Abstract
Objective
To study the impact on outcomes of direct admission versus emergency room (ER) admission in patients with ST‐segment elevation myocardial infarction (STEMI)
Design
Nationwide observational registry of STEMI patients
Setting
369 intensive care units in France.
Interventions
Patients were categorised on the basis of the initial management pathway (direct transfer to the coronary care unit or catheterisation laboratory versus transfer via the ER).
Main outcome measures
Delays between symptom onset, admission and reperfusion therapy. Mortality at five days and one year.
Results
Of 1204 patients enrolled, 66.9% were admitted direct and 33.1% via the ER. Bypassing the ER was associated with more frequent use of reperfusion (61.7% v 53.1%; p = 0.001) and shorter delays between symptom onset and admission (244 (interquartile range 158) v 292 (172) min; p < 0.001), thrombolysis (204 (150) v 258 (240) min; p < 0.01), hospital thrombolysis (228 (156) v 256 (227) min, p = 0.22), and primary percutaneous coronary intervention (294 (246) v 402 (312) min; p < 0.005). Five day mortality rates were lower in patients who bypassed the ER (4.9% v 8.6%; p = 0.01), regardless of the use and type of reperfusion therapy. After adjusting for the simplified Thrombolysis in Myocardial Infarction (TIMI) risk score, admission via the ER was an independent predictor of five day mortality (odds ratio 1.67, 95% confidence interval 1.01 to 2.75).
Conclusions
In this observational analysis, bypassing the ER was associated with more frequent and earlier use of reperfusion therapy, and with an apparent survival benefit compared with admission via the ER.
Keywords: acute myocardial infarction, emergency room, reperfusion therapy, percutaneous coronary intervention
The management of patients with acute ST segment elevation myocardial infarction (STEMI) is centred on the fastest possible implementation of reperfusion therapy. Many patients with symptoms of STEMI are identified in a prehospital setting—at their home, workplace or in public areas—by emergency physicians or paramedics. These patients are usually initially brought to the emergency room (ER) before being admitted to a coronary care unit (CCU). However, for patients who contact the medical system upstream of the hospital and are diagnosed with STEMI before arriving at the hospital, it is possible to bypass the ER and admit them directly to the CCU or even directly to a catheterisation laboratory, if available.
In France, most patients with STEMI who present out‐of‐hospital are managed by mobile intensive care units staffed by paramedics and a physician. The patient, their relatives or friends, a physician or a paramedic may call a centralised phone system for medical emergencies, which will usually dispatch a mobile intensive care unit to the patient. The staff in the mobile intensive care unit can then assess the patient, record an ECG, start initial treatment immediately (which may include prehospital thrombolysis) on site, and then transfer the patient to hospital. Once the decision regarding hospital transfer has been made, however, the patient may be dispatched by the mobile intensive care unit to the hospital ER or transferred direct to the CCU or even to the catheterisation laboratory, thereby bypassing the ER. The decision to direct the patient to the CCU or the ER rests on mobile intensive care unit physicians and may vary according to local practice, CCU availability or patient characteristics. There have been few studies on patient profiles, impact on delays, implementation of reperfusion therapy and clinical outcomes related to these decisions.1,2,3 Therefore we analysed data from a large French contemporary nationwide cohort of patients with STEMI to compare patients admitted directly to a CCU (or to a catheterisation laboratory), bypassing the ER, with those admitted via the ER.
METHODS
Population
The population and methods of the USIC 2000 registry have been described in detail elsewhere.1 Briefly, the objective of the study was to gather complete and representative data on the management and outcome of patients admitted to intensive care units for definite acute myocardial infarction over a one month period in France, regardless of the type of institution to which the patients were admitted (that is, university hospitals, public hospitals or private clinics). Of the 443 centres that treated patients with acute myocardial infarction nationwide at that time, 369 (83%) participated in the study. One physician responsible for the study was recruited in each centre; they completed a case record form for each patient meeting the inclusion criteria and admitted to the intensive care unit during the recruitment period. The physicians in charge of the patients took care of them according to their usual practice and independently from the study. The methods for this prospective registry are similar to those of a previous survey carried out in France five years earlier,4,5 although more data were collected in the current registry.
