) | 31 (7) | 32 (7) |
| Plasma BNP (pg/ml) | 244 (133) | 154 (95)**† | 239 (147) | 218 (94) |
| NYHA functional class | ||||
| I/II/III | 0/7/13 | 7/12/1**† | 0/8/12 | 2/13/5* |
*p<0.05, **p<0.001 v baseline; †p<0.05 v furosemide group at 6 months.
BNP, brain natriuretic peptide; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NYHA, New York Heart Association.
Figure 2 Comparison of echocardiographic findings during treatment in the two groups. 6M, after six months of treatment; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume.
Comparison of plasma BNP concentration
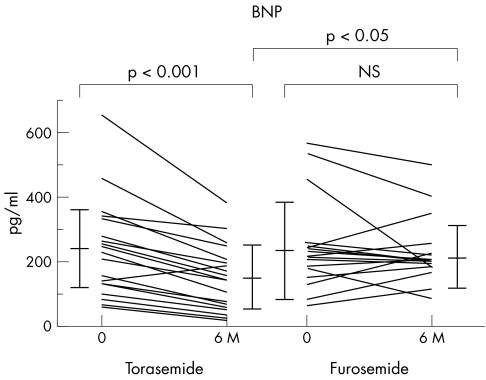
Table 3 and fig 3 show plasma BNP concentrations. In patients receiving torasemide, the plasma BNP concentration decreased significantly after six months compared with the baseline values (p < 0.001). In contrast, BNP values at baseline and after six months did not differ significantly in the patients receiving furosemide. Furthermore, the plasma BNP concentration of patients receiving torasemide was significantly lower than that of patients receiving furosemide after six months (p < 0.05).
Figure 3 Comparison of plasma brain natriuretic peptide (BNP) concentration during treatment in the two groups. 6M, after six months of treatment.
Comparison of NYHA functional class
Table 3 summarises the NYHA functional class of the patients. Patients in both groups improved after six months of treatment compared with the baseline values (in patients receiving torasemide, p < 0.001; in patients receiving furosemide, p < 0.05). After treatment, the NYHA functional class of patients in the torasemide group was better than in the furosemide group (p < 0.05).
Relationship between percentage change of TDS and of left ventricular volume
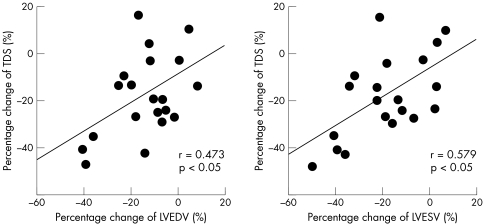
The percentage change of TDS evaluated by 123I‐MIBG scintigraphy was significantly correlated with the percentage change of LVEDV from baseline to six months (r = 0.473, p < 0.05) and of LVESV (r = 0.579, p < 0.01) after torasemide treatment (fig 4). In contrast, these changes were not related in the patients receiving furosemide.
Figure 4 Correlations between percentage change in total defect score (TDS) evaluated by iodine‐123 meta‐iodobenzylguanidine scintigraphy and percentage change in left ventricular end diastolic volume (LVEDV) and left ventricular end systolic volume (LVESV) after treatment with torasemide.
DISCUSSION
Recently, it was reported that aldosterone is produced in the ventricles of the failing human heart28 and that the aldosterone synthase gene is expressed in cardiac tissue.29 Furthermore, aldosterone has been reported to induce the expression of ACE mRNA in cultured neonatal cardiocytes.30 In that study, Harada et al30 discussed that the expression of ACE mRNA is significantly inhibited by an aldosterone receptor blocker, spironolactone. Torasemide is a loop diuretic, and this drug is known to have an antialdosteronergic effect.7,8 Therefore, torasemide, as well as spironolactone, may inhibit the RAAS and have a cardioprotective effect.
Activation of the RAAS promotes structural remodelling of the heart and the progression of heart failure.31 A long‐term increase in LVEF has been identified as a marker of beneficial left ventricular remodelling manifested by reduced chamber volume,32 and this change is associated with improvement in the rate of survival.33 Yamato et al4 reported that torasemide attenuates left ventricular remodelling in patients with CHF in comparison with furosemide treatment. Moreover, very interestingly, Lopez et al5 reported that torasemide's abilities differ from furosemide's in reversing myocardial fibrosis and reducing collagen synthesis in patients with CHF. In our study, left ventricular volume and cardiac function improved with torasemide treatment compared with furosemide treatment. In our study, torasemide treatment also improved the symptoms of heart failure, as measured by changes in NYHA functional class.
