Abstract
Objective
To explore metabolic syndrome as a possible risk factor for development of heart failure (HF).
Design
Community‐based cohort study.
Setting
Uppsala, Sweden.
Participants
2314 50‐year‐old men free from HF, myocardial infarction and valvular disease at baseline were enrolled between 1970 and 1974 and were followed up until the age of 70. A modified National Cholesterol Education Program definition of metabolic syndrome was used with body mass index in the place of waist circumference.
Main outcome measure
First hospitalisation for HF.
Results
In multivariable Cox proportional hazards models adjusted for established risk factors for HF (hypertension, diabetes, ECG left ventricular hypertrophy, smoking and body mass index), the presence at baseline of metabolic syndrome (hazard ratio 1.66, 95% confidence interval (CI) 1.02 to 2.70) was a predictor of subsequent HF. This relation was even stronger after adjustment for the presence of an acute myocardial infarction during follow up in addition to the other established risk factors for HF (hazard ratio 1.80, 95% CI 1.11 to 2.91).
Conclusion
Metabolic syndrome was a significant predictor of HF, independent of established risk factors for HF including an interim myocardial infarction, during two decades of follow up in a community‐based sample of middle‐aged men. This implies that metabolic syndrome provides important risk information beyond that of established risk factors for HF.
Heart failure (HF) is a major cause of morbidity and mortality. The predominant causes of HF are hypertension and coronary heart disease. Other established risk factors for HF are left ventricular hypertrophy, valvular heart disease, diabetes, cigarette smoking and obesity.1,2,3
Some of these established risk factors, such as hypertension, diabetes, obesity and dyslipidaemia, tend to cluster in certain people. This is the basis for the so‐called metabolic syndrome, for which insulin resistance has been proposed to be of key pathogenetic importance.4,5 Metabolic syndrome, defined by the current National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III),6 has been associated with an increased risk for coronary heart disease in community‐based samples,7,8 in primary preventive settings9 and in clinical trial settings.10
Insulin resistance—and its clinical surrogate, metabolic syndrome—may have direct myocardial effects in addition to their atherogenic effects, as insulin resistance has been related to both left ventricular systolic11 and diastolic12 dysfunction, as well as left ventricular remodelling.13,14,15 In a previous cross‐sectional study based on the NHANES III (Third National Health and Nutrition Examination Survey) cohort,16 the presence of HF was significantly associated with metabolic syndrome. In a recent study, insulin resistance, measured with the euglycaemic insulin clamp technique, was shown to be a risk factor for HF, independent of diabetes, obesity and other established HF risk factors.17 The impact of metabolic syndrome on incident HF is not known but, given the current obesity epidemic, this question is of great importance. Enhancing knowledge about the clinical usefulness of metabolic syndrome is a priority, stressed in a recent joint statement from the American Diabetes Association and the European Association for the Study of Diabetes.18
We hypothesised that the prevalence of metabolic syndrome increases the risk of subsequent HF, taking established HF risk factors, including a myocardial infarction and hypertension, into account. Thus, our objective was to analyse metabolic syndrome as a possible predictor of HF in a community‐based sample of middle‐aged men free from HF, prior acute myocardial infarction and valvular disease at baseline, after adjustment of established risk factors for HF at baseline and interim myocardial infarction during follow up.
METHODS
Study sample
The study was based on the ULSAM (Uppsala Longitudinal Study of Adult Men) cohort, which originated as a health investigation mainly aimed at identifying metabolic risk factors for cardiovascular disease. All 50‐year‐old men living in Uppsala County in 1970–4 were invited to participate. Of the 2841 invited men, 82% (2322 men) participated in the investigation.19 In addition to regular repeat examinations, annual updates on mortality and in‐hospital morbidity based on national registers have been added to the data. The ULSAM study is described in detail on the internet (http://www.pubcare.uu.se/ULSAM/). None of the participants had a diagnosis of HF in the hospital discharge register before baseline. Seven participants were excluded because of prior myocardial infarction, and one participant because of valvular disease at baseline; thus 2314 men were eligible for the investigation. All participants gave written consent and the ethics committee of Uppsala University approved the study.
