Abstract
Objective
To examine the relationship with outcome of plasma haemoglobin and glucose concentrations, measured soon after first hospital admission with chronic heart failure (CHF), in standard clinical practice.
Methods and results
Hospital records of 528 patients (43% women, mean age 70 years) with first hospital admission for CHF were reviewed. During follow up (mean 1257 days, range 520–1800), 240 (45%) patients died. On admission, 140 of 528 (27%) and at discharge 179 of 472 survivors (38%) were receiving treatment for diabetes. World Health Organization criteria for anaemia were met by 39% of men and 43% of women. Lower haemoglobin (hazard ratio 0.879, 95% confidence interval (CI) 0.828 to 0.933, p < 0.0001) and higher plasma glucose (hazard ratio 1.034, 95% CI 1.008 to 1.061, p = 0.009) had univariate association with all‐cause mortality. On multivariate analysis, compared with patients with a normal haemoglobin for their sex, hazard ratio was 1.415 (95% CI 1.087 to 1.841, p = 0.010) for those with low haemoglobin. All‐cause mortality fell linearly for haemoglobin up to 159 g/l, above which mortality increased. Glucose above the highest quartile (> 10 mmol/l) was an independent predictor of mortality (hazard ratio 1.966, 95% CI 1.376 to 2.810, p = 0.0002). In survivors of the index admission the association between glucose and mortality was linear, the relationship being stronger for patients without diabetes.
Conclusions
Lower haemoglobin and higher plasma glucose are associated with all‐cause mortality in CHF. Higher glucose is associated with mortality irrespective of diabetic status.
Despite recent advances in the identification and management of chronic heart failure (CHF) the prognosis for patients remains poor. In the modern treatment era, morbidity and mortality after the initial hospitalisation remain high: 25–50% of patients are readmitted within 3–6 months of discharge1 and one‐year case fatality is about 40%.2
Recent guidelines highlight the estimation of prognosis as a key element in the management of patients with CHF.3 Numerous biochemical, haemodynamic, functional, demographic, electrophysiological and psychological factors have been identified as markers of prognosis.4,5,6,7,8,9,10,11,12,13,14,15 Such reports have often been based on highly selected populations, such as those recruited to clinical trials,12 those with advanced heart failure9,10,11 or those referred for transplant assessment.7,12 Similarly, many studies have assessed invasive and non‐invasive investigations not routinely available to the vast majority of patients with CHF.6,7,11,13,15 These issues have led to attempts to develop prognostic models applicable in routine clinical practice and based on routine clinical variables.14
In CHF clinical trials16,17 and in population‐based analyses,18 anaemia is prevalent and of prognostic importance. Similarly, a concomitant diagnosis of diabetes has been reported to confer adverse prognosis in CHF.4,14 However, the prognostic power of absolute values of haemoglobin and glucose (as opposed to the diagnosis of anaemia or diabetes) has not been reported in a population with heart failure seen in routine practice. The current study aimed at examining the relationship with outcome of blood concentrations of haemoglobin and glucose, measured routinely soon after first‐ever hospital admission with heart failure.
METHODS
Patient cohort
The strategy for patient identification in this historical cohort study has been described elsewhere.2 We used routine hospital data to identify, for residents of Leicestershire, first‐ever hospitalisations with a discharge coding of heart failure. First admissions were those occurring between 1 April 1998 and 31 March 2001 of patients with no previous heart failure‐related hospitalisation.
The diagnosis of CHF was validated by review of case records and required documentation of appropriate symptoms (shortness of breath, peripheral oedema or fatigue) and physical findings such as pulmonary rales, a gallop rhythm or peripheral oedema. If doubt remained then chest radiography documented to show pulmonary oedema or a clear improvement in symptoms after diuretic treatment was accepted.
We identified 629 patients with a first recorded diagnosis of heart failure and for whom case records were available. One hundred and one (16%) patients were excluded in view of insufficient evidence for a new diagnosis of CHF. Baseline clinical characteristics relating to the index heart failure admission of the remaining 528 patients, including demographic features, clinical history and physical examination, were abstracted from the case notes by a single investigator (JN) and entered into a bespoke database. A history of coronary heart disease (angina or myocardial infarction), stroke or chronic obstructive pulmonary disease was recorded. Diabetes was recorded for patients giving a history thereof and for those treated with insulin, oral hypoglycaemia drugs or dietary restriction. A history of hypertension was recorded for patients giving a history thereof or prescribed hypertension drugs at the time of admission. The first recorded mean red blood cell volume and concentrations of haemoglobin, plasma sodium, potassium, creatinine and plasma glucose, taken on the day of admission, were retrieved from the records and verified through the hospital laboratory database.