123I‐MIBG is an analogue of the adrenergic neurone‐blocking agent guanethidine, which is thought to use the same myocardial uptake and release mechanisms as norepinephrine.34 Cardiac 123I‐MIBG imaging therefore seems to be a useful tool for detecting abnormalities of the myocardial adrenergic nervous system in patients with CHF.9,10,11 Furthermore, myocardial uptake of 123I‐MIBG has been shown to be a strong prognostic indicator for overall mortality in patients with CHF.10,11,12 In patients with non‐ischaemic CHF, a large proportion of the decrease in the uptake of norepinephrine is probably due to the loss of neuronal norepinephrine uptake in the failing myocardium. However, some of the reduction appears to be functional (that is, reversible) and is mediated by hormonal factors, including angiotensin and aldosterone. Struthers et al35 reported that once norepinephrine is taken up by cardiac myocytes, it is rapidly metabolised and inactivated so that uptake is equivalent to local removal by the myocardium.
Several reports have suggested that ACE inhibitors,13,14,23 β blockers,12,14,15,16 spironolactone17,18 and angiotensin receptor blockers 19,21,24 can improve cardiac sympathetic nerve activity in patients with CHF, on the basis of cardiac 123I‐MIBG scintigraphy findings. However, there have been no reports on the effects of torasemide treatment on cardiac sympathetic nerve activity in patients with CHF compared with furosemide treatment. In this study, we examined whether torasemide improves cardiac sympathetic nerve activity in patients with CHF by using 123I‐MIBG scintigraphy, and found that TDS, H/M ratio and WR improved with torasemide treatment and did not change significantly with furosemide treatment. Moreover, the percentage change of TDS was significantly correlated with the percentage change of LVEDV and of LVESV from baseline to six months among patients receiving torasemide.
Plasma BNP concentration is a useful prognostic indicator in patients with CHF,36 as it is a ventricular hormone.37 Plasma BNP concentration is reported to correlate with abnormalities of LVEF and left ventricular end diastolic pressure,37 as well as with left ventricular mass.38 The decrease in plasma BNP after treatment with torasemide was therefore caused by decreased left ventricular filling pressure, improved left ventricular remodelling, or both factors. The decrease in BNP during torasemide treatment may also reflect improvement in left ventricular diastolic function secondary to the effects of this drug on cardiac hypertrophy and fibrosis. Treatment of CHF guided by plasma BNP concentration has been reported to reduce cardiovascular events,36 so a decrease in BNP may be associated with a better outcome as was the case in the TORIC study.3
In general, patients with high blood pressure have impaired cardiac sympathetic nerve activity, and the use of agents has proved beneficial, as shown by 123I‐MIBG scintigraphy.39 In this study, systolic and diastolic blood pressure did not differ significantly after six months of treatment in either group (in patients receiving torasemide, 133 (15) mm Hg v 132 (14) mm Hg, and 73 (8) mm Hg v 72 (9) mm Hg, respectively; in patients receiving furosemide, 131 (16) mm Hg v 129 (14) mm Hg, and 72 (9) mm Hg v 71 (9) mm Hg, respectively). We therefore believe that torasemide can improve cardiac sympathetic nerve activity and left ventricular performance in patients with CHF, and that this effect is independent of a blood pressure‐lowering effect.
The small number of patients with non‐ischaemic CHF in this study was a limitation. In addition, this study included patients with heart failure with various aetiologies (dilated cardiomyopathy, valvular heart disease and hypertensive heart disease). Patients with these diseases did not differ significantly in their response to torasemide, although 123I‐MIBG scintigraphic and echocardiographic parameters tended to improve in patients with DCM in comparison with other diseases (data not shown). We therefore speculate that torasemide can ameliorate cardiac sympathetic nerve activity and left ventricular performance in patients with non‐ischaemic CHF, irrespective of its aetiology.
Furthermore, our patients took lower doses of ACE inhibitors and diuretics than those used in previously reported trials.3,5,40 However, the doses of these agents are generally lower in Japan than in other countries. Enalapril at a dose of 5–10 mg once daily and perindopril 2–4 mg are considered to be effective and safe for the treatment of Japanese patients with heart failure.13,23,24 Furosemide at a dose of 20–40 mg once daily and torasemide 4–8 mg are also considered to be effective and safe.4 Moreover, in our study, NYHA functional class of both groups improved after treatment compared with the baseline, and the body weight of both groups had decreased similarly. The doses of these agents in this study therefore were not too low and were effective for our Japanese patients with heart failure.
Tsutamoto et al6 reported that, unlike furosemide, torasemide reduces plasma procollagen type III N‐terminal peptide (PIIINP) concentration, a biochemical marker of fibrosis, as well as aldosterone concentration in patients with CHF. Lopez et al5 described that torasemide treatment decreases C‐terminal peptide of procollagen type I in patients with chronic heart failure. We did not measure plasma PIIINP or procollagen type I in this study. Further studies are necessary to clarify the effects of torasemide on cardiac sympathetic nerve activity and PIIINP or procollagen type I in patients with CHF.
