Abstract
Objectives
To establish the prevalence of preserved left ventricular (LV) systolic function (PSF) in 435 consecutive symptomatic patients referred to a heart failure clinic and to examine their ventilatory response to exercise when compared with 134 control volunteers.
Methods
216 (50%) patients had systolic heart failure (SHF) (ejection fraction < 45%). 51 (11%) had an immediately apparent alternative causes of breathlessness and 168 (39%), with no obvious other cause of breathlessness, were divided into those with PSF and diastolic dysfunction (DD) (PSFDD; n = 113 or 26% of referrals) and those without DD (PSFN; n = 55 or 13% of referrals). The controls were divided into those with (CDD; n = 32) and those without (CN; n = 102) echocardiographic evidence of DD.
Results
Patients with SHF had lower peak oxygen consumption (pV̇o2), steeper slope of minute ventilation (V̇e) to carbon dioxide production, lower exercise time and shorter 6 min walk test than PSF patients and controls. PSFDD patients had lower pV̇o2, exercise time and 6 min walk test than CDD, although their echocardiograms were not different. Exercise capacity did not differ between PSFDD and PSFN patients. The slope relating V̇e to symptoms (Borg/V̇e slope) was less steep in those with SHF than in PSFDD (0.17 (0.04) v 0.20 (0.08), p < 0.05) and in PSFN (0.19 (0.10), p < 0.05), implying greater symptoms of breathlessness for a given level of V̇e. Both PSF groups had a steeper slope than CDD (0.14 (0.09), p < 0.05 for both comparisons).
Conclusions
Patients with PSF have exercise tolerance intermediate between that of patients with SHF and controls. Exercise tolerance is similar in PSFDD and PSFN. Both groups have worse exercise tolerance than CDD. PSFDD and PSFN patients seem to experience a greater awareness of V̇e than CDD and patients with SHF.
Chronic heart failure is a syndrome characterised by exercise intolerance and symptoms of breathlessness and fatigue.1 A diagnosis of chronic heart failure is usually associated with evidence of impaired left ventricular (LV) function, often on echocardiography. Depending on the population studied, up to one half of patients with symptoms of chronic heart failure do not have impaired LV systolic function and have been deemed to have diastolic heart failure (DHF) or heart failure with preserved systolic function (PSF).2 Such patients are generally older and are more likely to be women, to have a history of hypertension and to have LV hypertrophy on echocardiography than those with systolic dysfunction.2
A positive diagnosis of DHF (rather than heart failure with PSF) usually depends on the presence of symptoms of chronic heart failure with PSF but also on objective evidence of impaired relaxation of the left ventricle. These include E:A wave reversal on transmitral Doppler, prolonged deceleration time of transmitral E wave or increased isovolumic relaxation time (IVRT).3 However, these changes are also seen as a consequence of normal ageing. One or more of these variables is commonly abnormal in older patients to the extent that some authors have suggested that the diastolic variables may not be helpful in diagnosing DHF.4
Patients with DHF may therefore have symptoms of other causes of breathlessness, or even reduced fitness,5 and have DHF diagnosed as a result of diastolic echocardiographic changes that are often seen in normal older people. Data are emerging that the diastolic impairment of normal ageing can be differentiated from diastolic impairment in patients presenting with breathlessness by using tissue Doppler imaging.6 Nevertheless, the definition of what constitutes DHF remains controversial.5
The objective of the present study was to establish the prevalence of echocardiographically determined diastolic impairment (that is, DHF) in patients referred to a specialist heart failure clinic and to describe these patients' exercise capacity when measured objectively by exercise testing with metabolic gas exchange. We also looked at how peak oxygen consumption (pV̇o2) relates to symptoms as judged by the New York Heart Association (NYHA) classification in DHF and by the relationship of minute ventilation (V̇e) to symptoms (Borg/V̇e slope).7 This ratio is greater in patients with systolic heart failure (SHF) than in controls7 and permits semiobjective assessment of symptoms during an exercise test.
