Abstract
Objectives
To determine concentrations of adiponectin and its predictive value on outcome in a cohort of patients with congestive heart failure (CHF).
Methods
Serum and clinical data were obtained for outpatients with clinically controlled CHF (n = 175). Serum concentrations of adiponectin, C reactive protein, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), interleukin (IL) ‐1β, IL‐6, IL‐8, IL‐10, IL‐12, tumour necrosis factor α and CD‐40 ligand were determined. The association of adiponectin with the clinical severity of CHF was sought as well as the predictive value of this adipokine on mortality, CHF hospitalisations or the occurrence of each of these end points.
Results
Concentrations of adiponectin were significantly increased in patients with CHF. Patients with higher New York Heart Association class had significantly higher serum concentrations of adiponectin. Adiponectin serum concentrations were lower in patients with diabetes and CHF as well as in patients with ischaemic cardiomyopathy. Serum adiponectin concentration was positively associated with age and NT‐proBNP but was negatively correlated with C reactive protein concentrations. Serum adiponectin above the 75th centile was found to be an independent predictor of total mortality, CHF hospitalisations or a composite of these end points over a two‐year prospective follow up.
Conclusion
Adiponectin is increased in CHF patients and predicts mortality and morbidity.
Adiponectin is an adipocyte‐derived cytokine with anti‐inflammatory, antidiabetic and antiatherogenic properties.1 It has been initially shown that circulating concentrations of adiponectin are reduced in patients with obesity and increase after weight loss.2 Moreover, adiponectin concentrations negatively correlate with fractional body fat, waist to hip ratio and intra‐abdominal fat.3 Subsequent studies have shown that adiponectin serum concentrations are also reduced in patients with diabetes, atherosclerosis and acute coronary syndrome.4,5,6,7,8 Of particular relevance is the correlation of adiponectin concentrations with markers of inflammation. It has been shown that adiponectin correlates negatively with C reactive protein (CRP), interleukin (IL) ‐6 and tumour necrosis factor (TNF) α, suggesting adiponectin may antagonise some of their proinflammatory properties.9,10 Experimentally, adiponectin has been shown to reduce atherosclerosis in apolipoprotein E‐deficient mice and to suppress robust inflammatory responses within the vessel wall, supporting a protective role of this adipokine.11
Congestive heart failure (CHF) is a complex syndrome of neurohormonal inflammatory dysregulation resulting in progressive cardiac remodelling. CHF is still associated with significant morbidity and mortality.12 A better understanding of factors governing the pathogenesis of CHF resulted in major advances in its treatment. Inhibition of both neurohormonal axes has accordingly become a mainstay in the management of patients with CHF.13 Mounting evidence supports the role of immune system activation in the pathogenesis and progression of CHF, exemplified by raised circulating concentrations of proinflammatory cytokines (TNFα, IL‐6) and acute phase reactants such as CRP (reviewed by Pugh et al14).
In the present study, we evaluated concentrations of the adipose tissue‐derived cytokine adiponectin in patients with clinically controlled CHF of various degrees of severity. Furthermore, we determined the association of adiponectin with various inflammatory and neurohormonal mediators known to be increased in patients with CHF and studied its prognostic value in the prediction of morbidity and mortality.
METHODS
Patient selection
One hundred and seventy‐five consecutive patients with clinically controlled CHF attending the outpatient clinic of the Tel Aviv Sourasky Medical Center were recruited. Patients with CHF in New York Heart Association (NYHA) functional classes II–IV were included. The local research ethics committee approved the study protocol and all patients gave written informed consent.
At baseline, patients had a full medical history taken, clinical examination performed and NYHA class assigned. Patients were followed up every 1–3 months or more often as required. Baseline serum was drawn at the initial visit and frozen at −80°C until assayed in the laboratory. Control, age‐matched healthy participants (n = 43) were also recruited and blood was similarly drawn and preserved.
Follow up
The study end points were all‐cause death, hospitalisations caused by heart failure, or the combined occurrence of each. Patients were followed up for a minimum of 24 months. No patients were lost to follow up.
Determination of circulating adiponectin
Fasting serum adiponectin was measured with the radioimmunoassay from Linco Research (St Charles, Missouri, USA) (human adiponectin sensitivity is 1 mg/l on a 100 μl sample size.) Assay range is 1–200 mg/l and specificity is < 0.01%.
