Abstract
Objective
To examine whether aggressive risk factor modification in chronic kidney disease (CKD) can limit the development of new ischaemia or reduce cardiac events.
Methods
Patients with CKD were randomly assigned to either an aggressive risk factor modification strategy (targeted treatment of hypertension, dyslipidaemia, homocysteine, haemoglobin and phosphate) or standard care. An intention to treat analysis was performed on 152 patients who had baseline dobutamine stress echocardiography (DSE), including 107 who had follow‐up DSE. Biochemical parameters, cardiac risk factors and investigations (ECG, two‐dimensional echocardiography) were recorded at baseline. New ischaemia was classed as new or worsening stress wall motion abnormality between follow‐up and baseline DSE. Patients were followed up for the development of new ischaemia or cardiac death, acute coronary syndrome and non‐fatal myocardial infarction over 1.8 years.
Results
The development of new ischaemia was common but not different between the standard and aggressively treated groups (15 (21%) v 18 (23%), p = 0.8). Independent predictors of new ischaemia were older age, abnormal ECG, higher systolic blood pressure and lower serum high density lipoprotein cholesterol, but not treatment arm. The standard and aggressively treated groups did not differ in cardiac event rate (10% v 13%, p = 0.6) or all‐cause mortality (10% v 19%, p = 0.2). In patients with an abnormal baseline DSE (non‐diagnostic, scar or ischaemia), the event rate was similar (22% v 20%, p = 0.9).
Conclusion
Aggressive risk factor modification in CKD does not limit the development of new ischaemia or reduce cardiac events in patients with an abnormal DSE.
Cardiac events and left ventricular abnormalities assessed by resting two‐dimensional echocardiography are a major cause of morbidity and mortality in patients with chronic kidney disease (CKD). Cardiovascular mortality is several orders of magnitude greater than in the general population, matched for age.1,2 Patients with CKD are prone to coronary events, partly because of clustering of risk factors (hypertension, diabetes, dyslipidaemia) but also because uraemia promotes an atherogenic milieu.3 Dialysis predisposes to the development of arterial disease,4 which may progress rapidly, and survival after events is poor.5 Dobutamine stress echocardiography (DSE) is an accurate diagnostic tool for coronary artery disease in patients with CKD6 and is a powerful predictor of cardiac events.7,8 A normal DSE does imply a favourable prognosis in this patient group, although the two largest prognostic studies7,9 suggest that the warranty is limited to 24 months, presumably due to accelerated atherosclerosis. Ischaemia is associated with an adverse outcome in CKD, as in other diseases, and therefore the development of new ischaemia at 24 months may not only be important as a marker of coronary disease progression but may also identify patients at risk of a new event. Although measures of vascular structure or function, such as intima media thickness or brachial artery activity, can provide prognostic information in a wide variety of patients including those with CKD,10 they do not provide data on the presence or absence of myocardial ischaemia.
In patients with CKD, many potential risk factors contribute to the development of cardiac disease,11 some of which are operative before dialysis is started.12 These include traditional risk factors (older age, dyslipidaemia, hypertension, diabetes and smoking), but there is increasing emphasis on CKD‐related risk factors such as hyperphosphataemia, vascular calcification, malnutrition, inflammation, hyperhomocystinaemia, anaemia or dialysis‐related factors. In particular, abnormalities of calcium phosphate metabolism have adverse prognostic effects13,14 and may contribute to vascular calcification.15 Moreover, studies with non‐calcium‐containing phosphate binders, such as Sevelamer, have shown beneficial effects on both coronary and aortic calcification.16 Nevertheless, data from interventional studies of multiple risk factor modification are limited. In this study of DSE in patients with CKD, we sought to establish whether aggressive risk factor modification can limit the development of new ischaemia or cardiac events.
METHODS
Study design
This was a prospectively planned cardiac imaging substudy of a larger study, powered for vascular structure and function end points.17 All patients receiving dialysis for CKD or those with an estimated glomerular filtration rate of 30 ml/min or less, calculated by the Cockcroft–Gault formula,18 were eligible for inclusion into the study. Exclusion criteria were life expectancy of less than six months or unwillingness to participate. Patients who declined the test or those with a recent (within three months) cardiac event or pacemaker were excluded from the DSE component. The study was a randomised, single‐centre study with 200 patients enrolled between April 2000 and August 2001, of whom 152 underwent two‐dimensional resting echocardiography and a baseline DSE. On the basis of a standard deviation of 0.2 of change in wall motion score index, a sample size of 75 in each group would allow a 10% change (for example, development of akinesis in one segment or hypokinesis in two) to be detected with a power of 80% at a probability of 0.05.