Examinations at baseline
Participants at 50 years of age underwent a structured interview; a questionnaire; blood sampling (after an overnight fast) for glucose, insulin and lipid determinations; an ECG; and a physical examination with measurement of supine blood pressure and anthropometric variables as described previously.19 Body mass index (BMI) was calculated as weight (in kg) divided by height (in metres) squared (kg/m2). Cholesterol and triglyceride concentrations in serum and high density lipoprotein were assayed by enzymatic techniques. The erythrocyte sedimentation rate (ESR) was determined by Westergren's method. Coding of smoking was based on interview reports. Supine systolic and diastolic blood pressures were measured twice in the right arm after a 10 min rest and means were calculated. The presence of hypertension at baseline was defined as systolic blood pressure ⩾ 140 mm Hg, diastolic blood pressure ⩾ 90 mm Hg or use of hypertension drugs, according to the current definition.20 In secondary analyses defined a priori, hypertension at baseline was defined as systolic blood pressure ⩾ 130 mm Hg, diastolic blood pressure ⩾ 85 mm Hg or use of hypertension drugs. The purpose of the analyses was to examine whether the predictive value of metabolic syndrome was driven only by the lower blood pressure cut off used in the NCEP metabolic syndrome criteria. The presence of diabetes at baseline was defined as fasting plasma glucose ⩾ 7.0 mmol/l or use of oral hypoglycaemia agents or insulin. ECG left ventricular hypertrophy was defined as high amplitude R waves according to the revised Minnesota code21 together with a left ventricular strain pattern.3 The presence of valvular disease (International Classification of Diseases (ICD), eighth revision codes 394–396 and 424 or ICD, ninth revision codes 394–397) and prior myocardial infarction (ICD‐8 code 410 or ICD‐9 code 410) was assessed from the hospital discharge register. The diagnosis of acute myocardial infarction was chosen as a proxy for coronary heart disease, as the precision of the myocardial infarction diagnosis in the Swedish hospital discharge register is high.22
Metabolic syndrome definition
The NCEP ATP III definition of metabolic syndrome6 was analysed in the present study, with a minor modification for use in an existing longitudinal cohort (table 1).
Table 1 Modified National Cholesterol Education Program Adult Treatment Pane III6 metabolic syndrome definition used in the present study.
| Metabolic syndrome present if ⩾3 of the following criteria fulfilled |
| • Fasting plasma glucose ⩾6.1 mmol/l (110 mg/dl) |
| • Blood pressure ⩾130/85 mm Hg or treatment |
| • Triglycerides ⩾1.7 mmol/l (150 mg/dl) |
| • High density lipoprotein cholesterol <1.04 mmol/l (40 mg/dl) |
| • Body mass index >29.4 kg/m2 |
Reference limits are given for men only.
As waist circumference was measured in only 479 men at the examination, the NCEP definition was modified by use of a BMI cut point instead of the criterion of waist circumference > 102 cm. In this subsample, a waist circumference of 102 cm corresponded to a BMI of 29.4 kg/m2 in a linear regression analysis (regression equation: BMI [kg/m2] = 0.298 × waist circumference [cm] − 1.027), which is similar to BMI cut points used in previous modified NCEP definitions of metabolic syndrome.8 BMI did not differ significantly between this subsample (25.2 (SD 3.1) kg/m2) and the rest of the cohort (25.0 (SD 3.3) kg/m2, p = 0.32). BMI may be considered a satisfactory substitute for waist circumference in middle‐aged men8 and has been used instead of waist circumference in modified NCEP criteria in several previous studies examining cardiovascular risk.7,8,10,23,24 Further, treatment for hypertension was included in the definition, which is an approach used in several previous studies.7,8,9,10,24
Follow‐up and outcome variables
The participants were followed up until they were 70 years old, an age chosen a priori to examine the effect of metabolic syndrome on HF incidence in middle‐aged men. The participants had a median follow up of 20.1 years (range 0.04–21.4 years), contributing to 43367 person years at risk. One hundred and fifteen men had a hospital discharge register diagnosis of HF between the entry to the ULSAM study and the age of 70. As a possible diagnosis of HF, we considered ICD HF codes 427.00, 427.10, 428.99 (ICD‐8) and 428 (ICD‐9). The medical records from the relevant hospitalisation were reviewed by two physicians (EI and LL), who classified the cases as definite, questionable or miscoded, blinded to the baseline data. All HF diagnoses were reviewed, independently of whether a patient experienced a myocardial infarction during the same admission. The classification relied on the definition proposed by the European Society of Cardiology.25 Thus, for HF to be classified as definite, the patient had to have symptoms and signs of HF and objective evidence of cardiac dysfunction at rest. In cases of doubt, the response to treatment directed towards HF was a useful check of the diagnosis. The review process has been described extensively.26 After this validation, 100 patients with definite HF were included in the present study (that is, 87% of the participants with an HF diagnosis coded in the hospital discharge register). None of the participants was lost to follow up.