Determination of N‐terminal pro‐brain natriuretic peptide concentrations
Serum N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) was measured by automated immunoassay (Elecsys proBNP, Roche Diagnostics, Germany). The test uses two polyclonal antibodies directed against NT‐proBNP: epitope 1, amino acids 1–21; and epitope 2, amino acids 39–50. The results are calibrated against a synthetic NT‐proBNP (amino acids 1–76). Results range from 5–35 000 pg/ml.
High sensitivity CRP concentrations
CRP was assayed according to the manufacturer's instructions (Dade Behring Inc). Briefly, the method uses polystyrene particles coated with monoclonal antibodies to CRP. These particles agglutinate with CRP. CRP concentration was determined according to the intensity of the scattered light in the nephelometer, compared with standards of known concentrations.
Determination of circulating inflammatory cytokines
Serum cytokines were measured serially by cytometric bead array (CBA) with a four‐colour FACSCalibur flow cytometer (Becton Dickinson, San Jose, California, USA). In CBA, six bead populations with distinct fluorescence intensities had been coated with capturing antibodies specific for the respective cytokines. These bead populations could be resolved in the fluorescence channels of the flow cytometer. The beads were incubated with 50 μl of serum and cytokines were captured by their corresponding beads. The cytokine‐captured beads were then mixed with phycoerythrin‐conjugated detection antibodies to form sandwich complexes. After incubation, washing and acquisition of fluorescence data, the results were generated in graphical format by the Becton Dickinson CBA software. The concentrations of IL‐1β, IL‐6, IL‐10, IL‐8, TNFα and IL‐12p70 were measured by using the inflammatory cytokine CBA kits (BD PharMingen, San Diego, California, USA). The coefficients of variation for all cytokine assays were < 10%. Their respective normal ranges have been derived from measurement of healthy subjects.
Determination of soluble CD‐40 ligand concentrations
Serum concentrations of CD‐40 ligand were determined by a commercial enzyme‐linked immunosorbent assay kit according to the manufacturer's instructions (R&D Systems).
Statistical analysis
Data are presented as mean (SEM) unless otherwise stated. Serum adiponectin concentrations were stratified according to NYHA class and values within CHF patient groups were compared with those obtained for healthy controls by a one‐way analysis of variance. Student's t test was used to compare concentrations of adiponectin between patients with ischaemic CHF, diabetes and chronic renal failure.
The association between baseline clinical characteristics and dichotomous adiponectin serum concentrations was analysed with the χ2 test for categorical variables. Event rates were compared by Kaplan–Meier curves calculated for adiponectin serum concentrations above compared with below the 75th centile of 14 mg/l. The independent predictive power of serum adiponectin, left ventricular ejection fraction (LVEF), NT‐proBNP, NYHA class, IL‐1β, IL‐6, IL‐10, IL‐8, TNFα and IL‐12p70 and other clinical and demographic variables for death, hospitalisations for CHF or either of each of these end points was tested by stepwise Cox proportional hazards regression analyses. A value of p < 0.05 was accepted as significant.
RESULTS
Table 1 presents baseline clinical characteristics and use of drugs.
Table 1 Clinical characteristics of the congestive heart failure outpatient cohort (n = 175).
| Age (years) | 71.1 (11.2) |
| Men | 135 (77%) |
| NYHA | 2.7 (0.53) |
| LVEF (%) | 36.1 (12.1%) |
| Hyperlipidaemia | 99 (55%) |
| Smoking | 65 (37%) |
| Hypertension | 101 (58%) |
| Diabetes mellitus | 62 (35%) |
| Ischaemic heart disease | 130 (74%) |
| Chronic atrial fibrillation | 52 (30%) |
| TIA/CVA | 20 (11%) |
| PTCA or CABG | 92 (53%) |
| ACE inhibitors/ARBs | 140 (80%) |
| Spironolactone | 77 (44%) |
| β blockers | 128 (73%) |
Data are mean (SD) or number (%).
ACE, angiotensin‐converting enzyme; ARB, angiotensinogen receptor blockers; CABG, coronary artery bypass surgery; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PTCA, percutaneous transluminal coronary angioplasty; TIA, transient ischaemic attack.