Randomisation was achieved by random numbers generated by SPSS for Windows V.11.0 (SPSS Inc, Chicago, Illinois, USA). The investigator was blinded by the use of double‐sealed opaque envelopes. Of the 152 patients, 72 were assigned to receive usual care (standard group) according to national guidelines and 80 were allocated to a strategy of aggressive risk factor modification (aggressively treated group) with a physician‐directed, nurse‐run programme in addition to usual care.17
Briefly, the intervention was the use of atorvastatin 20–80 mg/day to attain a low density lipoprotein (LDL) cholesterol target < 2.0 mmol/l; folic acid 30 mg/day, alternate monthly hydroxycobalamin and pyridoxine 25 mg/day to attain a homocysteine < 15 μmol/l; protocol‐driven erythropoietin replacement and iron supplementation to attain a haemoglobin target of 110–125 g/l; stepwise introduction of hypertension agents with angiotensin‐converting enzyme inhibitors or receptor blockers as the preferred treatment to attain a target blood pressure < 140/90 mm Hg; smoking cessation; and use of aspirin in all patients apart from those in whom it was contraindicated. Patients received calcium carbonate as the preferred treatment, and aluminium hydroxide was added if phosphate control was poor; a subset of patients in the aggressively treated group (n = 23) who had hypercalcaemia or poor phosphate control despite maximal conventional treatment received Sevelamer (Genzyme Corp, Cambridge, Massachusetts, USA). All patients were seen at about two‐monthly intervals and those in the aggressively treated group were reviewed every month in the nurse‐led clinic. All patients gave written, informed consent, and the study was approved by the Human Ethics Committee of the University of Queensland and Princess Alexandra Hospital. Fasting serum was collected for biochemical analysis at baseline and at 24 months. DSE was performed at baseline and at 24 months and wall motion scores were recorded. Patients who underwent renal transplantation during the course of the study were excluded from follow‐up DSE.
Clinical assessment
Baseline demographic data, including a thorough assessment of risk factor status and history of cardiovascular disease, was recorded, cardiovascular drugs were documented, and a 12‐lead ECG was reviewed. Blood pressure, averaged from three seated measurements taken after a 5 min rest, was measured before dialysis on a short (two‐day) break in all patients undergoing haemodialysis. An abnormal ECG was defined as showing ST change, a bundle branch block pattern or a prolonged corrected QT interval. Diabetes was defined by a history of this diagnosis or by use of oral hypoglycaemic agents or insulin. Previous cardiac event was defined as a history of documented myocardial infarction, coronary artery bypass surgery, percutaneous coronary intervention or hospital admission with acute coronary syndrome (ischaemic chest pain with or without ECG changes suggestive of ischaemia with no rise in cardiac enzymes).
Biochemical analysis
Blood for biochemical analyses was obtained from fasting venous samples and taken at baseline and 24 months. Serum total cholesterol, triglycerides, albumin, phosphate, calcium, creatinine and haemoglobin concentration were measured by standard laboratory techniques. High density lipoprotein (HDL) cholesterol was measured as a homogeneous assay in liquid phase on a Hitachi 747 autoanalyser (Boehringer Mannheim, Mannheim, Germany) and the Friedewald equation was used to calculate LDL cholesterol concentrations. For homocysteine analysis, the specimen was centrifuged within 1 h of collection and measured with high performance liquid chromatography. C reactive protein was assayed with a Beckman Coulter IMMAGE C reactive protein test.
Resting two‐dimensional echocardiography
Left ventricular mass was calculated by the area–length method and ejection fraction by Simpson's biplane method. Left ventricular mass was indexed to height in metres,2,7 which is more accurate than body surface area.19
Dobutamine stress echocardiography
DSE was performed by a standard dobutamine–atropine protocol20 at baseline and follow up. The patient was prepared in the usual way for stress testing, and an intravenous cannula was inserted into a proximal arm vein. Resting echocardiographic images were obtained in the parasternal long and short axis and apical two‐ and four‐chamber views. The images were acquired in digital format online, as well as being stored on videotape. Each patient was under continuous clinical, ECG and echocardiographic monitoring, and dobutamine was infused at doses of 5, 10, 20, 30 and 40 μg/kg/min. The test was stopped in the presence of severe side effects, severe chest pain or ischaemia, or the completion of the protocol. If the target heart rate (85% of age‐predicted maximum) was not achieved at maximum dose in the absence of these end points, atropine (0.25 mg/min) was given to a total of 1 mg at the discretion of the physician performing the test. After the test, a physician blinded to the clinical data interpreted the ECG and echocardiographic responses. Ischaemia was identified on the ECG in the presence of 0.1 mV of ST segment depression at 0.08 ms after the J point. Those patients whose resting ECGs showed extensive repolarisation abnormalities, including those due to left ventricular hypertrophy and left bundle branch block, were excluded from ECG analysis. A DSE was reported as normal if the patient was stressed maximally (to > 85% predicted heart rate) and no wall motion abnormalities were observed at rest or with stress. Negative tests at a submaximal heart rate and with rate–pressure product < 21 000 were identified as non‐diagnostic.