METHODS
We studied 435 consecutive patients referred for assessment of breathlessness by their general practitioner to a heart failure clinic between July 2000 and July 2003. Each patient had a remote history of breathlessness thought to be caused by heart failure for which diuretic drugs had at some time in the past been started All patients were in a stable situation, were clinically not fluid overloaded and had no recent (< 4 weeks) change in heart failure drugs. We excluded patients in atrial fibrillation. We also investigated 134 controls of a similar age chosen at random from the patient lists of local general practitioners with no history or symptoms of cardiovascular disease. All the control participants gave informed, written consent. The study was approved by the local ethics committee.
Echocardiography
Each participant underwent echocardiographic examination with a GE Vingmed Vivid FiVe scanner (Horten, Norway) equipped with 2.5 MHz phased array transducers. All Doppler echocardiographic recordings were obtained during normal respiration.
LV end diastolic diameter, LV end systolic diameter, and interventricular septum and LV posterior wall thickness at end diastole were measured from parasternal M mode echocardiography of the left ventricle. LV end diastolic and end systolic volumes were calculated with the modified Simpson's rule (biplane), and the standard formula was applied to give LV ejection fraction.
Doppler echocardiography
Pulsed wave Doppler studies were performed in apical views. Mitral flow velocities were recorded from an apical four‐chamber view with the sample volume positioned adjacent to the tip of either the mitral or tricuspid leaflets in diastole. Care was taken to obtain the smallest possible angle between the direction of transvalvular flow and the ultrasound beam.
Peak velocity of early filling (E), peak velocity of atrial filling (A), and the E:A ratio were calculated for both transmitral and transtricuspid flow. Deceleration time of early filling and IVRT were measured from the transmitral Doppler spectrum. Deceleration time was calculated as the time between peak E wave and the upper deceleration slope extrapolated to the baseline. IVRT was measured by placing the sample volume between the anterior mitral leaflet and LV outflow tract.
All patients were stratified according to the presence or absence of significant LV systolic dysfunction (ejection fraction > 45%). Patients with PSF were then classified into those with (PSFDD) and without (PSFN) signs of diastolic dysfunction (DD) (ejection fraction > 45%, and at least one of E:A wave reversal on transmitral Doppler (< 0.5), a prolonged deceleration time of transmitral E wave (> 280 ms) or an increased IVRT (> 105 ms)).3 We also assessed left atrial diameter in the parasternal long axis view. Controls were similarly divided into those with (CDD) and without (CN) evidence of DD.
Exercise testing
Participants described their own NYHA symptom class. Each person was then invited to perform a 6 min walk test8 and incremental treadmill exercise testing with metabolic gas exchange according to a Bruce protocol modified by the addition of a stage 0 at onset consisting of 3 min of exercise at 1.61 km/h (1 mph) with a 5% gradient. Participants were encouraged to exercise to exhaustion. During the tests participants wore a tightly fitting facemask to which was connected a capnograph and a sample tube enabling online V̇e and metabolic gas exchange measurements (Jaeger Oxycon Delta, Würtzburg, Germany). A respiratory exchange ratio (carbon dioxide production (V̇co2) to oxygen consumption (V̇o2) (RER)) > 1 was taken to indicate a maximal effort. Standard spirometry (forced expiratory volume in 1 s and forced vital capacity) was performed before the exercise test. The participants were asked to score their symptoms of breathlessness or fatigue between 0 and 10 (0 being no symptoms and 10 being the maximum) on a standard scale of perceived exertion9 at the end of each stage during the test. The Borg/V̇e slope for each participant was plotted.7 Exercise tests were reported by a single investigator blinded to the results of the echocardiograms.
We initially established three groups, consisting of the control group and two patient groups (patients with symptoms of breathlessness): those with SHF and those with PSF. To investigate further the influence of diastolic variables we then divided patients with PSF into PSFDD (or DHF) and PSFN. We divided the controls in the same way (CDD and CN).