Conclusion
TDS, H/M ratio and WR determined by 123I‐MIBG scintigraphy significantly improved after six months of torasemide treatment. In addition, echocardiographic parameters improved with this treatment. In contrast, these parameters did not change significantly with furosemide treatment. Moreover, the percentage change of TDS was significantly correlated with the percentage change of LVEDV and of LVESV from baseline to six months in patients receiving torasemide. These findings indicate that, compared with furosemide treatment, torasemide treatment can improve cardiac sympathetic nerve activity and attenuate left ventricular remodelling in patients with CHF.
ACKNOWLEDGEMENTS
The authors thank Takayoshi Honjo, Akira Nakaya, Hiromitsu Takahashi, Hiroyuki Takada and Takehiro Ishikawa for their technical assistance.
Abbreviations
ACE - angiotensin‐converting enzyme
BNP - brain natriuretic peptide
CHF - congestive heart failure
H/M - heart to mediastinum count ratio
LVEDV - left ventricular end diastolic volume
LVEF - left ventricular ejection fraction
LVESV - left ventricular end systolic volume
MIBG - meta‐iodobenzylguanidine
NYHA - New York Heart Association
PIIINP - procollagen type III N‐terminal peptide
RAAS - renin–angiotensin–aldosterone system
SPECT - single‐photon emission computed tomography
TDS - total defect score
TORIC - TORasemide In Chronic heart failure
WR - washout rate
Footnotes
Competing interests: None declared.
References
- 1.Ho K K, Pinsky J L, Kannel W B.et al The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993226A–13A. [DOI] [PubMed] [Google Scholar]
- 2.American College of Cardiology, American Heart Association Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995922764–2784. [DOI] [PubMed] [Google Scholar]
- 3.Cosin J, Diez J, TORIC investigators Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail 20024507–513. [DOI] [PubMed] [Google Scholar]
- 4.Yamato M, Sasaki T, Honda K.et al Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J 200367384–390. [DOI] [PubMed] [Google Scholar]
- 5.Lopez B, Querejeta R, Gonzalez A.et al Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004432028–2035. [DOI] [PubMed] [Google Scholar]
- 6.Tsutamoto T, Sakai H, Wada A.et al Torasemide inhibits transcardiac extraction of aldosterone in patients with congestive heart failure. J Am Coll Cardiol 2004442252–2253. [DOI] [PubMed] [Google Scholar]
- 7.Uchida T, Yamanaga K, Nishikawa M.et al Anti‐aldosteronergic effect of torasemide. Eur J Pharmacol 1991205145–150. [DOI] [PubMed] [Google Scholar]
- 8.Goodfriend T L, Ball D L, Oelkers W.et al Torasemide inhibits aldosterone secretion in vitro. Life Sci 199863PL45–PL50. [DOI] [PubMed] [Google Scholar]
- 9.Henderson E B, Kahn J K, Corbett J R.et al Abnormal I‐123 metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988781192–1199. [DOI] [PubMed] [Google Scholar]
- 10.Ogita H, Shimonagata T, Fukunami M.et al Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart 200186656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merlet P, Valette H, Dubois‐Rande J L.et al Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 199233471–477. [PubMed] [Google Scholar]
- 12.Yamazaki J, Muto H, Kabano T.et al Evaluation of beta‐blocker therapy in patients with dilated cardiomyopathy: clinical meaning of iodine 123‐metaiodobenzylguanidine myocardial single‐photon emission computed tomography. Am Heart J 2001141645–652. [DOI] [PubMed] [Google Scholar]
- 13.Takeishi Y, Atsumi H, Fujiwara S.et al ACE inhibition reduces cardiac iodine‐123‐MIBG release in heart failure. J Nucl Med 1997381085–1089. [PubMed] [Google Scholar]
- 14.Toyama T, Aihara Y, Iwasaki T.et al Cardiac sympathetic activity estimated by 123I‐MIBG myocardial imaging in patients with dilated cardiomyopathy after beta‐blocker or angiotensin‐converting enzyme inhibitor therapy. J Nucl Med 199940217–223. [PubMed] [Google Scholar]
- 15.Toyama T, Hoshizaki H, Seki R.et al Efficacy of carvedilol treatment on cardiac function and cardiac sympathetic nerve activity in patients with dilated cardiomyopathy: comparison with metoprolol therapy. J Nucl Med 2003441604–1611. [PubMed] [Google Scholar]
- 16.Kasama S, Toyama T, Hoshizaki H.et al Dobutamine gated blood pool scintigraphy predicts the improvement of cardiac sympathetic nerve activity, cardiac function, and symptoms after treatment in patients with dilated cardiomyopathy. Chest 2002122542–548. [DOI] [PubMed] [Google Scholar]