Detection of ischaemia
Digital images were stored on magneto‐optical disk and analysed offline in a digitised quad‐screen display by at least two trained observers using the 16‐segment model of the American Society of Echocardiography.21 Regional myocardial performance was scored on the basis of wall thickening as normal, mildly hypokinetic, severely hypokinetic, and akinetic or dyskinetic. Scar was defined as a wall motion abnormality at rest that did not change with stress, and ischaemia was identified by new or worsening wall motion abnormalities with stress. Baseline DSEs that were non‐diagnostic and those showing scar or ischaemia were considered abnormal. A new or worsening stress wall motion abnormality between baseline and follow‐up DSE was classed as new ischaemia.
Ten randomly selected DSE studies were reanalysed by one of the observers blinded to the results of the first analysis. For rest and stress images, a wall motion score index was derived as the sum of the scores divided by the number of visualised segments. The coefficient of variance was calculated.
Follow up
Follow up was obtained by clinic review or, for patients living remotely, by telephone contact. Cardiac events were (1) non‐fatal myocardial infarction defined as raised cardiac enzymes (troponin I) and one of the following: ischaemic chest pain, development of pathological Q waves on the ECG or ECG changes suggestive of cardiac ischaemia; (2) acute coronary syndrome defined as no rise in cardiac enzymes with ischaemic chest pain with or without ECG changes suggestive of ischaemia; and (3) cardiac death defined as fatal myocardial infarction or cardiac arrest. Total mortality included death from any cause.
Data analysis
All data are expressed as mean (SD) or frequency (%), unless otherwise stated. Groups were compared by the two‐tailed independent t test or paired t test for continuous variables and the χ2 test, Wilcoxon signed rank test or McNemar's test, as appropriate. Event rates were compared by a Cox proportional hazards model. Two different regression analyses were performed: binary logistic regression was used to determine the predictors of new ischaemia and a Cox regression analysis was used for predictors of events. Data were examined by an intention to treat analysis. Data were statistically analysed with SPSS for Windows V.11.0.
RESULTS
Patient characteristics
At baseline, 152 patients underwent DSE. Table 1 shows the clinical characteristics of the study population. Fifty‐three patients were not yet undergoing dialysis (mean glomerular filtration rate 19.4 (7.4) ml/min) and 99 were dialysis dependent (58% haemodialysis, 42% peritoneal dialysis). Patient characteristics did not differ significantly between the standard and aggressively treated groups (table 2).
Table 1 Clinical characteristics of patients at entry into study.
| Age (years) | 54.2 (15.0) |
| Men | 43% |
| Body mass index (kg/m2) | 26.9 (5.0) |
| Peripheral vascular disease | 19% |
| Abnormal ECG | 26% |
| Risk factors | |
| Family history of premature CVD | 30% |
| Current smoking | 7% |
| Hypertension | 91% |
| Hyperlipidaemia | 56% |
| Diabetes | 26% |
| Previous cardiac event | 32% |
| hs‐CRP (mg/l) | 6.0 (1.6–9.0) |
| Lipid profile | |
| Total cholesterol (mmol/l) | 4.91 (1.4) |
| HDL cholesterol (mmol/l) | 1.10 (0.36) |
| LDL cholesterol (mmol/l) | 2.81 (0.90) |
| Triglyceride (mmol/l) | 2.20 (2.20) |
| Blood pressure (mm Hg) | |
| Systolic | 144 (21) |
| Diastolic | 84 (13) |
| Renal factors | |
| Renal replacement therapy | 65% |
| Duration of dialysis (years) | 1.6 (2.5) |
| Glomerular filtration rate (ml/min) | 19.4 (7.4) |
| Haemoglobin (g/l) | 109.5 (16.2) |
| Homocysteine (μmol/l) | 25.9 (13.2) |
| Phosphate (mmol/l) | 1.8 (0.5) |
| Albumin (g/l) | 37.9 (4.6) |
| Drugs | |
| β blocker | 31% |
| ACE inhibitor | 43% |
| Calcium channel blocker | 42% |
| Statin | 70% |
| Platelet inhibitors | 42% |
| Resting echocardiographic measurements | |
| Ejection fraction (%) | 57 (11) |
| LVMI2.7 (g/m2.7) | 54 (17) |
Continuous variables are expressed as mean (SD) or median (interquartile range) and categorical variables as percentages.
CVD, cardiovascular disease; HDL, high density lipoprotein; hs‐CRP, high sensitive C reactive protein; LDL, low density lipoprotein; LVMI, left ventricular mass index.