Patient selection
All consecutive patients admitted to the participating centres from 1–30 November 2000 were included in the registry if they had elevated serum markers of myocardial necrosis higher than twice the upper limit of normal for creatine kinase, creatine kinase‐MB or troponins, and symptoms compatible with acute myocardial infarction for 30 min and/or electrocardiographic changes on at least two contiguous leads with pathological Q waves (⩾ 0.04 s) and/or persisting ST elevation or depression > 0.1 mV, or a presumed new left bundle branch block on the first ECG recorded. ECGs were interpreted by local investigators at each site. The time from symptom onset to admission to the intensive care unit had to be < 48 h for the patient to be included in the registry. The present analysis, however, focuses on patients with a confirmed diagnosis of STEMI who were admitted within 12 h of the onset of symptoms. Patients who were transferred from another hospital were excluded. Patients gave informed consent for participation in the survey and for follow‐up. Specific instructions were given to participating physicians not to exclude patients in a critical condition or dying and to gather consent from the patient or their family at a later time.
Data collection and definitions
Cardiovascular history, current medications at the time of admission, risk factors, in‐hospital clinical course (including maximal Killip class) and initial diagnostic and therapeutic management were recorded for each patient. Prehospital thrombolysis was defined as initiating thrombolytic treatment before admission to hospital. For this analysis, direct admission was defined as admission to either the CCU or the catheterisation laboratory. Admission via the ER implies that patients were initially transported to the ER, regardless of the duration of their stay in the ER and of whether they were subsequently transported to the CCU or to another department. Time to admission was defined as time between symptom onset and actual arrival in the CCU or catheterisation laboratory (not time to registration in the hospital database), as recorded per patient's chart or investigator notes. The time of thrombolysis was defined as the time of starting the infusion or bolus injection of the drug. The time of primary percutaneous coronary intervention (PCI) was the time of arterial puncture.
A subsequent analysis focused on patients who received reperfusion therapy at any time during the first 12 h after symptom onset. Reperfusion therapy was defined as prehospital or hospital thrombolysis or primary PCI.
Statistical analysis
All continuous variables are given as mean (SD). All categorical variables are described using absolute and relative frequency distributions. Comparisons between groups were made with one‐way analysis of variance (ANOVA), with unpaired t tests for continuous variables and χ2 tests for discrete variables. Multivariable stepwise logistic regression analysis was used to assess the independent predictive value of baseline parameters (age, sex, previous medical history, risk factors, Killip class, infarct location and delays) on five day mortality. Cox multivariable regression analysis was used for assessing predictors of one year mortality. Odds ratios (ORs) and hazard ratios (HRs) are reported with 95% confidence intervals (CIs) and p values. For both multivariable analyses, variables with a value of p < 0.15 on univariable analyses were included in the models. Additional multivariable regression analyses were performed, adjusting for the simplified TIMI (Thrombolysis In Myocardial Infarction) risk score for STEMI.6 Survival curves were generated with the Kaplan‐Meier method and compared through the use of log‐rank tests. For all tests, a value of p < 0.05 was considered significant.
RESULTS
Patient characteristics
Overall, 1922 patients were admitted to the CCU within 48 h of symptom onset, of which 1204 fulfilled the criteria for this analysis (confirmed diagnosis of STEMI and admission within 24 h of symptom onset). Of these 1204 patients, 66.9% were admitted direct to the CCU or catheterisation laboratory whereas 33.1% were taken to the ER before admission to the CCU. Patients admitted via the ER tended to have slightly more severe baseline characteristics than those who bypassed the ER, and a higher average Killip class upon admission and with higher TIMI risk scores (table 1).
Table 1 Patient baseline characteristics and in‐hospital management of the total cohort of patients admitted within 12 h of onset of symptoms (n = 1204).