Statistical analysis
To assess the difference between the NYHA classes and between the groups, we used analysis of variance (Statview; SAS Institute, Cary, North Carolina, USA). In assessing differences between categorical data we used the χ2 test. A value of p < 0.05 was taken to be significant. We have presented unadjusted p values rather than correcting for multiple comparisons.10,11
RESULTS
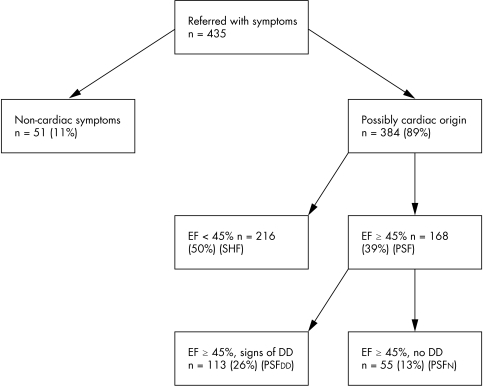
Four hundred and thirty‐five patients were referred for assessment. Each referred patient went through a standardised assessment as described above. The results presented here are assembled from a retrospective analysis of these systematically collected data. Fifty‐one patients (11%) had a definite non‐cardiac cause of breathlessness (significant airways disease with abnormal lung function (n = 31), severe arthritis (n = 7), anaemia (n = 7), pneumonia (n = 4) and lung cancer (n = 2)). This left a study group of 384. Of these patients, 216 (56%; 50% of referred patients) had an ejection fraction < 45% on echocardiography (SHF) and 168 (44%; 39% of referred patients) had PSF. Of those with PSF, 113 (26% of those referred) had symptoms of heart failure and echocardiographic evidence of DD (PSFDD or DHF) and 55 (13% of referred patients) had symptoms but no signs of systolic or diastolic impairment (PSFN) (fig 1).
Figure 1 Breakdown of 435 patients referred to the heart failure clinic with systolic heart failure (SHF), preserved systolic function (PSF) with diastolic dysfunction (DD), and preserved systolic function without diastolic dysfunction (PSFN). Non‐cardiac breathlessness was caused by chronic airways disease (31), arthritis (7), anaemia (7), pneumonia (4) and lung cancer (2). EF, ejection fraction.
In the study population (384 patients), 67 had concomitant conditions reducing the quality of their exercise test: 29 had arthritis, 32 had chronic airways disease, and 6 had a previous stroke. Thirty patients had a test unsuitable for analysis (not able to exercise past stage 1). These patients' test data were excluded from further analysis, leaving 287 patients: 186 in the SHF group, 61 in the PSFDD group, and 40 in the PSFN group. Diagnostic criteria for DD were E:A reversal in 37%, prolonged IVRT in 43% and prolonged deceleration time in 49% (table 1).
Table 1 Characteristics of patients and controls stratified by presence or absence of significant left ventricular systolic dysfunction.