Statistical methods
Data are given as mean (SD) and percentages. The prognostic value of the prevalence of metabolic syndrome for HF incidence was investigated by Cox proportional hazards analyses. Proportional hazards assumptions were confirmed both graphically and by Schoenfeld's tests. We investigated three sets of models in a hierarchical fashion: model A, unadjusted analyses; model B, multivariable‐adjusted analyses of the following baseline covariates: hypertension, diabetes, ECG left ventricular hypertrophy, smoking and BMI; and model C, covariates as in model B, with the addition of interim myocardial infarction during follow up, modelled as a time‐dependent covariate.
The current clinical definition of hypertension20 was used as a covariate in the primary analyses. As this definition uses higher cut‐off values than the NCEP metabolic syndrome criterion of hypertension, we also used an alternative definition of hypertension in secondary analyses, in which all the above mentioned analyses were repeated. In these secondary analyses, hypertension at baseline was defined as systolic blood pressure ⩾ 130 mm Hg, diastolic blood pressure ⩾ 85 mm Hg or use of hypertension drugs. In further secondary analyses, we added ESR as a covariate to all models to investigate whether the effect of metabolic syndrome was mediated partly by inflammation. Interaction terms were investigated for metabolic syndrome and the other covariates. Two‐tailed 95% confidence intervals (CIs) and p values were given, with p < 0.05 regarded as significant. We used the statistical software package STATA V. 8.2 (StataCorp, College Station, Texas, USA).
RESULTS
The incidence for HF during the follow‐up period was 2.3/1000 person years at risk (5.3/1000 person years at risk among those with metabolic syndrome at baseline and 1.7/1000 person years at risk among those without). Table 2 shows the participant characteristics at baseline.
Table 2 Baseline characteristics of the cohort.
| Developed HF (n = 100) | Did not develop HF (n = 2214) | |
|---|---|---|
| Hypertension | 66% | 42% |
| Diabetes | 11% | 5% |
| ECG left ventricular hypertrophy | 4% | 2% |
| Current cigarette smoking | 56% | 51% |
| MI during follow up | 42% | 10% |
| Body mass index (kg/m2) | 27.0 (3.9) | 24.9 (3.2) |
| Serum cholesterol (mmol/l) | 7.1 (1.6) | 6.9 (1.3) |
| LDL cholesterol (mmol/l) | 5.6 (1.5) | 5.3 (1.2) |
| HDL cholesterol (mmol/l) | 1.2 (0.3) | 1.4 (0.4) |
| Serum triglycerides (mmol/l) | 2.3 (1.3) | 1.9 (1.2) |
| Fasting plasma glucose (mmol/l) | 5.8 (1.2) | 5.6 (1.0) |
| Metabolic syndrome | 39% | 17% |
Values are mean (SD) or percentage.
HDL, high density lipoprotein; HF, heart failure; LDL, low density lipoprotein; MI, myocardial infarction.
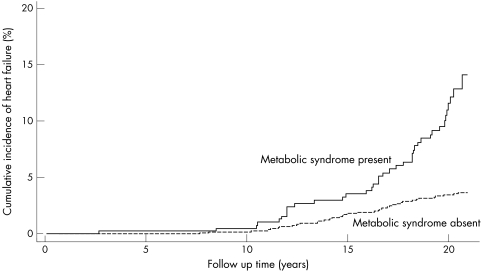
In an unadjusted Cox proportional hazards analysis, the presence of metabolic syndrome increased the risk for HF more than threefold (table 3, model A). Figure 1 presents a cumulative HF incidence plot for metabolic syndrome. After adjustment for established risk factors for HF (hypertension, diabetes, ECG left ventricular hypertrophy, smoking and BMI), metabolic syndrome remained as a significant predictor of HF (table 3, model B).