| Characteristic | Direct admission to CCU | Admission via ER | p Value |
|---|---|---|---|
| n = 806 (66.9%) | n = 398 (33.1%) | ||
| Age (years)* | 64.1 (14) | 64.6 (15) | 0.56 |
| Age ⩾70 years | 309 (38.3%) | 175 (44.0%) | 0.06 |
| Sex (female) | 194 (24.1%) | 120 (30.2%) | 0.025 |
| Hypertension | 350 (43.4%) | 180 (45.2) | 0.55 |
| Diabetes | 155(19.2%) | 78 (19.6%) | 0.88 |
| Hypercholesterolaemia | 327 (41.3%) | 159 (40.3%) | 0.72 |
| Current smoker | 301 (37.4%) | 131 (33.2%) | 0.15 |
| History of myocardial infarction | 115 (14.3%) | 65 (16.3%) | 0.34 |
| History of congestive heart failure | 35 (4.3%) | 19 (4.8%) | 0.72 |
| History of stroke | 30 (3.7%) | 21 (5.3%) | 0.20 |
| History of CABG | 25 (3.1%) | 14 (3.5%) | 0.70 |
| Previous PCI | 63 (7.9%) | 32 (8.0%) | 0.91 |
| Peripheral artery disease | 58 (7.2%) | 34 (8.6%) | 0.41 |
| Renal insufficiency | 27 (3.4%) | 21 (5.3%) | 0.10 |
| Anterior infarct | 292 (36.3%) | 162 (40.8%) | 0.13 |
| Killip class at admission | 0.01 | ||
| Missing | 3 | 0 | |
| I | 659 (82.1%) | 305 (76.6%) | |
| II–III | 124 (15.4%) | 88 (22.1%) | |
| IV | 20 (2.5%) | 5 (1.3%) | |
| TIMI risk score* | |||
| All patients | 3.1 (2.3) | 3.5 (2.6) | 0.03 |
| Excluding patients with prehospital lysis | 3.3 (2.4) | 3.5 (2.6) | 0.11 |
| Medical treatment 48 h after admission | |||
| Oral antiplatelet agents | 772 (95.8%) | 377 (94.7%) | 0.41 |
| Glycoprotein IIb/IIIa inhibitors | 194 (24.1%) | 55 (15.5%) | 0.001 |
| β blockers | 608 (75.4%) | 288 (72.4%) | 0.25 |
| ACE inhibitors | 323 (40.1%) | 157 (39.4%) | 0.83 |
| Intravenous inotropes | 42 (5.2%) | 26 (6.5%) | 0.35 |
| Statins | 405 (50.2%) | 163 (41.0%) | 0.002 |
| In‐hospital use of angiography/revascularisation | |||
| Coronary angiography | 686 (85.1%) | 301 (75.6%) | <0.001 |
| PCI at any time during hospital stay (including primary PCI) | 554 (69.9%) | 212 (55.6%) | <0.001 |
| PCI (after first 24 h) | 182 (23.0%) | 107 (28.1%) | 0.06 |
| CABG | 15 (1.9%) | 15 (3.8%) | <0.05 |
Data are number (%) unless otherwise indicated.
*Data presented as mean (SE).
ACE, angiotensin‐converting enzyme; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCU, coronary care unit; ER, emergency room; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Delays and in‐hospital management
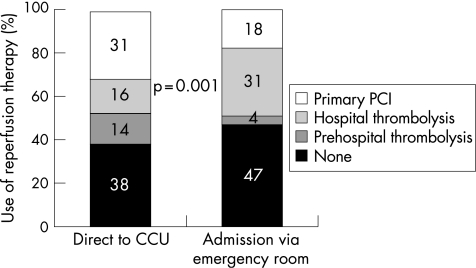
Direct transfer to the CCU was associated with a shorter delay between symptom onset and admission to the CCU or catheterisation laboratory (244 (interquartile range 158) compared to 292 (172) min; p < 0.001) (table 2). The use of any reperfusion therapy was higher among patients admitted direct to the CCU (61.7% v 53.1%; p = 0.001) due to a higher rate of use of prehospital thrombolysis and primary PCI than in patients admitted via the ER (fig 1). The in‐hospital management of patients was similar between the two groups (table 1), except for a higher early use of glycoprotein IIb/IIIa inhibitors and statins in patients admitted direct to the CCU. There was more frequent use of coronary angiography in patients admitted direct, as well as more frequent use of PCI, but this was related to the more frequent use of primary PCI. After the first 24 h the use of PCI was similar in both groups. Slightly more patients underwent coronary artery bypass grafting (CABG) among those who were admitted via the ER compared with those admitted direct.