We identified mortality in the cohort from routine death certification records provided to the local strategic health authority by the Office for National Statistics, censored at 31 March 2003. Survival was measured from the date of the first admission to the date of death. The principal outcome measure was all‐cause mortality.
Statistical analysis
Data are presented as mean (SD) for continuous variables and as proportions for categorical variables. We used Cox proportional hazards modelling to examine for an association between potential prognostic determinants and all‐cause mortality, with consideration of duration of follow up, proportionality of event occurrence, censoring, and time to event. To examine for linearity of observed associations between continuous variables and outcome, continuous variables were recoded into dummy categorical variables by quartiles. Multivariate backwards stepwise regression analysis was used, incorporating variables with univariate association of p < 0.10. Kaplan–Meier estimates were used to calculate survival over time, with differences evaluated by the log rank test. A two sided p < 0.05 was considered significant.
Patient characteristics by quartiles of haemoglobin and glucose were calculated, with differences tested by χ2. To minimise loss of power and introduction of bias by excluding patients with incomplete data,19 missing continuous variables were imputed by using the expectation‐maximisation method, based on the correlation between variables with absent values and all other variables as estimated from the set of complete subjects.
RESULTS
Patient characteristics
The study cohort comprised 528 patients, 43% female, with an average age at admission of 70 years (table 1). During follow up (mean 1257 days, range 520–1800), 240 (45%) patients died, 56 (11%) during the index admission. A history of heart failure was documented in only 12%, although 40% were prescribed loop diuretics at admission. Hypertension, coronary heart disease and current or previous smoking were each documented for about one third of patients. One hundred and forty of 528 (27%) patients at admission and 179 of 472 (38%) of those surviving to discharge were receiving insulin, oral hypoglycaemic or dietary treatment for diabetes.
Table 1 Demographic details of 528 index admissions and 472 survivors to discharge.
| Admission | Discharge | |
|---|---|---|
| Number | 528 | 472 |
| Men/women | 302 (57%)/226 (43%) | |
| Mean age (range) (years) | 70 (43–89) | |
| Medical history | ||
| Angina | 122 (23%) | |
| MI | 111 (21%) | |
| MI or angina | 186 (35%) | |
| Hypertension | 197 (37%) | |
| Hyperlipidaemia | 30 (6%) | |
| CVA | 57 (11%) | |
| Any diabetes | 140 (27%) | |
| Diet | 47 (9%) | |
| OHA | 16 (3%) | |
| Insulin | 77 (15%) | |
| Renal failure | 10 (2%) | |
| Malignancy | 15 (3%) | |
| Heart failure | 62 (12%) | |
| Current smoker | 83 (16%) | |
| Former smoker | 105 (20%) | |
| Prescribed treatment known | 518 (98%) | 472 (100%) |
| Diuretic | 237 (46%) | 352 (75%) |
| Loop diuretic | 210 (40%) | 346 (73%) |
| Thiazide | 27 (5%) | 6 (1%) |
| ACEI/ARB | 145 (27%) | 284 (60%) |
| Aspirin | 148 (29%) | 210 (45%) |
| β blocker | 66 (13%) | 78 (16%) |
| Digoxin | 36 (7%) | 76 (16%) |
| Statin | 35 (7%) | 76 (16%) |
| No drugs | 126 (24%) | 0 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CVA, cerebrovascular accident (stroke or transient ischaemic attack); MI, myocardial infarction; OHA, oral hypoglycaemic agent.
As might be expected in a relatively elderly cohort of hospitalised patients, plasma creatinine exceeded the upper limit of the normal range in one third of patients. Hyponatraemia at admission was recorded for 15% of patients. Hypokalaemia and hyperkalaemia were uncommon. Anaemia, as defined by the hospital normal ranges (men, haemoglobin 130–180 g/l; women, 115–165 g/l), was recorded in 118 of 302 (39%) of men and 72 of 226 (32%) of women. Applying the World Health Organization (WHO) criteria of anaemia, haemoglobin < 130 g/l in men and < 120 g/l in women, increased the prevalence of anaemia in women to 43%.