| SHF (n = 186) | PSFDD (n = 61) | PSFN (n = 40) | CDD (n = 32) | CN (n = 102) | p Value (SHF v PSFDD) | |
|---|---|---|---|---|---|---|
| Age (years) | 69 (13) | 69 (13) | 64 (16)†† | 72 (15) | 63 (12)** | NS |
| Men | 56% | 48% | 58%† | 35% | 52** | <0.05 |
| Height (cm) | 174 (5.5) | 175 (6.5) | 175 (9.2) | 175 (11.2) | 177 (8.2) | 0.74 |
| Weight (kg) | 80.6 (15.9) | 84.6 (9.7) | 83.5 (13.4) | 85.2 (12.1) | 82.5 (8.8) | 0.65 |
| FEV1 (% expected) | 90.0 (17.4) | 87.2 (21.4) | 88.3 (21.5) | 102 (25.4) | 116.0 (23.4)* | <0.02 |
| FVC (% expected) | 80.0 (22.9) | 77.4 (19.6) | 79.5 (21.6) | 89 (24.3) | 95.0 (22.6) | 0.02 |
| LVEDD (cm) | 6.3 (1.0) | 5.2 (0.9) | 5.1 (0.8) | 5.1 (0.8) | 5.0 (0.7) | <0.0001 |
| LVEF (%) | 32.8 (8.2) | 54.5 (7.6) | 57.3 (8.9) | 60.0 (9.6) | 62.1 (11.6) | <0.0001 |
| LA diameter (cm) | 4.9 (1.2) | 4.8 (1.3) | 3.8 (1.2)† | 4.4 (1.4) | 3.9 (1.2)* | 0.45 |
| E:A ratio | 1.5 (1.2) | 0.8 (0.8) | 1.0 (0.9)† | 0.8 (0.8) | 1.1 (0.3)** | <0.01 |
| Deceleration time (ms) | 185 (66) | 236 (35) | 215 (49)† | 251 (65) | 214 (44)* | <0.005 |
| IVRT (ms) | 93 (34) | 118 (32) | 96 (35)† | 113 (28) | 85 (20) * | <0.05 |
| Drugs | ||||||
| Furosemide‡ | 62 (37%) | 56 (32%) | 32 (46%) | 0 | 0 | |
| β blockers | 154 | 46 | 0 | 1 | 3 | |
| ACEI/AIIA | 91/16 | 28/7 | 4/2 | 0/0 | 0/0 | |
| Thiazide | 20 | 14 | 8 | 3 | 1 | |
| Spironolactone | 36 | 8 | 0 | 0 | 0 |
Values are mean (SD), number (%) or number.
*p<0.05, **p<0.02 for difference between CN and CDD; †p<0.05, ††p<0.02 for difference between PSFDD and PSFN; ‡mean daily dose of furosemide equivalent is given (1 mg bumetanide is equivalent to 40 mg furosemide).
ACEI, angiotensin‐converting enzyme inhibitor; AIIA, angiotensin II inhibitor; CDD, controls with diastolic dysfunction; CN, controls with normal diastolic function; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IVRT, isovolumic relaxation time; LA, left atrial; LVEDD, left ventricular end diastolic dimension from M mode echocardiography; LVEF, left ventricular ejection fraction; PSFDD, preserved systolic function with diastolic dysfunction; PSFN, preserved systolic function without diastolic dysfunction; SHF, systolic heart failure.
Patients excluded because of poor exercise test data did not differ in ejection fraction or NYHA class from those included, although older patients were more likely to refuse to walk on the treadmill or have a test unsuitable for analysis. Including the data from patients not achieving peak made no difference to the results, but we elected nevertheless to exclude these from the final analysis. Patients and controls were similar in age, height and weight. Although the patients with PSFDD and PSFN had non‐dilated ventricles and a normal ejection fraction, their LV ejection fraction was lower than that of the controls. Left atrial diameter was greater in patients with PSFDD and SHF than in patients with PSFN and controls. Patients with PSFDD and PSFN were of a similar age to those with systolic dysfunction but more were women (p < 0.05). Table 1 shows the characteristics of the participants undergoing exercise testing. CDD were older (p < 0.05) and more likely than CN to be women. Indices of DD were not different between patients with PSFDD and CDD.