Table 3 Heart failure incidence in relation to metabolic syndrome and established risk factors in the total sample (n = 2314) free from heart failure, myocardial infarction and valvular disease at baseline.
| Model A | Model B | Model C | |
|---|---|---|---|
| Metabolic syndrome (present v absent) | 3.33 (2.23 to 4.98)*** | 1.66 (1.02 to 2.70)* | 1.80 (1.11 to 2.91)* |
| Hypertension (present v absent) | 2.87 (1.90 to 4.35)*** | 2.05 (1.32 to 3.19)*** | 2.00 (1.29 to 3.09)** |
| Diabetes (present v absent) | 2.26 (1.21 to 4.24)* | 1.36 (0.71 to 2.62) | 1.23 (0.63 to 2.40) |
| ECG LVH (present v absent) | 2.83 (1.04 to 7.71)* | 1.79 (0.65 to 4.94) | 1.15 (0.41 to 3.21) |
| Current cigarette smoking (present v absent) | 1.36 (0.92 to 2.02) | 1.50 (1.01 to 2.24)* | 1.30 (0.87 to 1.95) |
| Body mass index (per 1 SD (3.2 kg/m2)) | 1.78 (1.51 to 2.09)*** | 1.45 (1.20 to 1.76)*** | 1.37 (1.12 to 1.66)** |
| Interim MI (present v absent) | 10.63 (7.05 to 16.02)*** | NA | 8.66 (5.71 to 13.14)*** |
Data are Cox proportional hazard ratios (95% CI), unadjusted (model A), adjusted for established risk factors for heart failure (hypertension, diabetes, ECG left ventricular hypertrophy (LVH), smoking and body mass index) at baseline (model B) and adjusted for these established risk factors and interim myocardial infarction (MI) during follow up (model C).
*p<0.05; **p<0.01; ***p<0.001 (p<0.05 was considered significant).
NA, not applicable.
Figure 1 Cumulative incidence of heart failure in the total cohort of patients free from heart failure, myocardial infarction and valvular disease at baseline, by the presence or absence of metabolic syndrome.
Evidence of myocardial infarction during the follow up was present in 267 of the participants in the total cohort and in 42 of the 100 patients with HF. Five of the patients with HF presented in the immediate post‐infarct period (within 30 days after the myocardial infarction). After adjustment for interim myocardial infarction in addition to the established baseline risk factors for HF, metabolic syndrome remained a significant predictor of subsequent HF (table 3, model C).
In the secondary analyses based on the alternative definition of hypertension (systolic blood pressure ⩾ 130 mm Hg, diastolic blood pressure ⩾ 85 mm Hg or drug), the presence of metabolic syndrome was a borderline significant predictor of HF after adjustment for established risk factors for HF (model B: hazard ratio 1.61, 95% CI 0.98 to 2.66). When interim myocardial infarction was also adjusted for in addition to the established baseline risk factors for HF, the presence of metabolic syndrome was a significant predictor of HF (model C: hazard ratio 1.74, 95% CI 1.06 to 2.83) in the model based on the alternative definition of hypertension. In further secondary analyses with ESR added as a covariate, the association between metabolic syndrome and HF was still strong and significant (hazard ratio 3.23, 95% CI 2.16 to 4.83 with ESR added to model A). When ESR was added to the model adjusted for established risk factors for HF including myocardial infarction during follow up (model C), metabolic syndrome remained a significant predictor of HF (hazard ratio 1.73, 95% CI 1.07 to 2.81). None of the interaction terms was significant.
DISCUSSION
Principal findings
In this community‐based cohort study of men free from HF, myocardial infarction and valvular disease at baseline, we showed for the first time that the presence of metabolic syndrome increased the risk of subsequent HF, when established HF risk factors and interim myocardial infarction during follow up were taken into account.
Previous studies
In a previous cross‐sectional study based on the NHANES III cohort,16 the presence of HF was significantly associated with both the NCEP ATP III and World Health Organization definitions of metabolic syndrome. To our knowledge, there are no previous longitudinal studies of the predictive value of metabolic syndrome for the incidence of subsequent HF. In a recent study of the present cohort, insulin resistance, measured with the reference standard euglycaemic insulin clamp technique, was an independent risk factor for HF, taking diabetes, obesity and other established HF risk factors into account.17 The cumbersome euglycaemic insulin clamp technique is unlikely ever to be used for clinical risk evaluation. The present study, which investigated a clinically feasible proxy for insulin resistance—that is, metabolic syndrome—is therefore an important clinical extension of that previous study and adds knowledge, as it examined a larger study sample of middle‐aged men with a longer follow up.