Prognosis
Greater age, lower systolic and diastolic blood pressures, lower plasma sodium, higher potassium and creatinine, lower haemoglobin and higher plasma glucose each had univariate association with increased risk of all‐cause mortality (table 2).
Table 2 Crude association of potential prognostic determinants with all‐cause mortality in 528 patients.
| Variable | Survivors | Deaths | Hazard ratio (95% CI) | p Value | Missing values |
|---|---|---|---|---|---|
| Number | 288 (55%) | 240 (45%) | |||
| Age (years) | 67 (10.3) | 72 (9.9) | 1.040 (1.026 to 1.053) | <0.0001 | 0 |
| Men | 168 (56%) | 134 (44%) | |||
| Women | 120 (54%) | 106 (46%) | 1.079 (0.836 to 1.392) | 0.560 | 0 |
| Medical history | |||||
| Angina | 66 (23%) | 56 (23%) | 0.990 (0.734 to 1.335) | 0.948 | 0 |
| Myocardial infarction | 54 (19%) | 57 (24%) | 1.253 (0.931 to 1.687) | 0.137 | 0 |
| Hypertension | 112 (39%) | 85 (35%) | 0.884 (0.678 to 1.151) | 0.360 | 0 |
| Stroke | 27 (9%) | 30 (13%) | 1.234 (0.841 to 1.809) | 0.282 | 0 |
| Diabetes | 69 (24%) | 74 (31%) | 1.275 (0.970 to 1.677) | 0.082 | 0 |
| COPD | 19 (7%) | 28 (12%) | 1.485 (1.001 to 2.202) | 0.049 | 0 |
| Physical examination | |||||
| Heart rate (beats/min) | 94 (24.2) | 94 (22.4) | 1.001 (0.996 to 1.007) | 0.622 | 40 (7.6%) |
| SBP (mm Hg) | 144 (27.1) | 139 (28.3) | 0.993 (0.989 to 0.998) | 0.007 | 59 (11.2%) |
| DBP (mm Hg) | 86 (18.5) | 80 (16.5) | 0.984 (0.977 to 0.992) | <0.0001 | 58 (11%) |
| Biochemical data | |||||
| Sodium (mmol/l) | 138 (4.4) | 137 (4.8) | 0.969 (0.943 to 0.995) | 0.020 | 1 (0.2%) |
| Potassium (mmol/l) | 4.1 (0.6) | 4.3 (0.8) | 1.374 (1.157 to 1.632) | <0.0001 | 1 (0.2%) |
| Creatinine (µmol/l) | 109 (51.9) | 141 (100.5) | 1.003 (1.002 to 1.004) | <0.0001 | 1 (0.2%) |
| Haemoglobin (g/l) | 132 (19) | 125 (22) | 0.879 (0.828 to 0.933) | <0.0001 | 2 (0.4%) |
| Plasma glucose (mmol/l) | 8.4 (3.9) | 9.4 (4.8) | 1.034 (1.008 to 1.061) | 0.009 | 96 (18.2%) |
| Drugs at baseline | |||||
| Aspirin | 84 (29) | 64 (27) | 0.962 (0.723 to 1.278) | 0.787 | 10 (2%) |
| ACEI/ARB | 83 (29) | 62 (26) | 0.917 (0.687 to 1.224) | 0.557 | 10 (2%) |
| Diuretic | 116 (40) | 121 (50) | 1.415 (1.099 to 1.822) | 0.007 | 10 (2%) |
| β blocker | 45 (16) | 21 (9) | 0.629 (0.402 to 0.984) | 0.042 | 10 (2%) |
| Statin | 25 (9) | 10 (4) | 0.572 (0.304 to 1.077) | 0.083 | 10 (2%) |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 3 shows the results of multivariate Cox modelling, with haemoglobin stratified by sex‐related lower limit of normal, and other continuous variables categorised by quartile for the entire cohort and for those patients surviving to discharge from the index admission. Within the cohort as a whole, greater age, lower systolic blood pressure, higher creatinine concentration, abnormal haemoglobin, glucose raised to the highest quartile, and prior diuretic were the variables retaining association with all‐cause mortality on multivariate analysis. The association between age and all‐cause mortality was linear: compared with patients in the lowest age quartile (< 63 years), for patients aged 63–70 years the adjusted hazard ratio for death was 1.575, for age 71–77 it was 1.873, and for patients aged > 77 years it was 2.330. There was a linear relationship between admission creatinine and all‐cause mortality. For the entire cohort and for patients surviving the index admission, creatinine in the highest quartile (> 133 µmol/l) was associated with a greater than twofold case fatality rate than that for the lowest quartile (< 85 µmol/l) (table 3). Although the risk of death was about 70% greater for lowest systolic blood pressure than for the highest in all patients, this relationship was less apparent for patients surviving to discharge.