Table 2 Delays from symptom onset to admission and from symptom onset to reperfusion therapy.
| Direct admission to CCU (%) | Admission via ER (%) | p Value | |
|---|---|---|---|
| Delay from symptom onset to admission (min) | |||
| All patients | 244 (158) | 292 (172) | <0.001 |
| Patients receiving reperfusion therapy | 215 (127) | 250 (146) | 0.002 |
| Patients with reperfusion therapy (prehospital thrombolysis excluded) | 245 (164) | 292 (172) | 0.001 |
| Delay from symptom onset to reperfusion therapy (min) | |||
| Symptom onset to start of thrombolysis | 204 (150) | 258 (240) | <0.01 |
| Symptom onset to thrombolysis (prehospital thrombolysis excluded) | 228 (156) | 256 (227) | 0.22 |
| Symptom onset to start of PCI | 294 (246) | 402 (312) | <0.01 |
CCU, coronary care unit; ER, emergency room; PCI, percutaneous coronary intervention.
Data shown as median (interquartile range).
Figure 1 Use of reperfusion therapy according to admission pathway. CCU, coronary care unit; PCI, percutaneous coronary intervention.
Outcomes
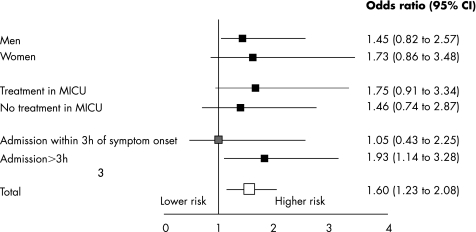
At five days, all‐cause mortality was 4.9% in patients admitted direct to the CCU compared with 8.6% (p = 0.01) in those admitted via the ER. By multivariable analysis (tables 3 and 4, fig 2), admission via the ER was an independent correlate of five day mortality when adjusting for the simplified TIMI risk score (OR 1.67, 95% CI 1.01 to 2.75) (fig 2). Subset analyses found that the benefit of bypassing the ER on adjusted five day mortality was consistent across sex, and was observed regardless of whether or not the patients had been treated in mobile intensive care units (fig 2). There was a non‐significant trend for a greater benefit of bypassing the ER in those patients with delays to therapy > 3 h as opposed to ⩽ 3 h after symptom onset.
Table 3 All‐cause mortality at day 5 with adjusted odds ratios.
| Five day mortality | Direct admission to CCU (%) | Admission via ER (%) | p Value | TIMI risk‐adjusted odds ratio | 95% CI | p Value |
|---|---|---|---|---|---|---|
| All patients (n = 1201) | 4.9 | 8.6 | 0.01 | 1.67 | 1.01 to 2.75 | 0.04 |
| Patients receiving reperfusion therapy (n = 787) | 3.8 | 8.5 | 0.007 | 2.47 | 1.25 to 4.86 | 0.009 |
| Patients receiving reperfusion therapy (pre‐hospital lysis excluded) (n = 634) | 4.5 | 8.7 | 0.03 | 2.15 | 1.09 to 4.16 | 0.03 |
CCU, coronary care unit; CI, confidence interval; ER, emergency room; TIMI, Thrombolysis In Myocardial Infarction.
Table 4 All‐cause mortality at one year, with adjusted hazard ratios.
| One year mortality | Direct admission to CCU (%) | Admission via ER (%) | p Value | TIMI risk‐adjusted hazard ratio | 95% CI | p Value |
|---|---|---|---|---|---|---|
| All patients (n = 1201) | 11.5 | 15.6 | <0.05 | 1.25 | 0.90 to 1.72 | 0.18 |
| Patients receiving reperfusion (n = 787) | 8.2 | 12.2 | 0.07 | 1.52 | 0.96 to 2.43 | 0.076 |
| Patients receiving reperfusion therapy (pre‐hospital lysis excluded) (n = 634) | 9.5 | 12.3 | 0.27 | 1.36 | 0.86 to 2.16 | 0.19 |
CCU, coronary care unit; CI, confidence interval; ER, emergency room; TIMI, Thrombolysis In Myocardial Infarction.
Figure 2 Independent predictors of five day mortality for patients admitted via the emergency room compared to those admitted direct, for the whole population and across selected subgroups, adjusting for the simplified TIMI risk score. MICU, mobile intensive care units.
