Abstract
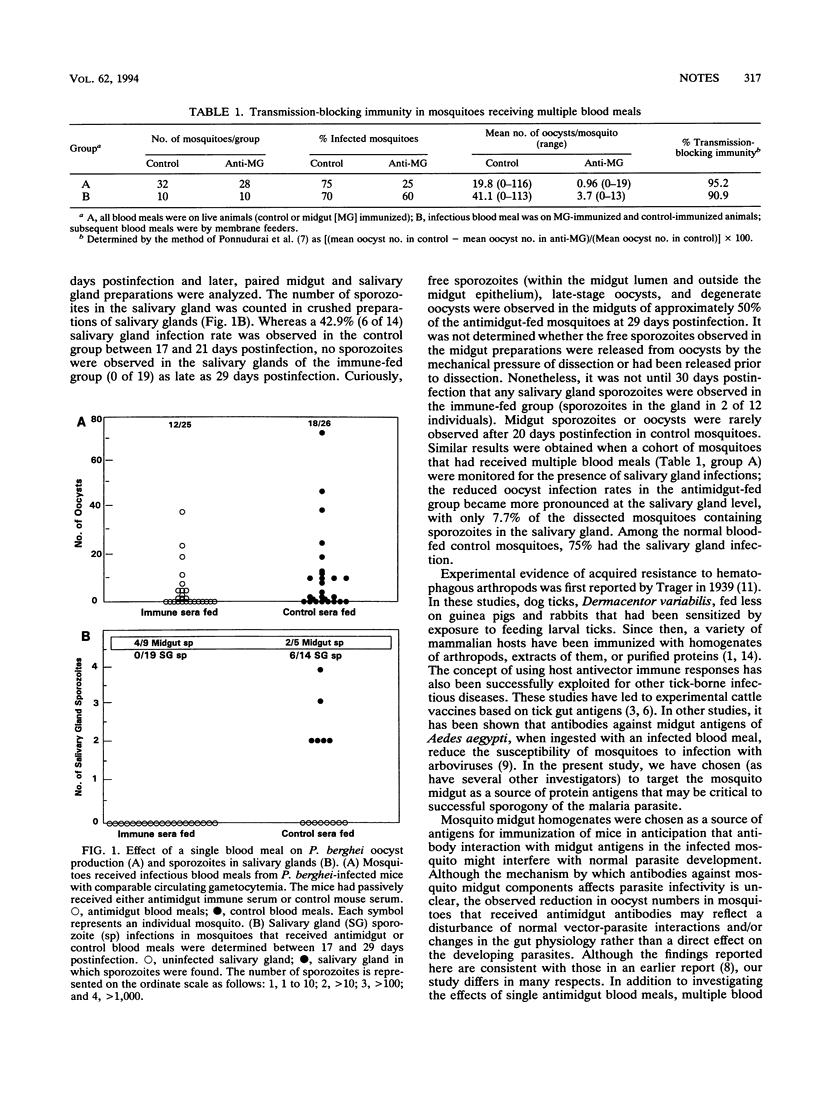
The mosquito midgut plays a central role in the development and subsequent transmission of malaria parasites. Using a rodent malaria parasite, Plasmodium berghei, and the mosquito vector Anopheles stephensi, we investigated the effect of anti-mosquito-midgut antibodies on the development of malaria parasites in the mosquito. In agreement with previous studies, we found that mosquitoes that ingested antimidgut antibodies along with infectious parasites had significantly fewer oocysts than mosquitoes in the control group. We also found that the antimidgut antibodies inhibit the development and/or translocation of the sporozoites. Together, these observations open an avenue for research toward the development of a vector-based malaria parasite transmission-blocking vaccine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger N. E., Cabrera E. J. An increase in death rate of Anopheles stephensi fed on rabbits immunized with mosquito antigen. J Econ Entomol. 1972 Feb;65(1):165–168. doi: 10.1093/jee/65.1.165. [DOI] [PubMed] [Google Scholar]
- Collins F. H., Zavala F., Graves P. M., Cochrane A. H., Gwadz R. W., Akoh J., Nussenzweig R. S. First field trial of an immunoradiometric assay for the detection of malaria sporozoites in mosquitoes. Am J Trop Med Hyg. 1984 Jul;33(4):538–543. doi: 10.4269/ajtmh.1984.33.538. [DOI] [PubMed] [Google Scholar]
- Meis J. F., Pool G., van Gemert G. J., Lensen A. H., Ponnudurai T., Meuwissen J. H. Plasmodium falciparum ookinetes migrate intercellularly through Anopheles stephensi midgut epithelium. Parasitol Res. 1989;76(1):13–19. doi: 10.1007/BF00931065. [DOI] [PubMed] [Google Scholar]
- Morrison W. I. Immunological control of ticks and tick-borne parasitic diseases of livestock. Parasitology. 1989;98 (Suppl):S69–S86. doi: 10.1017/s0031182000072267. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T., van Gemert G. J., Bensink T., Lensen A. H., Meuwissen J. H. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans R Soc Trop Med Hyg. 1987;81(3):491–493. doi: 10.1016/0035-9203(87)90172-6. [DOI] [PubMed] [Google Scholar]
- Ramasamy M. S., Ramasamy R. Effect of anti-mosquito antibodies on the infectivity of the rodent malaria parasite Plasmodium berghei to Anopheles farauti. Med Vet Entomol. 1990 Apr;4(2):161–166. doi: 10.1111/j.1365-2915.1990.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Ramasamy M. S., Sands M., Kay B. H., Fanning I. D., Lawrence G. W., Ramasamy R. Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med Vet Entomol. 1990 Jan;4(1):49–55. doi: 10.1111/j.1365-2915.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Ramasamy M. S., Srikrishnaraj K. A., Wijekoone S., Jesuthasan L. S., Ramasamy R. Host immunity to mosquitoes: effect of antimosquito antibodies on Anopheles tessellatus and Culex quinquefasciatus (Diptera: Culicidae). J Med Entomol. 1992 Nov;29(6):934–938. doi: 10.1093/jmedent/29.6.934. [DOI] [PubMed] [Google Scholar]
- Vaughan J. A., Azad A. F. Passage of host immunoglobulin G from blood meal into hemolymph of selected mosquito species (Diptera: Culicidae). J Med Entomol. 1988 Nov;25(6):472–474. doi: 10.1093/jmedent/25.6.472. [DOI] [PubMed] [Google Scholar]
- Vaughan J. A., Wirtz R. A., do Rosario V. E., Azad A. F. Quantitation of antisporozoite immunoglobulins in the hemolymph of Anopheles stephensi after bloodfeeding. Am J Trop Med Hyg. 1990 Jan;42(1):10–16. doi: 10.4269/ajtmh.1990.42.10. [DOI] [PubMed] [Google Scholar]
- Wikel S. K. Immune responses to arthropods and their products. Annu Rev Entomol. 1982;27:21–48. doi: 10.1146/annurev.en.27.010182.000321. [DOI] [PubMed] [Google Scholar]
- de Castro J. J., Newson R. M. Host resistance in cattle tick control. Parasitol Today. 1993 Jan;9(1):13–17. doi: 10.1016/0169-4758(93)90154-8. [DOI] [PubMed] [Google Scholar]