Table 2 Risk factor characteristics of standard and aggressively treated groups.
| Parameter | Aggressive treatment | Standard treatment | p Value |
|---|---|---|---|
| Number | 80 | 72 | |
| Mean age (years) | 53 (15) | 56 (15) | 0.9 |
| Men | 31 (39%) | 34 (47%) | 0.3 |
| Dialysis dependent | 55 (69%) | 44 (61%) | 0.3 |
| Current smoking | 4 (5%) | 7 (10%) | 0.3 |
| Hypertension | 74 (93%) | 65 (90%) | 0.6 |
| Hyperlipidaemia | 47 (59%) | 38 (53%) | 0.5 |
| Diabetes | 21 (26%) | 19 (26%) | 0.9 |
| Previous cardiac event | 26 (33%) | 22 (31%) | 0.8 |
Continuous variables are expressed as mean (SD) and categorical variables as percentages.
Changes in risk factors
Changes in modifiable risk factors were analysed on the basis of intention to treat (table 3).
Table 3 Comparison of changes in risk factors in standard and aggressively treated groups.
| Variable | Treatment group | Baseline | Follow up | p Value* | Change | p Value† |
|---|---|---|---|---|---|---|
| Total cholesterol (mmol/l) | Standard | 4.85 (1.14) | 4.43 (1.1) | 0.002 | 0.42 (0.13) | 0.05 |
| Aggressive | 4.96 (1.6) | 4.1 (1.18) | <0.001 | 0.85 (0.17) | ||
| Triglyceride (mmol/l) | Standard | 2.16 (2.58) | 1.95 (1.54) | 0.30 | 0.21 (0.2) | 0.99 |
| Aggressive | 2.22 (1.86) | 2.01 (1.4) | 0.23 | 0.2 (1.7) | ||
| HDL cholesterol (mmol/l) | Standard | 1.11 (0.35) | 1.23 (0.35) | 0.002 | −0.11 (0.03) | 0.88 |
| Aggressive | 1.09 (0.36) | 1.2 (0.36) | <0.001 | −0.11 (0.03) | ||
| LDL cholesterol (mmol/l) | Standard | 2.79 (0.78) | 2.34 (0.78) | <0.001 | 0.44 (0.1) | 0.02 |
| Aggressive | 2.82 (1.05) | 1.98 (0.88) | <0.001 | 0.82 (0.12) | ||
| Homocysteine (μmol/l) | Standard | 25.8 (14.4) | 24.7 (11.0) | 0.55 | 0.3 (1.9) | 0.004 |
| Aggressive | 25.9 (12.4) | 19.0 (6.8) | <0.001 | 7.0 (1.3) | ||
| CRP (mg/l) | Standard | 5.6 (1.4–8.0) | 7.8 (3.1–15.0) | 0.01 | 13.5 (7.6) | 0.18 |
| Aggressive | 6.0 (1.8–9.9) | 6.5 (3.0–12.0) | 0.001 | 3.2 (2.0) | ||
| Systolic BP (mm Hg) | Standard | 142 (22) | 143 (21) | 0.89 | 0 (3) | 0.1 |
| Aggressive | 146 (21) | 140 (21) | 0.01 | 6 (2) | ||
| Diastolic BP (mm Hg) | Standard | 81 (14) | 80 (12) | 0.45 | 1 (2) | 0.07 |
| Aggressive | 86 (12) | 81 (12) | 0.001 | 5 (1) | ||
| Haemoglobin (g/l) | Standard | 109.2 (15.4) | 111.5 (16.1) | 0.38 | −2.3 (2.6) | 0.65 |
| Aggressive | 109.7 (17.1) | 110.5 (14.4) | 0.71 | −0.8 (2.1) | ||
| Phosphate (mmol/l) | Standard | 1.7 (0.4) | 1.8 (0.6) | 0.17 | −0.1 (0.1) | 0.05 |
| Aggressive | 1.8 (0.5) | 1.7 (0.5) | 0.15 | 0.1 (0.1) | ||
| Albumin (g/l) | Standard | 38.2 (3.9) | 37.2 (5.2) | 0.1 | 1.0 (0.6) | 0.07 |
| Aggressive | 37.5 (5.1) | 38.1 (4.8) | 0.35 | −0.5 (0.6) | ||
| Aspirin use | Standard | 23 (32%) | 17 (37%) | 0.3 | 5% | <0.001 |
| Aggressive | 40 (50%) | 40 (76%) | <0.001 | 26% | ||
| Smoking | Standard | 7 (10%) | 2 (3%) | 0.2 | 70% | 0.02 |
| Aggressive | 4 (5%) | 0 | NS | 100% |
Data expressed as mean (SD), median (interquartile range) or percentage.
*Baseline v follow up; †standard v aggressively treated groups.
BP, blood pressure; CRP, C reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein.
A small percentage of patients smoked before study entry. In the standard group this decreased from 10% to 3% and in the aggressively treated group from 5% to 0%.
Use of aspirin increased 26% in the aggressively treated group and 5% in the standard group.
Total cholesterol and LDL cholesterol decreased significantly and HDL cholesterol increased in both the standard and aggressively treated groups. The mean change from baseline to follow up differed between the groups for total cholesterol (p = 0.05) and LDL cholesterol (p = 0.02), but not HDL cholesterol (p = 0.88). The difference in mean change in triglycerides was not significant between groups.