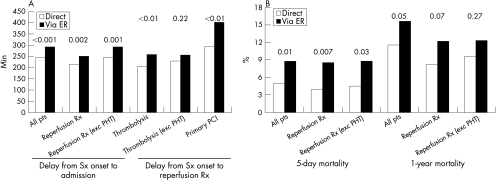
One year follow‐up data were obtained in 91% of patients (99% had one month follow‐up available and 94% had six month follow‐up available). One year all‐cause mortality was lower in patients admitted direct to the CCU compared with those admitted via the ER (11.5% v 15.6%; p < 0.05) (fig 3A), although, after multivariable analysis adjusting for the simplified TIMI risk score, admission via the ER was not an independent predictor of one year mortality (table 4).
Figure 3 (A) Delays between symptom onset and admission or start of reperfusion therapy. (B) Five day and one year mortality. ER, emergency room; PCI, percutaneous coronary intervention; PHT, pre‐hospital thrombolysis; Rx, therapy; Sx, symptom.
Analysis of patients who received reperfusion therapy
In order to summarise the main results, delays and mortality are displayed in fig 3 for each of the subsets. Among the whole cohort, 787 (65.4%) patients received reperfusion therapy. Admission always occurred earlier in the group transported direct to the CCU compared with those admitted via the ER; direct transfer to the CCU was also associated with consistently shorter delays between symptom onset and the start of thrombolysis (although the difference was not significant when patients receiving pre‐hospital thrombolysis were excluded) or the start of primary PCI (table 2). To exclude the possibility that the differences between groups were solely driven by a more frequent use of pre‐hospital thrombolysis in the group which bypassed the ER, we reanalysed the data after excluding these 153 patients.
After five days, total mortality was lower in patients who bypassed the ER (3.8% v 8.5%; p = 0.007), even after exclusion of patients who received pre‐hospital thrombolysis. The survival benefit was consistent regardless of whether reperfusion was attempted by primary PCI or intravenous thrombolysis. For patients who were treated by primary PCI, in‐hospital mortality was 3.1% v 9.0% (p = 0.02, OR 3.07, 95% CI 1.04 to 9.03) for patients bypassing the ER versus being admitted via the ER, respectively. Likewise, for patients treated with thrombolysis, in hospital mortality was 4.6% v 8.1% (p = 0.12, OR 1.85, 95% CI 0.8 to 4.32) for patients bypassing the ER versus being admitted via the ER, respectively.
One year follow‐up data were obtained in 92% of patients initially treated with reperfusion therapy. At one year, cumulative all‐cause crude and risk‐adjusted mortality showed a consistent but not significant trend in favour of patients bypassing the ER.
In order to summarise the main results, delays and mortality are displayed for each of the subsets in fig 3.
DISCUSSION
For patients with STEMI, direct transportation to the CCU or the catheterisation laboratory, as opposed to admission via the ER, was strongly associated with more frequent use of reperfusion therapy, shorter delays to admission and shorter delays to implementation of reperfusion therapy. All of these factors are expected to be associated with improved clinical outcomes in STEMI and are therefore likely to account for at least a large fraction of the improved short‐term and one year survival seen in patients bypassing the ER. While, in theory, a well organised ER system may achieve as high a rate of reperfusion in as rapid a fashion and be associated with as low a mortality as that achieved by bypassing the ER, large‐scale longitudinal analyses concur in showing that suboptimal delays to reperfusion therapy and missed opportunities for reperfusion persist.7,8 Bypassing the ER provides a practical and simple method for reducing delays to therapy and improving reperfusion therapy rates.
Another important finding of this analysis is that bypassing the ER was associated with apparent improved survival at five days and possibly at one year. One year mortality was low in all groups compared with recent unselected cohorts,3 possibly because of the relatively high rate of use of PCI in this group. This disparity was not explained by differences in baseline characteristics, which were remarkably similar and indeed persisted after multivariable adjustment for differences in baseline characteristics.