Metabolic syndrome is a known predictor of coronary heart disease,7,8,9 cardiovascular diseases combined7,9,27,28 and total mortality.7,9,28 Thus, metabolic syndrome is a strong predictor of those atherosclerosis‐driven end points. In the present study, we sought to investigate the impact of metabolic syndrome on non‐ischaemic HF, by using the accepted approach of excluding patients with myocardial infarction before baseline and adjusting for myocardial infarction during follow up,29,30 as well as adjusting for established risk factors for HF, most of which are also risk factors for ischaemic heart disease.
Possible mechanisms
We speculated that metabolic syndrome may have direct myocardial effects, apart from its effects on atherosclerosis. Indeed, in the present study, the presence of metabolic syndrome predicted HF incidence independently of baseline and interim myocardial infarction and baseline variables that are powerful risk factors for atherosclerotic disease. Metabolic syndrome has numerous plausible direct myocardial effects, which are related to insulin resistance and accompanying hyperinsulinaemia. Firstly, insulin may function as a growth factor in the myocardium, a notion supported by experimental observations of increased myocardial mass and decreased cardiac output in rats with sustained hyperinsulinaemia.31 Secondly, hyperinsulinaemia activates the sympathetic nervous system,32 which is a presumed causal factor for HF.33 Thirdly, insulin resistance has recently been shown to increase the trophic effects of angiotensin II on cellular hypertrophy and collagen production34 in patients with hypertension, which leads to myocardial hypertrophy and fibrosis,33 both key substrates for HF. Fourthly, advanced glycosylation end products are produced at a greatly accelerated pace in insulin‐resistant people, which leads to increased collagen cross linking and myocardial stiffness. A previous study has shown that treating diabetic dogs with a collagen cross‐link breaker, such as metformin, can improve ventricular function and reverse myocardial stiffness.35 Moreover, a recent small clinical trial found decreased left ventricular mass and improved left ventricular diastolic filling after treatment with a cross‐link breaker.36
Another possible explanation of how metabolic syndrome may predict HF independently of the established risk factors is that the cut‐off level for blood pressure is lower in the NCEP definition of metabolic syndrome than in the current definition of hypertension.20 Blood pressures in the high normal range (systolic 130–139 mm Hg or diastolic 85–89 mm Hg) are captured by the NCEP definition. High blood pressure is the most important risk factor for HF on a population level,3 and a blood pressure in the high normal range has previously been shown to increase the risk of subsequent cardiovascular events.37 To evaluate this possible explanation of the association between metabolic syndrome and HF, we performed secondary analyses adjusted for hypertension defined with the same cut‐off levels as in the NCEP definition of metabolic syndrome. In these analyses, metabolic syndrome was a borderline significant predictor of HF, and the hazard ratio was a little lower (1.61, as compared with 1.66 in the primary model). This indicates that the blood pressure level explains some, but not all, of the risk for HF contained in metabolic syndrome. When we adjusted for interim myocardial infarction during the follow up, metabolic syndrome was a significant predictor of HF after adjustment of all established risk factors, including hypertension according to the alternative definition. The hazard ratio for metabolic syndrome was higher in the models adjusted for myocardial infarction during follow up, implying that myocardial infarction was a negative confounder for the association between metabolic syndrome and HF. This may indicate that the hypertension component of metabolic syndrome is important in the relationship between metabolic syndrome and HF, especially in people who experience a myocardial infarction. In people without myocardial infarction during follow up, other parts of metabolic syndrome seem to be more important—for example, those reflecting insulin resistance.
There is substantial evidence of an association between metabolic syndrome and inflammation,8,23 and inflammation has been shown to predict HF, both in this cohort38 and by others.39 To investigate whether the effect of metabolic syndrome was mediated partly by inflammation, we performed secondary analyses with ESR added to the statistical models. The results were very similar with and without inclusion of ESR, indicating that inflammation may not be an important confounder or mediator of the association between metabolic syndrome and HF.
Strengths and limitations
The strengths of this study include the large population, the long follow‐up period and the detailed characterisation of the cohort. Furthermore, all cases of HF were validated, limiting the inclusion of false positive cases.
As some of the established HF risk factors are included in the definition of metabolic syndrome, the bar is high for evidence of an independent effect of metabolic syndrome on HF incidence. Nevertheless, we chose this approach to mimic the clinical situation, in which the status of the established risk factors is presumed to be known. Consequently, robust statistical methods that can handle some collinearity were used. The clinical perspective was also the reason for modelling the established risk factors as dichotomous variables, as this is how they are handled in clinical decision making.