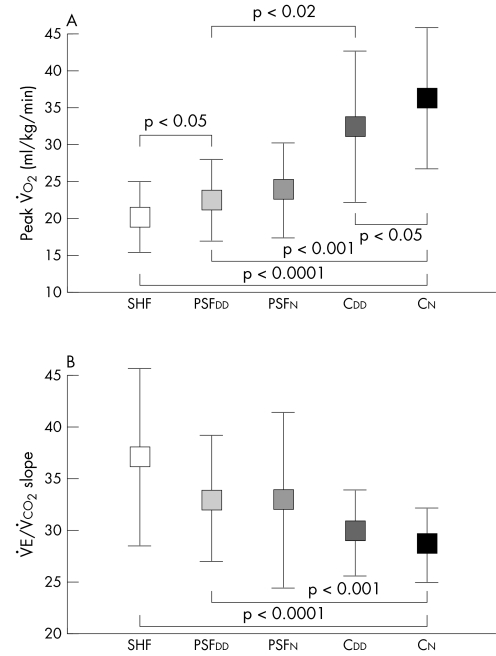
Table 2 and fig 2 show the exercise variables and symptom class for the five groups of participants. The NYHA class was better for patients with PSF than for those with reduced LV function (SHF group). Exercise capacity (pV̇o2 and exercise time) was lower and the V̇e/V̇co2 slope steeper in those with impaired systolic ventricular function than in patients with PSF. Exercise capacity and ventilatory response to exercise were similar in breathless patients with normal hearts (PSFN) to patients in the PSFDD (DHF) group. Patients in the PSFDD group had significantly lower pV̇o2 (p < 0.02), exercise time (p < 0.05) and 6 min walk test distance than CDD (p < 0.02) and a steeper V̇e/V̇co2 slope (p < 0.05), even though the diastolic variables were not different (table 2). The 6 min walk distance was greater in the PSFDD and PSFN groups than in SHF, although still lower than in the controls. The CDD group performed less well than CN on exercise testing. To compensate for the higher number of women in the PSFDD group, we repeated the above analyses within the sexes. The patterns described above for the exercise variables remained significant for both men and women.
Table 2 Symptom scores and exercise results.
| SHF (n = 186) | PSFDD (n = 61) | PSFN (n = 40) | CDD (n = 32) | CN (n = 102) | p Value (SHF v PSFDD) | |
|---|---|---|---|---|---|---|
| NYHA class I | 8% | 18% | 0% | 100% | 100% | |
| NYHA class II | 65% | 62% | 34% | 0 | 0 | |
| NYHA class III | 26% | 20% | 6% | 0 | 0 | |
| pV̇o2 (ml/kg/min) | 20.0 (4.8)** | 22.5 (5.5)‡‡ | 23.8 (6.4) | 32.4 (10.2)† | 36.2 (9.6) | <0.01 |
| V̇e/V̇co2 slope | 37.0 (8.6)** | 33.0 (6.1)‡‡ | 32.8 (8.4) | 29.8 (4.2) | 28.5 (3.6) | <0.001 |
| RER | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.87 |
| Exercise time (s) | 455 (198)** | 562 (261)‡‡ | 571 (281) | 698 (292)† | 846 (285) | <0.001 |
| 6 min walk (m) | 221 (137)** | 275 (147)‡‡ | 273 (164) | 402 (168)† | 472 (114) | <0.01 |
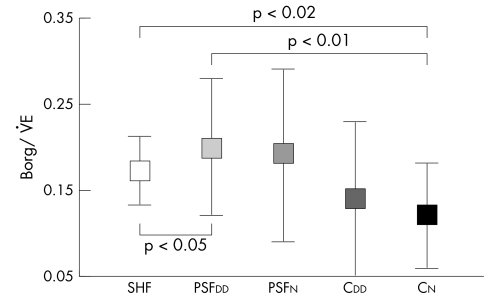
| Borg/V̇e slope | 0.17 (0.04)* | 0.20 (0.08)‡ | 0.19 (0.10) | 0.14 (0.09) | 0.12 (0.07) | <0.05 |
| Reason for stopping (B/F) | 79/107* | 28/33 | 12/28 | 21/11 | 42/60 | <0.001 |
Values are mean (SD) or percentage.
*p<0.02, **p<0.0001 for difference between SHF and CN; †p<0.05 for difference between CDD and CN; ‡p<0.01, ‡‡p<0.001 for difference between PSFDD and CN.