In the aggressively treated group systolic (p = 0.01) and diastolic blood pressures (p = 0.001) decreased from baseline to follow up. Between groups the mean change was non‐significantly greater in the aggressive than in the standard group.
Homocysteine concentrations improved more in the aggressively treated group than in the standard group (p < 0.001). Between groups the mean change was greater in the aggressively treated group (p = 0.004). Serum phosphate did not improve significantly in either group, but the mean change from baseline to follow up was significantly greater for the aggressively treated patients. Median C reactive protein increased in both groups but the mean change was not different between groups. Haemoglobin and albumin were not significantly different between the two groups.
Dobutamine response
All DSE tests were interpretable and table 4 shows the results. In 152 baseline studies, 16 patients developed side effects to dobutamine. Eight patients stopped the test early due to chest pain, hypotension or arrhythmia. No patients had major or life threatening side effects. In those patients with ischaemia, five (21%) had angina with ST changes provoked by dobutamine. Independent baseline predictors of an abnormal baseline DSE were a previous cardiac event (β = 4.8, 95% confidence interval (CI) 2.1 to 10.9, p < 0.001), treatment with a β blocker (β = 3.3, 95% CI 1.4 to 7.7, p = 0.05) and a lower resting ejection fraction (β = 0.96, 95% CI 0.92 to 1.0, p = 0.03).
Table 4 Results of dobutamine stress echocardiography (DSE) at baseline and follow up.
| Variable | Baseline DSE | Follow‐up DSE |
|---|---|---|
| Number of patients | 152 | 107 |
| Resting systolic BP (mm Hg) | 143 (27) | 135 (25) |
| Resting diastolic BP (mm Hg) | 81 (16) | 73 (14) |
| Peak systolic BP (mm Hg) | 161 (38) | 148 (39) |
| Peak diastolic BP (mm Hg) | 75 (20) | 69 (18) |
| Resting heart rate (beats/min) | 78 (14) | 77 (13) |
| Peak heart rate (beats/min) | 134 (21) | 134 (20) |
| Submaximal heart rate (<85% age‐predicted maximum heart rate) | 57 (38%) | 29 (27%) |
| Use of atropine | 60 (40%) | 29 (27%) |
| Chest pain | 10 (7%) | 8 (8%) |
| ST segment change | 14 (9%) | 20 (19%) |
| Findings | ||
| Normal | 95 (62%) | 57 (53%) |
| Non‐diagnostic | 20 (13%) | 8 (7%) |
| Scar | 13 (9%) | 19 (18%) |
| Ischaemia | 24 (16%) | 23 (22%) |
Continuous variables expressed as mean (SD) and categorical variables as percentages.
BP, blood pressure.
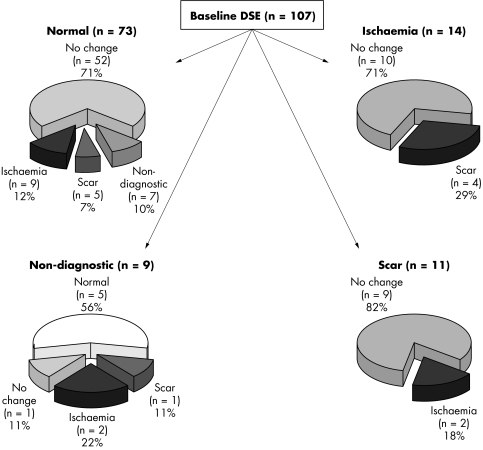
During the follow‐up period of a mean of 1.8 years, 22 patients died, 12 underwent transplantation and 21 withdrew their consent, transferred to another unit or were not able to have a DSE due to a recent cardiac event. Ten patients who died had a DSE at 12 months, which was used in the final analysis. Thus, 107 patients had a follow‐up DSE. One patient developed ventricular tachycardia secondary to inferior myocardial infarction during the test, but there were no other serious side effects. In those patients with ischaemia at follow‐up (n = 23), four (17%) had angina and 11 (48%) had ST changes provoked by dobutamine. At baseline 62% of studies were normal and this proportion decreased to 53% (p = 0.002) at follow up. The percentage with scar increased from 9% to 18% (p = 0.04) and with ischaemia from 16% to 22% (p = 0.05). Scar and ischaemia combined changed significantly from baseline to follow up (25% to 40%, p < 0.001). Figure 1 shows the changes in paired DSE findings from baseline to follow up.
Figure 1 Results of paired dobutamine stress echocardiography (DSE) at baseline and follow up.
For resting studies the coefficient of variation for wall motion score index was 8% (95% CI 5.9 to 16.4) and for stress studies, 3% (95% CI 2.0 to 5.4).
New ischaemia
In comparing baseline with follow‐up studies in all 152 patients by using intention to treat analysis, new ischaemia was identified in 33 (22%). Between standard and aggressively treated groups, the numbers of patients with new ischaemia were similar (15 v 18, p = 0.8).