In the subset of patients who underwent reperfusion therapy, bypassing the ER was still an independent correlate of improved five day survival and was associated with a trend for benefit up to a year, suggesting that shorter delays to therapy are indeed key to this benefit. In fact, previous data support the concept that prehospital identification and triage of patients with STEMI is associated with shorter treatment delays,2,9 especially if the patients can be triaged directly to centres equipped for PCI.3
This observation has important implications for the organisation of prehospital care of patients with STEMI. It suggests that systems should be implemented that allow for direct communication between prehospital caregivers and CCUs or catheterisation laboratories, leading to early triage of patients with STEMI to these sites, bypassing the ER. It is likely that electrocardiographic teletransmission systems are one key element in this scheme.10,11
These observations were made in France, a country in which emergency physicians are usually present in the mobile intensive care unit in the prehospital setting. However, they have relevance to other settings. Experience in prehospital thrombolysis has demonstrated that properly trained primary care physicians and paramedics are able to identify patients with STEMI and to deliver prehospital thrombolysis safely, effectively and in a timely fashion.12,13,14 In fact, international comparisons in the ASSENT‐3 PLUS (Assessment of the Safety and Efficacy of a New Thrombolytic‐3 Plus) trial of prehospital thrombolysis demonstrated that countries participating in the trial in which physicians were involved in the prehospital care performed no better than countries in which the identification of patients and the administration of prehospital thrombolysis was carried out by paramedics.15 Therefore, diagnosis of STEMI and triage or implementation of reperfusion therapy can be done effectively and safely16 in the prehospital setting by properly trained paramedics,17 do not specifically require physicians, and are feasible in a wide variety of settings.18,19 In fact, in the present setting, the survival benefit of bypassing the ER was consistently observed regardless of whether or not the patient had actually been cared for by a mobile intensive care unit.
The magnitude of the survival advantage of direct admission is very large, suggesting that the benefit derived from more frequent and earlier implementation of reperfusion therapy may be larger than that derived from many sophisticated mechanical and pharmacological interventions currently tested in the management of patients with STEMI. These observations are consistent with previous reports that use of emergency medical services is associated with greater and significantly faster receipt of reperfusion therapies, and was linked to a similar range of reduction in hospital mortality.2,20 This finding emphasises the need to focus attention on organisational aspects of prehospital and early hospital care in these patients. In this respect, continuous measurement of reperfusion rates and time delays (delays to admission and implementation of reperfusion therapy) are probably the most important indicators of quality of care.21,22 Structured programmes should be available to guide medical and paramedical personnel, both prehospital and in the ER, in determining where to take patients with suspected STEMI and how to “fast‐track” them towards reperfusion therapy, as recommended in the European Society of Cardiology and American College of Cardiology/American Heart Association guidelines for the management of STEMI.23,24
Limitations of this analysis
There are several limitations to this analysis. Although 83% of the French CCUs participated in this survey, participation was voluntary and we cannot exclude the possibility that our sample was biased towards centres with superior performance. We also are not able to provide information regarding patients transferred direct to the CCU with suspected STEMI but in whom this diagnosis was later disproved. In addition, our ability to adjust for the impact of measured and unmeasured confounders on survival is limited. Because this is not a randomised study, it is possible that part of the survival advantage in favour of the group admitted direct to the CCU may be related to selection bias or confounding, such as an intrinsically lower risk at baseline (indeed, TIMI risk scores were slightly higher in the group admitted via the ER), prehospital evaluation and treatment initiation or unmeasured differences in hospital management in favour of patients bypassing the ER. Likewise, triage decisions may have been influenced by co‐morbidities or unmeasured characteristics. In addition, there are other factors which may impact on outcomes and could not be controlled for in this analysis, such as time of day, type of destination hospital, or type of healthcare environment (such as rural versus urban). However, the mere fact that bypassing the ER was associated with shorter delays to implementation of reperfusion therapy could be regarded as sufficient for recommending it. The sample size is relatively limited and represents a one month survey. Finally, we are not able to assign specific causes to the early and late deaths, nor can we provide information regarding infarct size or left ventricular function; therefore the exact mechanisms of the survival benefit of direct admission remains hypothetical, although there is a host of data to demonstrate that earlier implementation of thrombolysis25,26 or primary PCI27 is associated with reduced mortality, and that prehospital thrombolysis is very effective in reducing mortality, particularly when patients are treated very early (for example, within the first 2 h) after symptom onset.28
Conclusions
In this registry, management of STEMI by bypassing the ER and allowing for direct admission to the CCU or the catheterisation laboratory was associated with more frequent use and earlier implementation of reperfusion therapy. Although this observational analysis has the potential for confounding, bypassing the ER was associated with an apparent substantial survival benefit at both five days and one year compared with patients admitted via the ER. These findings of shorter delays and improved survival suggest that pathways should be established for patients with STEMI to bypass the ER.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the physicians and nurses participating in the USIC 2000 study. This study was supported by an unrestricted educational grant from Aventis Pharma France (now sanofi‐aventis). Dr Sophie Rushton‐Smith provided editorial assistance in the preparation of this manuscript and was funded by Association Naturalia et Biologia.