Table 3 Relationship of clinical variables with all‐cause mortality in entire cohort and in 472 survivors of index admission: multivariate analysis.
| Predictor | All patients (n = 528) | Survived index admission (n = 472) | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (years) | ||||
| <63 | 1.00 | 1.00 | ||
| 63–70 | 1.575 (1.030 to 2.407) | 0.036 | 1.756 (1.079 to 2.858) | 0.024 |
| 71–77 | 1.873 (1.238 to 2.834) | 0.003 | 2.182 (1.356 to 3.510) | 0.001 |
| >77 | 2.330 (1.542 to 3.520) | 0.0006 | 2.550 (1.577 to 4.123) | 0.0001 |
| Systolic blood pressure (mm Hg) | ||||
| >158 | 1.00 | 1.00 | ||
| 140–158 | 1.588 (1.094 to 2.304) | 0.015 | 1.480 (0.988 to 2.216) | 0.057 |
| 122–139 | 1.158 (0.770 to 1.743) | 0.481 | 0.954 (0.605 to 1.506) | 0.954 |
| <122 | 1.727 (1.174 to 2.541) | 0.006 | 1.454 (0.942 to 2.243) | 0.091 |
| Plasma creatinine (µmol/l) | ||||
| <85 | 1.00 | 1.00 | ||
| 85–104 | 1.452 (0.955 to 2.208) | 0.081 | 1.224 (0.771 to 1.943) | 0.392 |
| 105 to 133 | 1.323 (0.869 to 2.016) | 0.192 | 1.256 (0.796 to 1.982) | 0.327 |
| >133 | 2.388 (1.589 to 3.588) | <0.0001 | 2.044 (1.303 to 3.206) | 0.002 |
| Plasma glucose (mmol/l) | ||||
| <6.0 | 1.00 | 1.00 | ||
| 6.0–7.6 | 1.193 (0.812 to 1.752) | 0.368 | 1.135 (0.718 to 1.792) | 0.588 |
| 7.7–10.0 | 1.188 (0.841 to 1.733) | 0.372 | 1.474 (0.965 to 2.253) | 0.073 |
| >10.0 | 1.966 (1.376 to 2.810) | 0.0002 | 2.186 (1.440 to 3.320) | 0.0002 |
| Haemoglobin | ||||
| Normal | 1.00 | 1.00 | ||
| Below normal range* | 1.415 (1.087 to 1.841) | 0.010 | 1.388 (1.025 to 1.878) | 0.034 |
| Diuretic on admission | 1.364 (1.055 to 1.763) | 0.018 | 1.541 (1.148 to 2.068) | 0.004 |
*Defined as 130 g/l for men and 115 g/l for women.
HR, hazard ratio.
Haemoglobin
As haemoglobin fell, the mean age of patients, the proportion of women and plasma creatinine each increased. Plasma sodium paralleled haemoglobin. Admission drugs did not vary by quartile of haemoglobin, although there was a trend to more prevalent diuretic use by patients with haemoglobin in the lowest quartile (first quartile 51% v fourth quartile 42%, p = 0.081).