Univariate predictors of new ischaemia (with p ⩽ 0.1) were older age (p < 0.001), abnormal ECG (p = 0.002), diabetes mellitus (p = 0.02), longer duration of diabetes mellitus in years (p = 0.07), lower HDL cholesterol (p = 0.08) and higher systolic blood pressure (p = 0.1). From this a multivariate model was produced and the independent predictors of new ischaemia were older age (odds ratio (OR) 1.06, 95% CI 1.02 to 1.10, p = 0.002), abnormal ECG (OR 2.77, 95% CI 1.12 to 6.91, p = 0.03), higher systolic blood pressure (OR 1.02, 95% CI 1.0 to 1.04, p = 0.05) and lower HDL cholesterol (OR 0.21, 95% CI 0.05 to 0.81, p = 0.02), but not treatment arm.
Cardiac events and mortality
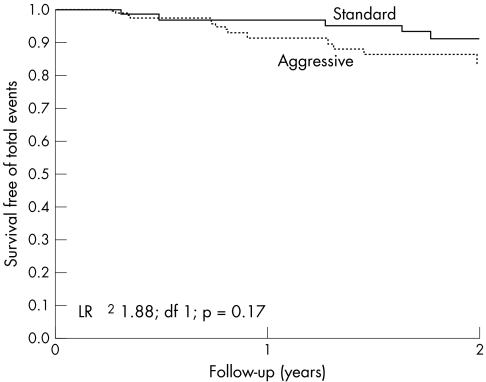
There were 17 cardiac events (four ST segment elevation myocardial infarctions, nine acute coronary syndromes and four cardiac deaths) and 22 deaths from all causes. The cardiac event rate (10% v 13%, p = 0.6) and all‐cause mortality (10% v 19%, p = 0.2) were similar between the standard and aggressively treated groups. Figure 2 shows the Kaplan–Meier survival curve.
Figure 2 Event‐free survival of patients by treatment group. LR, likelihood ratio.
Fifty seven (37%) patients had an abnormal (non‐diagnostic, scar or ischaemia) baseline DSE: 27 in the standard group and 30 in the aggressively treated group. Among those patients with abnormal baseline DSE, there were six cardiac events in both groups giving a similar event rate (22% v 20%, p = 0.9). Six patients in the standard group and seven in the aggressively treated group died, with similar event rates (22% v 23%, p = 0.7).
In the Cox regression model (χ2 = 22.9, p < 0.001), univariate predictors (with p ⩽ 0.1) of mortality were renal replacement therapy (p = 0.09), a longer duration of renal replacement therapy (p = 0.08), higher serum phosphate (p < 0.001), and higher left ventricular mass index (p = 0.05). The only independent predictor of mortality was higher baseline serum phosphate (OR 6.2, 95% CI 2.9 to 13.7, p < 0.001).
DISCUSSION
This study has shown the value of sequential DSE in the detection of new ischaemia in a group of high‐risk patients and supports the evidence that in patients with CKD the “warranty” of a normal DSE is limited. In this study risk factor modifications differed significantly between the two groups but this did not translate into differences in event rate in patients with an abnormal baseline DSE or the development of new ischaemia.
Clinical predictors in CKD
For the clinical aspects of this study, we identified serum phosphate as the only predictor of total mortality, which has been shown to be strongly associated with increased risk of death in patients with CKD.13 Higher systolic blood pressure was a predictor of new ischaemia, which is in contrast to studies showing that a low blood pressure is associated with mortality.22 The likely explanation for this is that a higher systolic blood pressure may be related directly to progressive atherosclerosis, whereas a lower blood pressure may be associated with heart failure.
Significance of DSE in CKD
Some studies have documented the diagnostic value of DSE in patients with CKD.6,7,8,23,24 The accuracy of DSE in CKD was first reported by Reis et al,8 who found a sensitivity of 95% and specificity of 86% in detection of significant coronary disease. Herzog et al6 found DSE to be less accurate, with a sensitivity of 75% and specificity of 76% in identification of angiographic coronary stenosis of 75% or greater, and West et al24 reported a negative predictive value of 92%. Other screening tests such as thallium scanning are less useful,25 probably reflecting false positives studies from ventricular hypertrophy.
DSE is already known to be a reliable predictor of outcome in patients with known or suspected coronary disease,26 as well as in the assessment of perioperative risk.27 Data on the use of DSE for risk stratification in patients with CKD are limited. In the current study, the annualised cardiac event rate for those who had a normal DSE was 2.5%. This is somewhat higher than published data on patients without CKD but analogous to previous studies investigating the prognostic value of DSE, which have reported a negative predictive value of 95% over 20 months.23 In the current study, 10 patients (59%) who had cardiac events had a non‐diagnostic study or ischaemia, similar to previous work showing that 73% of those who had events had either a non‐diagnostic study or ischaemia on baseline DSE.7 However, a normal DSE may miss late events,7 which may reflect progressive disease, seen in this study as a new ischaemia rate of about 22%, despite almost half of these patients having a normal DSE at baseline. Indeed, the largest published prognostic study suggests that the warranty of a normal scan is limited to two years: the event rate at one and two years after a normal baseline DSE is reported to be about 4% but by year 3 this more than doubles to about 10%.7 This may have implications for timing of repeat DSE testing, particularly for patients who are awaiting transplantation. In this regard, no prospective randomised studies have compared revascularisation with medical treatment in these patients, and so an optimal management strategy after identification of ischaemia by DSE remains to be defined.