Abbreviations
ASSENT‐3 PLUS - Assessment of the Safety and Efficacy of a New Thrombolytic‐3 Plus
CABG - coronary artery bypass grafting
CCU - coronary care unit
CI - confidence interval
ER - emergency room
HR - hazard ratio
OR - odds ratio
PCI - percutaneous coronary intervention
STEMI - ST‐segment elevation myocardial infarction
TIMI - Thrombolysis In Myocardial Infarction
Footnotes
This study was supported by an unrestricted educational grant from Aventis Pharma France (now sanofi‐aventis). One of the co‐authors is an employee of Aventis (now sanofi‐aventis). The study was, however, planned, organised and analysed, and this report was written and submitted, independently of Aventis Pharma.
Competing interests: PGS: Consultant and speaker for sanofi‐aventis. Institution receives a research grant from sanofi‐aventis. J‐PC: Consultant and speaker for sanofi‐aventis. P Goldstein: Speaker for Boehringer Ingelheim and sanofi‐aventis. P Guéret: Consultant for sanofi‐aventis. J‐MJ: Speaker and consultant for sanofi‐aventis and Boehringer Ingelheim. GH: Consultant for sanofi‐aventis. LV: Full‐time employee of sanofi‐aventis. ND: speaker for sanofi‐aventis. Chair of the USIC 2000 registry Steering Committee. ED, PS, ZK, DB, J‐ML, YC, SRS: None.
References
- 1.Hanania G, Cambou J P, Gueret P.et al Management and in‐hospital outcome of patients with acute myocardial infarction admitted to intensive care units at the turn of the century: results from the French nationwide USIC 2000 registry. Heart 2004901404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canto J G, Zalenski R J, Ornato J P.et al Use of emergency medical services in acute myocardial infarction and subsequent quality of care: observations from the National Registry of Myocardial Infarction 2. Circulation 20021063018–3023. [DOI] [PubMed] [Google Scholar]
- 3.Terkelsen C J, Lassen J F, Norgaard B L.et al Mortality rates in patients with ST‐elevation vs. non‐ST‐elevation acute myocardial infarction: observations from an unselected cohort, Eur Heart J 20052618–26. [DOI] [PubMed] [Google Scholar]
- 4.Danchin N, Vaur L, Genes N.et al Management of acute myocardial infarction in intensive care units in 1995: a nationwide French survey of practice and early hospital results. J Am Coll Cardiol 1997301598–1605. [DOI] [PubMed] [Google Scholar]
- 5.Danchin N, Vaur L, Genes N.et al Treatment of acute myocardial infarction by primary coronary angioplasty or intravenous thrombolysis in the “real world”: one‐year results from a nationwide French survey. Circulation 1999992639–2644. [DOI] [PubMed] [Google Scholar]
- 6.Morrow D A, Antman E M, Giugliano R P.et al A simple risk index for rapid initial triage of patients with ST‐elevation myocardial infarction: an InTIME II substudy. Lancet 20013581571–1575. [DOI] [PubMed] [Google Scholar]
- 7.Nallamothu B K, Bates E R, Herrin J.et al Times to treatment in transfer patients undergoing primary percutaneous coronary intervention in the United States: National Registry of Myocardial Infarction (NRMI)‐3/4 analysis. Circulation 2005111761–767. [DOI] [PubMed] [Google Scholar]
- 8.Eagle K A, Mehta R H, Nallamothu B K.et al Global reperfusion therapy in acute myocardial infarction—1999 to 2004: we're getting better, but we have a long way to go. The Global Registry of Acute Coronary Events. Circulation 2005112(suppl)II794–II795. [Google Scholar]
- 9.Millar‐Craig M W, Joy A V, Adamowicz M.et al Reduction in treatment delay by paramedic ECG diagnosis of myocardial infarction with direct CCU admission. Heart 199778456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grim P, Feldman T, Martin M.et al Cellular telephone transmission of 12‐lead electrocardiograms from ambulance to hospital. Am J Cardiol 198760715–720. [DOI] [PubMed] [Google Scholar]
- 11.Karagounis L, Ipsen S K, Jessop M R.et al Impact of field‐transmitted electrocardiography on time to in‐hospital thrombolytic therapy in acute myocardial infarction. Am J Cardiol 199066786–791. [DOI] [PubMed] [Google Scholar]