- 17.Kasama S, Toyama T, Kumakura H.et al Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med 2002431279–1285. [PubMed] [Google Scholar]
- 18.Kasama S, Toyama T, Kumakura H.et al Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol 200341574–581. [DOI] [PubMed] [Google Scholar]
- 19.Kasama S, Toyama T, Kumakura H.et al Addition of valsartan to an angiotensin‐converting enzyme inhibitor improves cardiac sympathetic nerve activity and left ventricular function in patients with congestive heart failure. J Nucl Med 200344884–890. [PubMed] [Google Scholar]
- 20.Kasama S, Toyama T, Kumakura H.et al Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity in patients with decompensated congestive heart failure. J Nucl Med 2004451108–1113. [PubMed] [Google Scholar]
- 21.Kasama S, Toyama T, Kumakura H.et al Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 200545661–667. [DOI] [PubMed] [Google Scholar]
- 22.Kasama S, Toyama T, Kumakura H.et al Effects of nicorandil on cardiac sympathetic nerve activity after reperfusion therapy in patients with first anterior acute myocardial infarction. Eur J Nucl Med Mol Imaging 200532322–328. [DOI] [PubMed] [Google Scholar]
- 23.Kasama S, Toyama T, Kumakura H.et al Effects of perindopril on cardiac sympathetic nerve activity in patients with congestive heart failure: comparison with enalapril. Eur J Nucl Med Mol Imaging 200532964–971. [DOI] [PubMed] [Google Scholar]
- 24.Kasama S, Toyama T, Hatori T.et al Comparative effects of valsartan and enalapril on cardiac sympathetic nerve activity and plasma brain natriuretic peptide in patients with congestive heart failure. Heart 200692625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerqueira M D, Weissman N J, Dilsizian V.et al Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002105539–542. [DOI] [PubMed] [Google Scholar]
- 26.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 27.Kasama S, Toyama T, Kumakura H.et al Dobutamine stress 99mTc‐tetrofosmin quantitative gated SPECT predicts improvement of cardiac function after carvedilol treatment in patients with dilated cardiomyopathy. J Nucl Med 2004451878–1884. [PubMed] [Google Scholar]
- 28.Mizuno Y, Yoshimura M, Yasue H.et al Aldosterone production is activated in failing ventricle in humans. Circulation 200110372–77. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura M, Nakamura S, Ito T.et al Expression of aldosterone synthase gene in failing human heart: quantitative analysis using modified real‐time polymerase chain reaction. J Clin Endocrinol Metab 2002873936–3940. [DOI] [PubMed] [Google Scholar]
- 30.Harada E, Yoshimura M, Yasue H.et al Aldosterone induces angiotensin‐converting‐enzyme gene expression in cultured neonatal rat cardiocytes. Circulation 2001104137–139. [DOI] [PubMed] [Google Scholar]
- 31.Cohn J N, Ferrari R, Sharpe N. Cardiac remodeling: concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 200035569–582. [DOI] [PubMed] [Google Scholar]
- 32.Francis G S, Cohn J N. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J 199043068–3075. [DOI] [PubMed] [Google Scholar]
- 33.Patten R D, Udelson J E, Konstam M A. Ventricular remodeling and its prevention in the treatment of heart failure. Curr Opin Cardiol 199813162–167. [PubMed] [Google Scholar]
- 34.Wieland D M, Wu J, Brown L E.et al Radiolabeled adrenergic neuron‐blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med 198021349–353. [PubMed] [Google Scholar]
- 35.Struthers A D. Aldosterone escape during angiotensin‐converting enzyme inhibitor therapy in chronic heart failure. J Card Fail 1996247–54. [DOI] [PubMed] [Google Scholar]
- 36.Tsutamoto T, Wada A, Maeda K.et al Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 199796509–516. [DOI] [PubMed] [Google Scholar]
- 37.Yasue H, Yoshimura M, Sumida H.et al Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation 199490195–203. [DOI] [PubMed] [Google Scholar]
- 38.Kohno M, Horio T, Yokokawa K.et al Brain natriuretic peptide as a cardiac hormone in essential hypertension. Am J Med 19929229–34. [DOI] [PubMed] [Google Scholar]
- 39.Sakata K, Shirotani M, Yoshida H.et al Comparison of effects of enalapril and nitrendipine on cardiac sympathetic nervous system in essential hypertension. J Am Coll Cardiol 199832438–443. [DOI] [PubMed] [Google Scholar]
- 40.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 19873161429–1435. [DOI] [PubMed] [Google Scholar]