Previously, only one nuclear perfusion study related the extent of change of ischaemia during treatment to predict outcome,28 and other studies with sequential stress echocardiography have focused on the effect of drugs on the detection of ischaemia.29 Sequential DSE testing was applied in a study of aggressive lipid lowering, where DSE showed a reduction in the number of ischaemic segments.30 Nonetheless, the sequential use of any test may present problems of test–retest variability, perhaps compounded by discordant DSE interpretation, particularly in patients with one‐ or two‐vessel coronary disease.31 In our echocardiography laboratory, we use standard reading criteria, and use of the same reviewers reduces heterogeneity.32
Study limitations
This study showed that increased targeting of cardiovascular risk factors had little impact on reduction of subsequent events and the reasons for this merit some discussion. Accelerated atherosclerosis is common in patients with CKD, arguably more so than in any other patient cohort. Limited intervention data are available on patients with CKD but, in contrast to studies showing benefits in those with normal renal function, current studies have not reduced cardiac events by risk factor modification.33,34 It is possible that in stage 4 or 5 CKD, the inflammatory and atherosclerotic processes have advanced beyond the point at which intervention can be effective, so that even in patients with normal DSE studies, progression to ischaemia or even infarction is likely. In our study, about 30% of patients who had a normal DSE at baseline developed abnormalities at two years, and this could not be altered by risk factor modification. Other factors such as inflammation, complement activation, oxidative stress or other risk factors for which the targets were not achievable, such as serum phosphate, may be more important than traditional risk factors. Although the study was adequately powered, another limitation was the relatively small sample size. A larger study would have to be multicentric, and care would be needed to reduce variability in DSE interpretation. Follow up was possible in only 107 patients due to either events or drop out. The use of an intention to treat analysis diluted the actual number of patients with new ischaemia. Previous work with 60 patients relating to standard compared with aggressive lipid lowering and sequential testing by DSE found a reduction in the number of ischaemic wall segments.30 We therefore believe that our study was suitably powered to assess the incidence of new ischaemia.
Lastly, combining three CKD groups (pre‐dialysis, haemodialysis and peritoneal dialysis) into a single group (to provide a reasonable sample size) may pose a limitation due to heterogeneity. Subgroup analysis showed no differences in the rates of new ischaemia between standard and aggressively treated groups in the 53 pre‐dialysis (29% v 20%, p = 0.7) and 99 dialysis (16% v 24%, p = 0.2) patients. However, these subgroups were underpowered and thus were not included in the final analysis.
Conclusion
New ischaemia identified by DSE is common in patients with CKD. This study found no reduction in new ischaemia or cardiac event rates in patients with DSE abnormalities with a risk factor intervention strategy. It highlights the need for the introduction of intervention at an earlier stage in the disease process, for further studies on emerging treatments targeting CKD‐specific risk factors such as hypercalcaemia, hyperphosphataemia and hyperparathyroidism, and for prospective randomised data on the impact of ischaemia assessed by DSE on outcome after revascularisation. The combination of earlier intervention, targeting CKD‐specific risk factors and revascularisation, may alter the prognosis from cardiovascular disease in this high‐risk population.