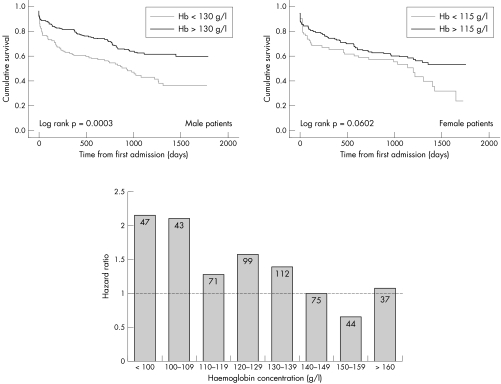
In the entire cohort and in those surviving to discharge, abnormal haemoglobin was independently associated with all‐cause mortality (table 3). Compared with patients with a normal haemoglobin for their sex, in those with a haemoglobin below the sex‐specific lower limit of normal the hazard of death was increased by 42% overall, with a greater influence of abnormal haemoglobin in male patients (fig 1). The nature of the anaemia, as assessed by the presence of normocytosis, microcytosis or macrocytosis, did not influence outcome.
Figure 1 Kaplan–Meier survival analysis by haemoglobin (Hb) stratified by sex (top) and hazard ratio by 10 g/l increments of haemoglobin (bottom).
Figure 1 also illustrates the hazard ratio for mortality associated with each 10 g/l increment in haemoglobin. For haemoglobin concentrations up to 159 g/l, there was a linear, inverse relationship between haemoglobin and all‐cause mortality with greater risk of adverse outcome for haemoglobin ⩾ 160 g/l.
Plasma glucose
The proportion of patients with diabetes increased from the lowest to highest quartile of admission glucose (⩽ 6.0 mmol/l, 16%; 6.0–7.6 mmol/l, 17%; 7.6–10.0 mmol/l, 24%; > 10.0 mmol/l, 51%; p < 0.0001).
For the entire cohort, glucose was an independent predictor of all‐cause mortality only when raised to the highest quartile—that is, > 10 mmol/l (hazard ratio 1.966, 95% confidence interval (CI) 1.376 to 2.810). In survivors of the index admission the association between plasma glucose and all‐cause mortality appeared linear, although also significant only for glucose > 10 mmol/l (table 3).
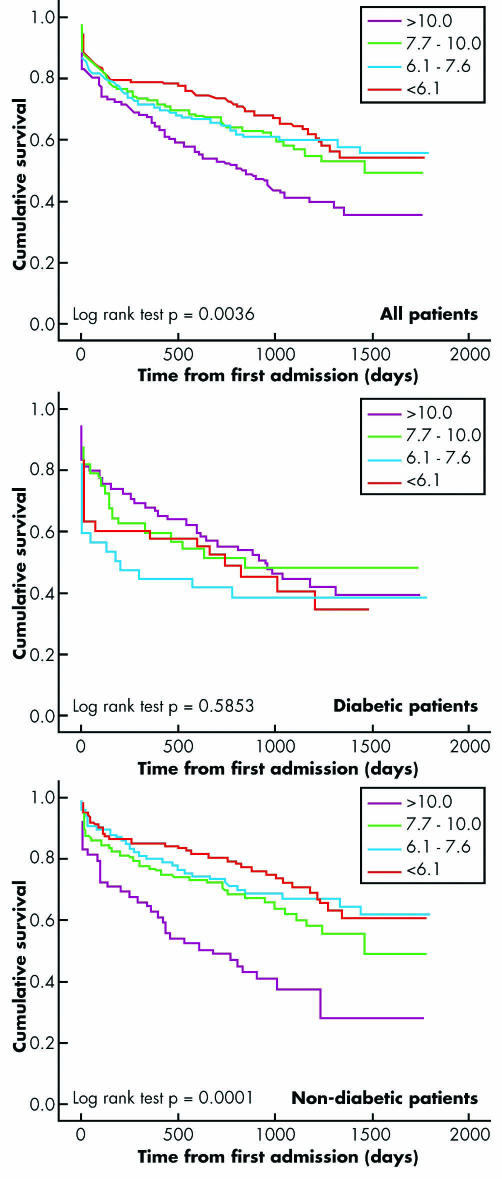
We tested for interaction between a diagnosis of diabetes and the association of increasing glucose with all‐cause mortality by stratifying crude and adjusted associations according to diabetic status, as defined by prescription of insulin or oral hypoglycaemia agents at either admission or discharge. Only in patients without diabetes and when raised to the highest quartile did glucose remain a significant predictor of death (odds ratio 2.490, 95% CI 1.521 to 4.08). Kaplan–Meier analysis confirmed this observation, indicating that for the entire cohort, glucose > 10.0 mmol/l was associated with increased mortality (p = 0.0036, log rank test). For non‐diabetic patients, higher mortality was observed for those with glucose in the highest quartile (p = 0.0001, log rank test) (fig 2). In patients with diabetes, glucose had no clear influence on survival (p = 0.5853, log rank test).