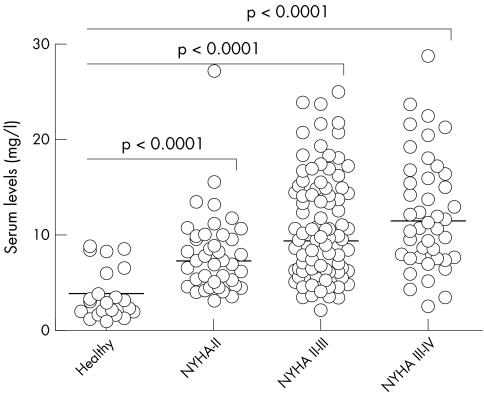
The more advanced the CHF was according to NYHA class, the higher the adiponectin concentrations were. Mean adiponectin serum concentrations in age‐matched healthy controls were 3.7 (SEM 0.6) mg/l. Patients with NYHA class II, II–III and III–IV had significantly increased serum concentrations of adiponectin (8.0 (6.7) mg/l, 10.5 (0.5) mg/l and 12.3 (0.9) mg/l, respectively, p < 0.0001 for all comparisons) (fig 1).
Figure 1 Serum adiponectin according to the severity of congestive heart failure. Serum adiponectin concentrations were determined by radioimmunoassay. Patients were stratified by the severity of heart failure expressed by New York Heart Association (NYHA) functional class. Results were compared by a one‐way analysis of variance. Horizontal lines indicate median serum concentrations.
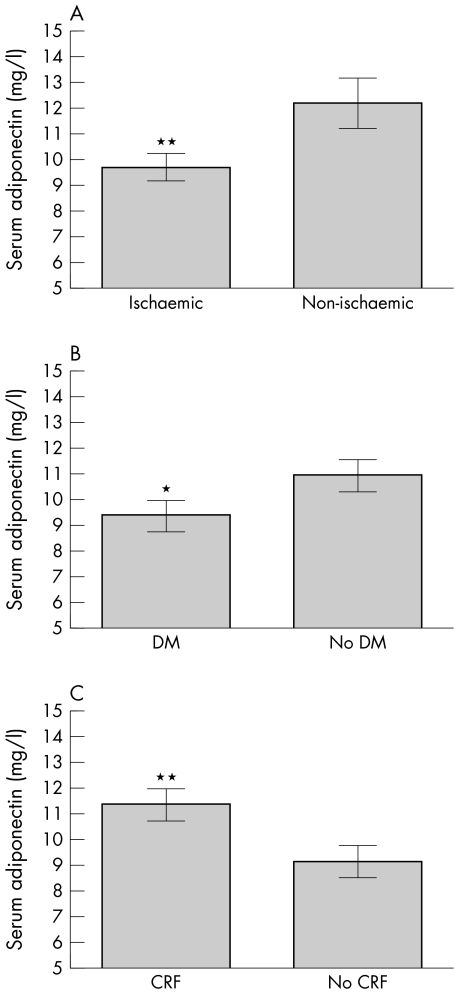
When patients with CHF were stratified according to whether they had ischaemic or non‐ischaemic CHF by evidence of coronary heart disease, the ischaemic group had significantly lower adiponectin serum concentrations (9.7 (0.4) mg/l) than the non‐ischaemic group (12.3 (0.9) mg/l, p = 0.009) (fig 2A). The presence of diabetes mellitus in patients with CHF was also associated with reduced serum concentrations of adiponectin (9.3 (0.6) mg/l) in patients with v 10.9 (0.6) mg/l without diabetes, p < 0.05) (fig 2B). Patients with CHF and chronic renal failure had significantly higher circulating adiponectin concentrations (11.4 (0.6) mg/l) than did patients with normal creatinine concentrations (9.1 (0.6) mg/l, p = 0.007) (fig 2C).
Figure 2 Serum adiponectin according to the presence of co‐morbidities. Patients were divided according to whether they had (A) ischaemic or non‐ischaemic cardiomyopathy by the presence of coronary artery disease, (B) diabetes (DM), or (C) chronic renal failure (CRF).*p < 0.05; **p < 0.01.