This study has some limitations. As we examined only men of the same age with a similar ethnic background, this study has an unknown generalisability to women and other age and ethnic groups. Non‐hospitalised patients with milder HF were not included in our end point, which would tend to bias the results towards the null hypothesis. Furthermore, as this study was initiated in the 1970s and the HF diagnosis was based on a review of medical records, it was not possible to distinguish between systolic and diastolic HF, as echocardiography was not available at the time of diagnosis for many of the patients. Another limitation of the study is that we used the presence of an interim myocardial infarction during follow up as a proxy for coronary heart disease. Even if this is an established method for examining “non‐ischaemic” HF,29,30 it would be assessed better in a more specific way—for example, by examining all participants with coronary angiography. However, this is not a feasible option in a large, population‐based epidemiological study. Furthermore, we believe that the presence of a symptomatic myocardial infarction is a good proxy for coronary heart disease, as it includes patients with the most advanced coronary heart disease.
Conclusions
The presence of metabolic syndrome increased the risk of subsequent HF, independently of established risk factors for HF including an interim myocardial infarction, in a community‐based sample of men through long follow up. This implies that metabolic syndrome provides important risk information beyond that of established risk factors for HF. This increased HF risk may be promoted by insulin resistance and accompanying hyperinsulinaemia. If our results are confirmed, the study indicates that metabolic syndrome may have direct myocardial effects in addition to its proatherosclerotic effects. Our findings add to the importance of identifying patients with metabolic syndrome, especially given the current obesity epidemic.
ACKNOWLEDGEMENTS
The work was supported by the Swedish Heart Lung Foundation (Hjärt‐Lungfonden) and Thuréus Foundation. The funding source was not involved in the work with the article.
Abbreviations
ATP III - Adult Treatment Panel III
BMI - body mass index
ESR - erythrocyte sedimentation rate
HF - heart failure
ICD - International Classification of Diseases
NCEP - National Cholesterol Education Program
NHANES III - Third National Health and Nutrition Examination Survey
ULSAM - Uppsala Longitudinal Study of Adult Men
Footnotes
Conflicts of interest: Lars Lind is a part time employee at AstraZeneca R&D, Mölndal, Sweden, and a part time employee of Uppsala University. AstraZeneca has no interests in this project and has not given any financial support. The study was initiated by the authors alone. Thus, none of the authors have any conflicts of interest.
Published Online First Date to follow
References
- 1.Eriksson H, Svärdsudd K, Larsson B.et al Risk factors for heart failure in the general population: the study of men born in 1913. Eur Heart J 198910647–656. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans J C, Levy D.et al Obesity and the risk of heart failure. N Engl J Med 2002347305–313. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Larson M G, Vasan R S.et al The progression from hypertension to congestive heart failure. JAMA 19962751557–1562. [PubMed] [Google Scholar]
- 4.Reaven G M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988371595–1607. [DOI] [PubMed] [Google Scholar]
- 5.Meigs J B. Invited commentary: insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol. 2000;152: 908–11; discussion 912, [DOI] [PubMed]
- 6.National Cholesterol Education Program Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 20021063143–3421. [PubMed] [Google Scholar]
- 7.Malik S, Wong N D, Franklin S S.et al Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 20041101245–1250. [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Gaw A, Scherbakova O.et al Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003108414–419. [DOI] [PubMed] [Google Scholar]
- 9.Lakka H M, Laaksonen D E, Lakka T A.et al The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA 20022882709–2716. [DOI] [PubMed] [Google Scholar]
- 10.Girman C J, Rhodes T, Mercuri M.et al The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol 200493136–141. [DOI] [PubMed] [Google Scholar]
- 11.Ärnlöv J, Lind L, Zethelius B.et al Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow‐up. Am Heart J 2001142720–724. [DOI] [PubMed] [Google Scholar]
- 12.Ärnlöv J, Lind L, Sundström J.et al Insulin resistance, dietary fat intake and blood pressure predict left ventricular diastolic function 20 years later. Nutr Metab Cardiovasc Dis 200515242–249. [DOI] [PubMed] [Google Scholar]