Figure 2 Kaplan–Meier survival curves for quartiles of glucose (mmol/l) for all patients (top) and patients with (middle) and without diabetes (bottom).
DISCUSSION
This is the first study in standard clinical practice to identify low haemoglobin and raised glucose as markers of adverse outcome in CHF. The strengths of the study lie in its representation of everyday clinical practice and its potential usefulness in this setting. We studied a relatively large population of patients, followed up over a long period and who had a large number of events. Moreover, we studied routinely measured laboratory variables. In particular we used absolute values of haemoglobin and glucose rather than labels of “anaemia” or “diabetes”. The current study also confirms the adverse prognosis associated with other clinical variables as seen in previous studies: greater age,5,14 raised creatinine5,14 and lower systolic blood pressure.4,5,7,14,20
Haemoglobin
The current study adds to the understanding of the relationship between haemoglobin and outcome in CHF. A previous population‐based study in CHF, dichotomising haemoglobin by the cohort median, found no independent association between haemoglobin and outcome.21 A population‐based study from Canada indicated a prevalence of anaemia of 17% and an association with adverse outcome.18 However, the diagnosis of anaemia was taken from hospital discharge coding and did not consider absolute value of haemoglobin.18 Other reports were from clinical trials populations.16,17,22 In a subgroup of the RENAISSANCE (Randomized Etanercept North American Strategy to Study Antagonism of Cytokines) trial,16 the prevalence of anaemia (haemoglobin < 120 g/l) was 12%, and the relationship between lower haemoglobin and increased mortality appeared linear. In a larger cohort recruited to the ELITE II (Losartan Heart Failure Survival Study),22 anaemia (haemoglobin < 125 g/l) was reported for 16.5% of participants. The relationship between haemoglobin and mortality was U shaped, centred on 145 g/l.
In the above clinical trials,16,17,22 low ejection fraction was a consistent inclusion criterion and most patients were men. In the current report in a population hospitalised for the first time with heart failure and drawn from standard clinical practice, anaemia was common. According to WHO criteria of anaemia, < 130 g/l in men and < 120 g/l in women, rates of anaemia were 39% and 43%, respectively. Moreover, admission haemoglobin was < 110 g/l in 17% of patients and < 120 g/l in 31%. Thus, a 10 g/l decrease in the threshold, from 120 g/l to 110 g/l, leads to a near doubling of the prevalence of anaemia. The importance of this observation lies in the linear, inverse association between haemoglobin and mortality.
As in previous reports, the predictive value of haemoglobin was independent of age and creatinine. Our patients with heart failure with lower haemoglobin were older and more likely to be women. However, we observed a stronger relationship with outcome in male patients, for whom haemoglobin below the sex‐specific cut off of 130 g/l was associated with adverse prognosis. The reasons for this novel observation are unclear. The lesser prognostic power of haemoglobin in women may reflect the greater prevalence of anaemia in these patients. Equally, our observations may be a chance finding. Prospective studies are required to clarify the relative importance of haemoglobin concentration to outcome in men and women with CHF.
Whether the association between anaemia and increased mortality is causal or whether anaemia is a marker of more advanced disease is uncertain. An association with more advanced disease in the current cohort is suggested by more prevalent diuretic use and higher creatinine concentrations by patients with the lowest haemoglobin. A contributory role of low haemoglobin to poor outcome is suggested by the improvement in outcome with correction of anaemia in advanced heart failure.23
Plasma glucose
Of previous studies reporting the relationship of diabetes and prognosis in CHF,4,7,11,12,14,24,25 most observed an adverse effect of the diagnosis.4,11,14,24,25 Other studies failed to find such an association.7,12 Additional studies,5,6,8,9,15 including population‐based analyses,5,15 did not report the influence of diabetic status on outcome.