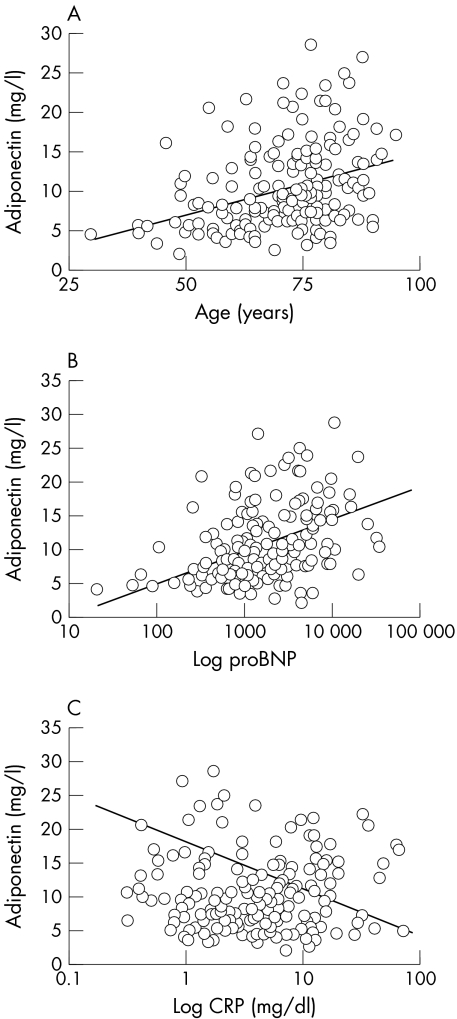
Serum adiponectin concentrations significantly and positively correlated with the age of patients with CHF (table 2, fig 3). Whereas a positive correlation was also evident with the severity of CHF estimated by NYHA class, no significant association was found between adiponectin serum concentrations and LVEF.
Table 2 Correlation of serum adiponectin with continuous clinical and laboratory variables.
| Parameter | Correlation coefficient (r) | p Value |
|---|---|---|
| Age | 0.36 | <0.0001 |
| NYHA class | 0.35 | <0.0001 |
| Ejection fraction | 0.10 | 0.08 |
| NT‐proBNP | 0.45 | <0.0001 |
| CD‐40 ligand | 0.008 | 0.45 |
| C reactive protein | −0.24 | <0.001 |
| Interleukin 12 | 0.007 | 0.46 |
| TNFα | 0.03 | 0.35 |
| Interleukin 10 | 0.13 | 0.05 |
| Interleukin 6 | 0.09 | 0.13 |
| Interleukin 1β | 0.04 | 0.29 |
| Interleukin 8 | 0.12 | 0.06 |
NYHA, New York Heart Association; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TNF, tumour necrosis factor.
Figure 3 Association of serum adiponectin with continuous determinants in patients with congestive heart failure (CHF). Adiponectin serum concentrations were correlated with (A) the age of patients with CHF, (B) their N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) concentration, and (C) their C reactive protein (CRP) concentration.
Adiponectin serum concentrations were significantly correlated with NT‐proBNP and negatively with CRP (table 2, fig 3). However, no association was found between adiponectin and either of the proinflammatory or anti‐inflammatory cytokines (TNFα, IL‐1β, IL‐12, IL‐10, IL‐6 or IL‐8), nor was adiponectin correlated with serum soluble concentrations of CD‐40 ligand.
Of the total 175 patients, 36 (20.5%) died during the two‐year follow up, whereas 40 (22.8%) were hospitalised for CHF deterioration and 64 (36.5%) reached the end point of either death or CHF hospitalisation within the follow‐up period.