- 13.Devereux R B, Roman M J, Paranicas M.et al Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 20001012271–2276. [DOI] [PubMed] [Google Scholar]
- 14.Rutter M K, Parise H, Benjamin E J.et al Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex‐related differences in the Framingham Heart Study. Circulation 2003107448–454. [DOI] [PubMed] [Google Scholar]
- 15.Sundström J, Lind L, Nyström N.et al Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation 20001012595–2600. [DOI] [PubMed] [Google Scholar]
- 16.Ford E S, Giles W H. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care 200326575–581. [DOI] [PubMed] [Google Scholar]
- 17.Ingelsson E, Sundström J, Ärnlöv J.et al Insulin resistance and risk of congestive heart failure. JAMA 2005294334–341. [DOI] [PubMed] [Google Scholar]
- 18.Kahn R, Buse J, Ferrannini E.et al The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005282289–2304. [DOI] [PubMed] [Google Scholar]
- 19.Ingelsson E, Ärnlöv J, Sundström J.et al Novel metabolic risk factors for heart failure. J Am Coll Cardiol 2005462054–2060. [DOI] [PubMed] [Google Scholar]
- 20.Anon World Health Organization‐International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 199917151–183. [PubMed] [Google Scholar]
- 21.Prineas R J, Crow R S, Blackburn H.The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Bristol: John Wright, 1982
- 22.Lindblad U, Råstam L, Ranstam J.et al Validity of register data on acute myocardial infarction and acute stroke: the Skaraborg Hypertension Project. Scand J Soc Med 1993213–9. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P M, Buring J E, Cook N R.et al C‐reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8‐year follow‐up of 14 719 initially healthy American women. Circulation 2003107391–397. [DOI] [PubMed] [Google Scholar]
- 24.Sundström J, Riserus U, Byberg L.et al Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006332878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Society of Cardiology Guidelines for the diagnosis of heart failure. The Task Force on Heart Failure of the European Society of Cardiology. Eur Heart J 199516741–751. [PubMed] [Google Scholar]
- 26.Ingelsson E, Ärnlöv J, Sundström J.et al The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 20057787–791. [DOI] [PubMed] [Google Scholar]
- 27.Rutter M K, Meigs J B, Sullivan L M.et al C‐reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 2004110380–385. [DOI] [PubMed] [Google Scholar]
- 28.Hunt K J, Resendez R G, Williams K.et al National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all‐cause and cardiovascular mortality in the San Antonio Heart Study. Circulation 20041101251–1257. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y T, Vaccarino V, Williams C S.et al Risk factors for heart failure in the elderly: a prospective community‐based study. Am J Med 1999106605–612. [DOI] [PubMed] [Google Scholar]
- 30.Vasan R S, Larson M G, Benjamin E J.et al Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 19973361350–1355. [DOI] [PubMed] [Google Scholar]
- 31.Holmäng A, Yoshida N, Jennische E.et al The effects of hyperinsulinaemia on myocardial mass, blood pressure regulation and central haemodynamics in rats. Eur J Clin Invest 199626973–978. [DOI] [PubMed] [Google Scholar]
- 32.Anderson E A, Hoffman R P, Balon T W.et al Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 1991872246–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell D S. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003262433–2441. [DOI] [PubMed] [Google Scholar]
- 34.Sartori M, Ceolotto G, Papparella I.et al Effects of angiotensin II and insulin on ERK1/2 activation in fibroblasts from hypertensive patients. Am J Hypertens 200417604–610. [DOI] [PubMed] [Google Scholar]
- 35.Jyothirmayi G N, Soni B J, Masurekar M.et al Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Ther 19983319–326. [DOI] [PubMed] [Google Scholar]
- 36.Little W C, Zile M R, Kitzman D W.et al The Effect of alagebrium chloride (ALT‐711), a novel glucose cross‐link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 200511191–195. [DOI] [PubMed] [Google Scholar]
- 37.Vasan R S, Larson M G, Leip E P.et al Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med 20013451291–1297. [DOI] [PubMed] [Google Scholar]
- 38.Ingelsson E, Ärnlöv J, Sundström J.et al Inflammation, as measured by the erythrocyte sedimentation rate, is an independent predictor for the development of heart failure. J Am Coll Cardiol 2005451802–1806. [DOI] [PubMed] [Google Scholar]
- 39.Vasan R S, Sullivan L M, Roubenoff R.et al Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 20031071486–1491. [DOI] [PubMed] [Google Scholar]