- 12.Rawles J M. Quantification of the benefit of earlier thrombolytic therapy: five‐year results of the Grampian Region Early Anistreplase Trial (GREAT). J Am Coll Cardiol 1997301181–1186. [DOI] [PubMed] [Google Scholar]
- 13.Rawles J. Magnitude of benefit from earlier thrombolytic treatment in acute myocardial infarction: new evidence from Grampian region early anistreplase trial (GREAT). BMJ 1996312212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawles J. Halving of mortality at 1 year by domiciliary thrombolysis in the Grampian Region Early Anistreplase Trial (GREAT). J Am Coll Cardiol 1994231–5. [DOI] [PubMed] [Google Scholar]
- 15.Welsh R C, Chang W C, Goldstein P.et al Time to treatment and the impact of a physician on pre‐hospital management of acute ST elevation myocardial infarction: insights from the ASSENT‐3 PLUS trial. Heart 2005911400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamfers E J, Schut A, Hertzberger D P.et al Prehospital versus hospital fibrinolytic therapy using automated versus cardiologist electrocardiographic diagnosis of myocardial infarction: abortion of myocardial infarction and unjustified fibrinolytic therapy. Am Heart J 2004147509–515. [DOI] [PubMed] [Google Scholar]
- 17.Foster D B, Dufendach J H, Barkdoll C M.et al Prehospital recognition of AMI using independent nurse/paramedic 12‐lead ECG evaluation: impact on in‐hospital times to thrombolysis in a rural community hospital. Am J Emerg Med 19941225–31. [DOI] [PubMed] [Google Scholar]
- 18.Weaver W D, Eisenberg M S, Martin J S.et al Myocardial Infarction Triage and Intervention Project—phase I: patient characteristics and feasibility of prehospital initiation of thrombolytic therapy. J Am Coll Cardiol 199015925–931. [DOI] [PubMed] [Google Scholar]
- 19.van't Hof A W, van de Weteringet al A quantitative analysis of the benefits of prehospital infarct angioplasty triage on outcome in patients undergoing primary angioplasty for acute myocardial infarction. Eur Heart J 2005(suppl K)K36–K40.
- 20.Terkelsen C J, Lassen J F, Norgaard B L.et al Reduction of treatment delay in patients with ST‐elevation myocardial infarction: impact of pre‐hospital diagnosis and direct referral to primary percutanous coronary intervention. Eur Heart J 200526770–777. [DOI] [PubMed] [Google Scholar]
- 21.Lambrew C T, Bowlby L J, Rogers W J.et al Factors influencing the time to thrombolysis in acute myocardial infarction. Time to Thrombolysis Substudy of the National Registry of Myocardial Infarction‐1. Arch Intern Med 19971572577–2582. [PubMed] [Google Scholar]
- 22.Hirvonen T P, Halinen M O, Kala R A.et al Delays in thrombolytic therapy for acute myocardial infarction in Finland. Results of a national thrombolytic therapy delay study. Finnish Hospitals' Thrombolysis Survey Group. Eur Heart J 199819885–892. [DOI] [PubMed] [Google Scholar]
- 23.Van de Werf F, Ardissino D, Betriu A.et al Management of acute myocardial infarction in patients presenting with ST‐segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 20032428–66. [DOI] [PubMed] [Google Scholar]
- 24.Antman E M, Anbe D T, Armstrong P W.et al ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004110e82–292. [PubMed] [Google Scholar]
- 25.Fibrinolytic Therapy Trialists' (FTT) Collaborative Group Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994343311–322. [PubMed] [Google Scholar]
- 26.Boersma E, Maas A C, Deckers J W.et al Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996348771–775. [DOI] [PubMed] [Google Scholar]
- 27.De Luca G, Suryapranata H, Zijlstra F.et al Symptom‐onset‐to‐balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 200342991–997. [DOI] [PubMed] [Google Scholar]
- 28.Steg P G, Bonnefoy E, Chabaud S.et al Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 20031082851–2856. [DOI] [PubMed] [Google Scholar]