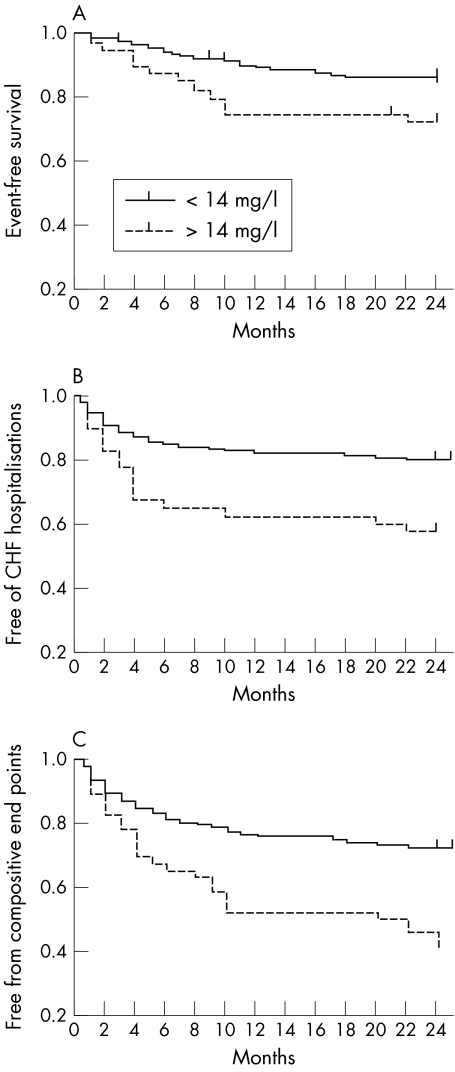
Kaplan–Meier analysis indicated differences in event‐free survival for death (fig 4A), hospitalisations caused by CHF (fig 4B), and the composite of death and CHF hospitalisation (fig 4C) when the group was divided according to concentrations above and below the 75th centile of serum adiponectin (set at 14 mg/l).
Figure 4 Survival curves according to baseline adiponectin concentrations above or below the 75th centile of 14 mg/l. Patients with congestive heart failure (CHF) had their baseline serum adiponectin evaluated and were followed up for two years for the occurrence of (A) all‐cause death, (B) hospitalisation for CHF, or (C) the first of the composite end points.
Multivariate analysis by stepwise Cox proportional hazards regression analysis (incorporating as putative predictors age, NYHA class, sex, LVEF, diabetes and serum TNFα, IL‐6, IL‐10, IL‐8, IL‐12 CD‐40 ligand, IL‐1β, CRP and NT‐proBNP) confirmed that serum adiponectin concentrations (determine dichotomously) remained independently predictive of death with a risk ratio of 2.2 (95% CI 1.1 to 5.9, p = 0.005). Similarly, and more significantly, serum adiponectin concentration was an independent predictor of CHF hospitalisation during the 24‐month follow up with a risk ratio of 2.4 (95% confidence interval (CI) 1.5 to 6.7, p = 0.001) and remained so when the composite of death and CHF hospitalisation was studied as a separate end point with a risk ratio of 2.5 (95% CI 1.7 to 5.6, p < 0.0001). Both NYHA class and NT‐proBNP also were independent predictors of prespecified end points of death, CHF hospitalisations and the combined occurrence of each.
DISCUSSION
Adiponectin is an adipokine produced principally by adipose tissue that is found abundantly in plasma.1 Recent literature suggests that adiponectin acts as an anti‐inflammatory and antiatherogenic cytokine that is involved in vascular remodelling.1 The purpose of this study was to determine the concentrations of adiponectin in the serum of patients with CHF and to study its predictive value. We have found that patients with CHF have significantly higher adiponectin concentrations than do healthy age‐matched controls. Moreover, patients with more severe forms of CHF evident by higher NYHA class had higher adiponectin concentrations than patients with milder clinical presentations.
We have also found that serum adiponectin increased with age, yet it did not correlate with LVEF. The current study cannot answer why the concentrations of adiponectin are higher in patients with CHF. As adiponectin apparently is a protective agent, however, we may speculate that it is secreted by the adipocytes in an attempt to counteract the robust activation of neurohumoral and inflammatory responses. Recent publications report reduced concentrations of adiponectin in patients with obesity, diabetes and atherosclerosis.2,3,4,5,6,7 Consistent with these reports, our study found that patients with evidence of obstructive coronary artery disease as the cause of CHF had lower serum concentrations of adiponectin than did patients with other forms of heart failure. Additionally, among the whole CHF cohort, patients with diabetes appeared to have lower concentrations of circulating adiponectin than patients without diabetes. Renal failure is a significant co‐morbidity in patients with CHF. A recent study has shown that patients with chronic renal failure have higher serum concentrations of adiponectin that was associated with the degree of proteinuria, suggesting that adiponectin negates, by its anti‐inflammatory properties, acute phase reactants. Herein, our results were similar in our CHF cohort—namely, that patients with chronic renal failure had significantly raised concentrations of adiponectin.