B/F, breathlessness or fatigue; Borg/V̇e slope, slope relating symptoms to minute ventilation; NYHA, New York Heart Association; pV̇o2, peak oxygen consumption; RER, peak respiratory exchange ratio; V̇e/V̇co2 slope, slope relating minute ventilation to carbon dioxide production.
Figure 2 (A) Peak oxygen consumption (pV̇o2) and (B) slope relating minute ventilation to carbon dioxide output (V̇e/V̇co2 slope) in patients with systolic heart failure (SHF), preserved systolic function with diastolic dysfunction (PSFDD) and preserved systolic function without diastolic dysfunction (PSFN) and in controls with diastolic dysfunction (CDD) and normal diastolic function (CN).
The mean inverse relationship between the V̇e/V̇co2 slope and pV̇o2 was greater in SHF than in PSFDD (DHF) (r = −0.51 (0.25) v −0.46 (0.18), p < 0.05). The relationship in those with symptoms and normal hearts (PSFN: r = −0.44 (0.26)) was the same as in the patients with DHF. There was no such relationship in controls.
Borg/V̇e slope was steeper in patients with PSFDD and PSFN than in those with SHF and both control groups, implying greater symptoms of breathlessness for a given level of V̇e (table 2, fig 3). CDD had a lower Borg/V̇e ratio than PSFDD patients (p < 0.05) and better exercise tolerance despite having similar echocardiographic variables of DD. Symptoms of breathlessness (Borg/V̇e slope) and LV ejection fraction were not related in any group.
Figure 3 Slope relating symptoms to minute ventilation (Borg/V̇e) in patients with systolic heart failure (SHF), preserved systolic function with diastolic dysfunction (PSFDD) and preserved systolic function without diastolic dysfunction (PSFN) and in controls with diastolic dysfunction (CDD) and normal diastolic function (CN).
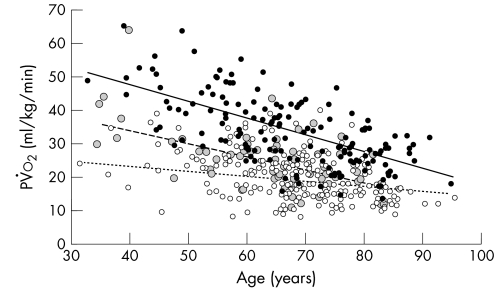
Figure 4 shows the relationship between pV̇o2 and age in patients with SHF, all breathless patients with PSF and all of the controls. In the control group and patients with PSF, the pV̇o2 and V̇e/V̇co2 slope deteriorated gradually with age and the slopes are not statistically different. This relationship is not seen in patients with SHF.
Figure 4 Peak oxygen consumption (pV̇o2) against age for patients with systolic heart failure (SHF) (unfilled circles), patients with preserved systolic function with diastolic dysfunction (PSFDD) (grey circles) and controls (solid circles). Regression lines for controls (solid line, r = 0.34), PSFDD (long dashes, r = 0.37) and SHF (short dashes, r = 0.07).
DISCUSSION
The present study showed that 40% of patients referred to a heart failure clinic with symptoms of breathlessness had PSF on echocardiography. Many of these (67%) had signs of diastolic impairment, but one third had no cardiac problem identified. Patients with PSF had a better exercise tolerance (when measured by pV̇o2) and a lower ventilatory response to exercise (V̇e/V̇co2 slope) than patients with systolic dysfunction, but patients with and those without evidence of DD did not differ significantly. However, the exercise tolerance of those presenting with breathlessness was worse than that of controls of similar age. Patients presenting with breathlessness and PSF are also more symptomatic for a given V̇e than both those with systolic dysfunction and controls, including those controls with similar degrees of DD.