Assessment of the influence of diabetes on outcome depends on the accuracy of the diagnosis and the completeness of its documentation. Interstudy differences in these areas, as well as differences in patient populations, probably contribute to inconsistent findings regarding the influence of diabetes on outcome in CHF. These difficulties are illustrated in our own cohort. Of 140 (27%) patients with diabetes at admission, 17 died during the index admission. By the time of discharge 179 of 472 survivors (38%) had documented diabetes. Even this approach, of using the recording of treatment rather than that of the diagnosis, relies on completeness of documentation and may have underestimated the true prevalence of diabetes. In view of these difficulties we chose to assess the influence on outcome of the routinely measured glucose concentration rather than diabetic status.
Our observation of an association between higher plasma glucose, measured soon after admission, and all‐cause mortality is novel. The relationship was more powerful in patients surviving to discharge—a point of potential clinical relevance. Moreover, this relationship was more evident in patients without diabetes—that is, not prescribed insulin or oral hypoglycaemia treatment, or given dietary advice. As our data were gathered via direct scrutiny of all available hospital records, our findings are unlikely to represent incomplete documentation of these treatments. Our data are more likely to indicate incomplete investigation of patients with raised plasma glucose during admission. Formal assessment of glucose tolerance probably would have classified many of the patients as having diabetes.
These findings have clear implications for patients with CHF. Plasma glucose, measured soon after admission for the first time with CHF, provides powerful prognostic information irrespective of diabetic status. Even very mildly raised glucose (7.7–10 mmol/l) was associated with higher all‐cause mortality in patients surviving the index admission. Diabetes is an independent risk factor for the development of heart failure26 and confers a worse prognosis once heart failure is established.27 Raised plasma glucose soon after myocardial infarction is associated with adverse outcome in patients both with and without diabetes28; furthermore, raised blood glucose is a strong independent predictor of mortality in patients without diabetes with chronic cardiovascular disease.29 Whereas intensive metabolic control has previously been shown to improve outcome for patients with raised blood glucose after acute myocardial infarction,30 a recent clinical trial failed to repeat this finding.31 Whether intensive metabolic control may improve prognosis in CHF merits further investigation with large‐scale prospective studies.
Conclusions
In standard clinical practice, at the time of first hospitalisation for heart failure, all‐cause mortality is inversely and linearly related to the concentration of haemoglobin. Raised plasma glucose is associated with adverse outcome, irrespective of diabetic status. The effect on outcome of intensive control of plasma glucose in CHF should be the subject of large‐scale, prospective studies.
Abbreviations
CHF - chronic heart failure
ELITE II - Losartan Heart Failure Survival Study
RENAISSANCE - Randomized Etanercept North American Strategy to Study Antagonism of Cytokines
WHO - World Health Organization
Footnotes
JDN was supported by Nuffield hospitals Leicester
Competing interests: None declared.
References
- 1.Cowie M R, Mosterd A, Wood D A.et al The epidemiology of heart failure. Eur Heart J 199718208–225. [DOI] [PubMed] [Google Scholar]
- 2.Blackledge H M, Tomlinson J, Squire I B. Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001. Heart 200389615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Society of Cardiology Task force for the diagnosis and treatment of chronic heart failure. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001221527–1560. [DOI] [PubMed] [Google Scholar]
- 4.Chin M H, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol 1997791640–1644. [DOI] [PubMed] [Google Scholar]
- 5.Cowie M A, Wood D A, Coats A J.et al Survival of patients with a new diagnosis of heart failure: a population based study. Heart 200083505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrutinio D, Lagioia R, Ricci A.et al Prediction of mortality in patients with mild to moderately symptomatic patients with left ventricular dysfunction: the role of the New York Heart Association classification, cardiopulmonary exercise testing, two‐dimensional echocardiography and Holter monitoring. Eur Heart J 1994151089–1095. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson K D, Schwartz S J, Chen T M.et al Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997952660–2667. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, Alexander J, Christopher E.et al Relationship of depression to increased risk of mortality and rehospitalisation in patients with congestive heart failure. Arch Intern Med 20011611849–1856. [DOI] [PubMed] [Google Scholar]