Interestingly, serum adiponectin concentrations were found to correlate significantly with serum NT‐proBNP and negatively with CRP concentrations, suggesting that the production of adiponectin by adipocytes parallels the activation of neurohormonal and inflammatory axes. These results are consistent with a recent publication suggesting a reciprocal association between adiponectin and CRP.9 However, unexpectedly, additional inflammatory cytokines such as TNFα and IL‐6, which are known to be increased in patients with CHF,15,16 did not correlate with adiponectin concentrations as has been shown in a recent study.17 These findings may be related to the significant co‐morbidities in this older cohort of patients with CHF that probably interact in a complex manner with innate and adaptive immune responses.
An additional interesting and potentially useful finding of this study was the predictive value ascribed to circulating adiponectin concentrations. We have found that at serum adiponectin concentrations above the 75th centile, patients with CHF are at a significantly increased risk of death, irrespective of other baseline clinical or laboratory findings. This independent predictive power was found to be even more significant with respect to “softer” end points such as either hospitalisation for CHF or a composite of death and CHF hospitalisation (fig 4).
In conclusion, we have shown that the serum concentrations of adiponectin, an antidiabetic, anti‐inflammatory and antiatherogenic cytokine, are increased in patients with CHF and positively correlate with the severity of the clinical syndrome. Adiponectin concentrations can also predict a higher likelihood of death and morbidity and serve as a potential marker for monitoring patients with CHF and predicting their outcome.
Abbreviations
CBA - cytometric bead array
CHF - congestive heart failure
CRP - C reactive protein
IL - interleukin
LVEF - left ventricular ejection fraction
NT‐proBNP - N‐terminal pro‐brain natriuretic peptide
NYHA - New York Heart Association
TNF - tumour necrosis factor
References
- 1.Haluzik M, Parizkova J, Haluzik M M. Adiponectin and its role in the obesity‐induced insulin resistance and related complications. Physiol Res 200453123–129. [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N.et al Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 199925779–83. [DOI] [PubMed] [Google Scholar]
- 3.Cnop M, Havel P J, Utzschneider K M.et al Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 200346459–469. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S.et al Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001861930–1935. [DOI] [PubMed] [Google Scholar]
- 5.Hotta K, Funahashi T, Arita Y.et al Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000201595–1599. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay R S, Funahashi T, Hanson R L.et al Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 200236057–58. [DOI] [PubMed] [Google Scholar]
- 7.Kumada M, Kihara S, Sumitsuji S.et al Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 20032385–89. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y.et al Novel modulator for endothelial adhesion molecules: adipocyte‐derived plasma protein, adiponectin. Circulation 19991002473–2476. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Kihara S, Funahashi T.et al Reciprocal association of C‐reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003107671–674. [DOI] [PubMed] [Google Scholar]
- 10.Matsubara M, Namioka K, Katayose S. Decreased plasma adiponectin concentrations in women with low‐grade C‐reactive protein elevation. Eur J Endocrinol 2003148657–662. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto Y, Kihara S, Ouchi N.et al Adiponectin reduces atherosclerosis in apolipoprotein E‐deficient mice. Circulation 20021062767–2770. [DOI] [PubMed] [Google Scholar]
- 12.Schrier R W, Abraham W. Hormones and hemodynamics in heart failure. N Engl J Med 1999341577–585. [DOI] [PubMed] [Google Scholar]
- 13.Anon Heart Failure Society of America. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction: pharmacological approaches, J Card Fail 19995357–382. [PubMed] [Google Scholar]
- 14.Pugh P J, Jones R D, Jones T H.et al Heart failure as an inflammatory condition: potential role for androgens as immune modulators. Eur J Heart Fail 20024673–680. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Kalman J, Mayer L.et al Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990323236–241. [DOI] [PubMed] [Google Scholar]
- 16.Lommi J, Pulkki K, Koskinen P.et al Haemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure. Eur Heart J 1997181620–1625. [DOI] [PubMed] [Google Scholar]
- 17.Engeli S, Feldpausch M, Gorzelniak K.et al Association between adiponectin and mediators of inflammation in obese women. Diabetes 200352942–947. [DOI] [PubMed] [Google Scholar]