Exercise capacity (however assessed) is an important prognostic indicator in patients with heart failure caused by LV systolic dysfunction. Patients with SHF have reduced pV̇o212 and an increased V̇e/V̇co2 slope.13,14 The V̇e/V̇co2 slope is abnormal throughout exercise15 and correlates inversely with pV̇o2, so that the greater the ventilatory response, the lower the exercise capacity.3,8 Peak V̇o2 and the V̇e/V̇co2 slope also relate independently to prognosis.16
Diastolic function is an important determinant of exercise capacity after myocardial infarction17 and in the setting of chronic heart failure with LV systolic dysfunction.18,19 One study that used a clinical diagnosis of DHF based on LV ejection fraction > 50% in 119 patients with breathlessness suggested that reductions of exercise capacity and abnormalities of the ventilatory response are similar in patients with DHF.20
The cause of impaired exercise tolerance in patients with heart failure is not clearly understood. Conventional measures of LV systolic function such as ejection fraction at rest have a poor correlation with pV̇o2 and the V̇e/V̇co2 slope. A tissue Doppler‐derived measure of systolic function has a closer relationship than ejection fraction,21 and tissue Doppler indices can be useful to differentiate diastolic changes associated with normal ageing from pathological changes.6 The correlation between tissue Doppler indices and exercise tolerance in patients with PSFDD has not been established.
Patients with severe SHF often have diastolic abnormalities on tissue Doppler scanning. The two types of heart failure therefore may merely be a spectrum of the same condition. In our patients, the pathological inverse relationship between the V̇e/V̇co2 slope and pV̇o2 seen in SHF22 was also seen in those with DHF and in patients with PSFN, although it was less steep. This has not been described before.
On the other hand, the age‐related deterioration in pV̇o2 seen in controls was also seen in breathless patients with PSF, unlike patients with SHF, where age is not closely related to pV̇o2. Furthermore, by using the semiobjective Borg/V̇e slope, we showed that, for a given V̇e, patients with DHF and those with PSFN are more symptomatic than those with SHF. We have also confirmed that there is no relation between symptoms of exercise intolerance as measured by the Borg/V̇e slope and LV ejection fraction. These data support the well‐known concept that the degree of cardiac function does not relate to symptoms or the ventilatory abnormalities in either SHF or DHF.
Patients who have DD identified using basic indices might therefore merely be symptomatic individuals who have brought themselves to the attention of their general practitioner and subsequently their cardiologist with a combination of symptoms of breathlessness and fatigue. In these patients imaging investigations might reveal appropriate age‐related cardiac function, combined with other non‐cardiac causes of impaired exercise tolerance, or merely reduced fitness, that are not significantly different to their more tolerant peers. Indeed in our symptomatic patients with DD the diastolic variables were not significantly different to the controls with abnormal diastolic function, although the exercise capacity was significantly reduced.
Study limitations
The study was based on a retrospective analysis of systematically collected data. During the enrolment period, indices of DD that are less load dependent became accepted, but were not collected for all of our patients. Our conclusions should be viewed in the light of this limitation.
Conclusions
Our study showed that patients with DD and symptoms of breathlessness (DHF) have an intermediate reduction in exercise tolerance between those with SHF and controls. The reduced pV̇o2 in patients with DHF does, however, correlate closely with age, unlike that in patients with SHF. We have also shown that patients with DHF and those with symptoms but no apparent signs of DHF are more symptomatic than those with SHF for a given V̇e.
Abbreviations
CDD - controls with diastolic dysfunction
CN - controls with normal diastolic function
DD - diastolic dysfunction
DHF - diastolic heart failure
IVRT - isovolumic relaxation time
LV - left ventricular
NYHA - New York Heart Association
PSF - preserved systolic function
PSFDD - preserved systolic function and diastolic dysfunction
PSFN - preserved systolic function without diastolic dysfunction
pV̇o2 - peak oxygen consumption
SHF - systolic heart failure
V̇co2 - carbon dioxide production
V̇e - minute ventilation
Footnotes
Financial support: None declared.
Competing interests: None declared.