- 9.Middlekauf H R, Stevenson W G, Stevenson L W. Prognostic significance of atrial fibrillation in advanced heart failure: a study of 390 patients. Circulation 19919440–48. [DOI] [PubMed] [Google Scholar]
- 10.Lee W H, Packer M. Prognostic significance of plasma sodium concentration and its modification by converting enzyme inhibition in patients with severe congestive heart failure. Circulation 199673257–267. [DOI] [PubMed] [Google Scholar]
- 11.Parameshwar J, Keegan J, Sparrow J.et al Predictors of prognosis in severe chronic heart failure. Am Heart J 1992123421–426. [DOI] [PubMed] [Google Scholar]
- 12.Brophy J M, Dagenias G R, McSherry F.et al A multivariate model for predicting mortality in patients with heart failure and systolic dysfunction. Am J Med 2004116300–304. [DOI] [PubMed] [Google Scholar]
- 13.Hullsman M, Berger R, Sturm B.et al Prediction of outcome by neurohumoral activation, the six minute walk test and the Minnesota Living with Heart Failure questionnaire in an outpatient cohort with congestive heart failure. Eur Heart J 200323886–891. [DOI] [PubMed] [Google Scholar]
- 14.Bouvy M L, Heerdink E R, Leufkens H G M.et al Predicting mortality in patients with heart failure: a pragmatic approach. Heart 200389605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney M T, Fox K A A, Lee A J.et al Predicting death due to progressive heart failure in patients with mild‐to‐moderate chronic heart failure. J Am Coll Cardiol 2002401801–1808. [DOI] [PubMed] [Google Scholar]
- 16.Anand I, McMurray J J V, Whitmore J.et al Anemia and its relationship to clinical outcome in heart failure. Circulation 2004110149–154. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Nye R, Levy W C. Anemia predicts mortality in severe heart failure. The Prospective Randomized Amlodipine Survival Evaluation (PRAISE). J Am Coll Cardiol 2003411933–1939. [DOI] [PubMed] [Google Scholar]
- 18.Esekowitz J A, McAlister F A, Armstrong P W. Anaemia is common in heart failure and is associated with poor outcomes. Circulation 2003107223–225. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Finkle W D. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 19951421255–1264. [DOI] [PubMed] [Google Scholar]
- 20.Cohn J N. Prognostic factors in heart failure: poverty amidst a wealth of variables. J Am Coll Cardiol 198914571–572. [Google Scholar]
- 21.Kalra P R, Collier T, Cowie M R.et al Haemoglobin concentration and prognosis in new cases of heart failure. Lancet 2003362211–212. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R, Francis D P, Pitt B.et al Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur Heart J 2004251021–1028. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg D S, Wexler D, Sheps D.et al The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Col Cardiol 2001371775–1780. [DOI] [PubMed] [Google Scholar]
- 24.Bart B A, Shaw L K, McCants C B.et al Clinical determinants of mortality in patients with angiographically diagnosed ischaemic or non‐ischaemic cardiomyopathy. J Am Coll Cardiol 1997301002–1008. [DOI] [PubMed] [Google Scholar]
- 25.Schindler D M, Kostis J B, Yusuf S.et al Diabetes mellitus, a predictor of mortality and morbidity in the studies of Left Ventricular Dysfunction (SOLVD) trials and registry. Am J Cardiol 1996771017–1020. [DOI] [PubMed] [Google Scholar]
- 26.He J, Ogden L G, Bazzano L A.et al Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med 2001161996–1002. [DOI] [PubMed] [Google Scholar]
- 27.Haas S J, Vos T, Gilbert R.et al Are β‐blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta‐analysis of large‐scale clinical trials. Am Heart J 2003146848–853. [DOI] [PubMed] [Google Scholar]
- 28.Stranders I, Diamant M, VanGelder R.et al Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes Mellitus. Arch Intern Med 2004164982–988. [DOI] [PubMed] [Google Scholar]
- 29.Port S C, Goodarzi M O, Boyle N G.et al Blood glucose: a strong risk factor for mortality in non‐diabetic patients with cardiovascular disease. Am Heart J 2005150209–214. [DOI] [PubMed] [Google Scholar]
- 30.Malmberg K, Ryden L, Efendic S.et al Randomised trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in patients with diabetes mellitus (DIGAMI study): effects on mortality at one year. J Am Coll Cardiol 19952657–65. [DOI] [PubMed] [Google Scholar]
- 31.Malmberg K, Rydén L, Wedel H.et al Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 200526650–661. [DOI] [PubMed] [Google Scholar]