References
- 1.Clark A L, Sparrow J L, Coats A J S. Muscle fatigue and dyspnoea in chronic heart failure: two sides of the same coin? Eur Heart J I995 1649–52. [DOI] [PubMed] [Google Scholar]
- 2.Vasan R S, Benjamin E J, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995261565–1574. [DOI] [PubMed] [Google Scholar]
- 3.Cleland J G, Tendera M, Adamus J.et al Perindopril for elderly people with chronic heart failure: the PEP‐CHF study. The PEP investigators. Eur J Heart Fail 19991211–217. [DOI] [PubMed] [Google Scholar]
- 4.Zile M R, Gaasch W H, Carroll J D.et al Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001104779–782. [DOI] [PubMed] [Google Scholar]
- 5.Caruana L, Petrie M C, Davie A P.et al Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ 2000321215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikitin N P, Witte K K, Clark A L.et al Color tissue Doppler‐derived long‐axis left ventricular function in heart failure with preserved global systolic function. Am J Cardiol 2002901174–1177. [DOI] [PubMed] [Google Scholar]
- 7.Witte K K A, Clark A L. The pattern of ventilation during exercise in chronic heart failure. Heart 200389610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyatt G H, Sullivan M J, Thompson P J.et al The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. CMAJ 1985132919–923. [PMC free article] [PubMed] [Google Scholar]
- 9.Borg G. Subjective effort and physical activities. Scand J Rehab 19786108–113. [PubMed] [Google Scholar]
- 10.Rigby A S. Statistical methods in epidemiology: I. Statistical errors in hypothesis testing. Disabil Rehabil 199820121–126. [DOI] [PubMed] [Google Scholar]
- 11.Perneger T V. What's wrong with Bonferroni adjustments. BMJ 19983161236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark A L, Poole‐Wilson P A, Coats A J S. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 1996281092–1102. [DOI] [PubMed] [Google Scholar]
- 13.Buller N P, Poole‐Wilson P A. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J 199063281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies S W, Emery T M, Watling M I L.et al A critical threshold of exercise capacity in the ventilatory response to exercise in chronic heart failure. Br Heart J 199165179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witte K K A, Clark A L. Is the elevated slope relating ventilation to carbon dioxide production in chronic heart failure a consequence of slow metabolic gas kinetics? Eur J Heart Fail 20014469–472. [DOI] [PubMed] [Google Scholar]
- 16.Cohn J N, Johnson G R, Shabetai R.et al Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norephinephrine as determinants of prognosis in heart failure. The V‐HeFT VA Cooperative Studies Group. Circulation 199387(suppl 6)V15–V16. [PubMed] [Google Scholar]
- 17.Miyashita T, Okano Y, Takaki H.et al Relation between exercise capacity and left ventricular systolic versus diastolic function during exercise in patients after myocardial infarction. Coron Artery Dis 200112217–225. [DOI] [PubMed] [Google Scholar]
- 18.Partenakis F I, Kanoupakis E M, Kochaidakis G E.et al Left ventricular diastolic filling pattern predicts cardiopulmonary determinants of function capacity in patients with congestive heart failure. Am Heart J 2000140338–344. [DOI] [PubMed] [Google Scholar]
- 19.Lapu‐Bula R, Robert A, De Kock M.et al Relation of exercise capacity to left ventricular systolic function and diastolic filling in idiopathic or ischaemic dilated cardiomyopathy. Am J Cardiol 199983728–734. [DOI] [PubMed] [Google Scholar]
- 20.Kitzman D W, Little W C, Brubaker P H.et al Pathophysiological characterisation of isolated diastolic heart failure in comparison to systolic heart failure. J Am Med Assoc 20022882144–2150. [DOI] [PubMed] [Google Scholar]
- 21.Witte K K A, Nikitin N P, Cleland J G F.et al Exercise tolerance and tissue Doppler imaging in chronic heart failure. Heart 2004901144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis D P, Shamim W, Davies L C.et al Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2) slope and peak VO(2). Eur Heart J 200021154–161. [DOI] [PubMed] [Google Scholar]