Abbreviations
CKD - chronic kidney disease
DSE - dobutamine stress echocardiography
HDL - high density lipoprotein
LDL - low density lipoprotein
OR - odds ratio
Footnotes
Supported in part by a project grant (102471) and Centre of Clinical Research Excellence award (219285), National Health and Medical Research Council, Canberra, Australia and funding from the Princess Alexandra Hospital Foundation
References
- 1.Foley R N, Parfrey P S, Harnett J D.et al Clinical and echocardiographic disease in patients starting end‐stage renal disease therapy. Kidney Int 199547186–192. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak M J, Levey A S. Epidemiology of cardiac disease in dialysis patients. Semin Dial 19991269–76. [Google Scholar]
- 3.Kennedy R, Case C, Fathi R.et al Does renal failure cause an atherosclerotic milieu in patients with end‐stage renal disease? Am J Med 2001110198–204. [DOI] [PubMed] [Google Scholar]
- 4.Bloembergen W E, Stannard D C, Port F K.et al Relationship of dose of hemodialysis and cause‐specific mortality. Kidney Int 199650557–565. [DOI] [PubMed] [Google Scholar]
- 5.Herzog C A, Ma J Z, Collins A J. Poor long‐term survival after acute myocardial infarction among patients on long‐term dialysis. N Engl J Med 1998339799–805. [DOI] [PubMed] [Google Scholar]
- 6.Herzog C A, Marwick T H, Pheley A M.et al Dobutamine stress echocardiography for the detection of significant coronary artery disease in renal transplant candidates. Am J Kidney Dis 1999331080–1090. [DOI] [PubMed] [Google Scholar]
- 7.Marwick T H, Lauer M S, Lobo A.et al Use of dobutamine echocardiography for cardiac risk stratification of patients with chronic renal failure. J Intern Med 1998244155–161. [DOI] [PubMed] [Google Scholar]
- 8.Reis G, Marcovitz P A, Leichtman A B.et al Usefulness of dobutamine stress echocardiography in detecting coronary artery disease in end‐stage renal disease. Am J Cardiol 199575707–710. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron S, Hillis G, Oh J K.et al Dobutamine stress echocardiography as a prognostic and diagnostic tool in patients with end stage renal failure. Circulation 2004110(SIII)511 [Google Scholar]
- 10.Fathi R, Haluska B, Isbel N.et al The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol 200443616–623. [DOI] [PubMed] [Google Scholar]
- 11.Pozzoni P, Pozzi M, Del Vecchio L.et al Epidemiology and prevention of cardiovascular complication in chronic kidney disease patients. Semin Nephrol 200424417–422. [DOI] [PubMed] [Google Scholar]
- 12.Parfrey P S, Foley R N. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 1999101606–1615. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Port F K. Calcium phosphate metabolism and cardiovascular disease in patients with chronic kidney disease. Semin Dial 200316140–147. [DOI] [PubMed] [Google Scholar]
- 14.Ganesh S K, Stack A G, Levin N W.et al Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001122131–2138. [DOI] [PubMed] [Google Scholar]
- 15.Giachelli C M. Vascular calcification mechanisms. J Am Soc Nephrol 2004152959–2964. [DOI] [PubMed] [Google Scholar]
- 16.Chertow G M, Burke S K, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 200262245–252. [DOI] [PubMed] [Google Scholar]
- 17.Isbel N M, Haluska B, Johnson D W.et al Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. Am Heart J 2006151745–753. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron 19761631–41. [DOI] [PubMed] [Google Scholar]
- 19.Zoccali C, Benedetto F A, Mallamaci F.et al Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 2001122768–2774. [DOI] [PubMed] [Google Scholar]
- 20.McNeill A J, Fioretti P M, el Said S M.et al Enhanced sensitivity for detection of coronary artery disease by addition of atropine to dobutamine stress echocardiography. Am J Cardiol 19927041–46. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong W F, Pellikka P A, Ryan T.et al Stress echocardiography: recommendations for performance and interpretation of stress echocardiography. Stress Echocardiography Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 19981197–104. [DOI] [PubMed] [Google Scholar]
- 22.Port F K, Hulbert‐Shearon T E, Wolfe R A.et al Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 199933507–517. [DOI] [PubMed] [Google Scholar]
- 23.Brennan D C, Vedala G, Miller S B.et al Pretransplant dobutamine stress echocardiography is useful and cost‐effective in renal transplant candidates. Transplant Proc 199729233–234. [DOI] [PubMed] [Google Scholar]
- 24.West J C, Napoliello D A, Costello J M.et al Preoperative dobutamine stress echocardiography versus cardiac arteriography for risk assessment prior to renal transplantation. Transpl Int 200013(Suppl 1)S27–S30. [DOI] [PubMed] [Google Scholar]
- 25.Herzog C A. Is there something special about ischemic heart disease in patients undergoing dialysis? Am Heart J 2004147942–944. [DOI] [PubMed] [Google Scholar]
- 26.Marwick T H, Case C, Sawada S.et al Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol 200137754–760. [DOI] [PubMed] [Google Scholar]
- 27.Poldermans D, Arnese M, Fioretti P M.et al Sustained prognostic value of dobutamine stress echocardiography for late cardiac events after major noncardiac vascular surgery. Circulation 19979553–58. [DOI] [PubMed] [Google Scholar]
- 28.Dakik H A, Kleiman N S, Farmer J A.et al Intensive medical therapy versus coronary angioplasty for suppression of myocardial ischemia in survivors of acute myocardial infarction: a prospective, randomized pilot study. Circulation 1998982017–2023. [DOI] [PubMed] [Google Scholar]
- 29.Longobardi G, Ferrara N, Abete P.et al Efficacy of transdermal nitroglycerin patches evaluated by dipyridamole‐induced myocardial ischemia in patients with coronary artery disease: comparison between continuous and intermittent schedule. Cardiovasc Drugs Ther 200216535–542. [DOI] [PubMed] [Google Scholar]
- 30.Fathi R, Haluska B, Short L.et al A randomized trial of aggressive lipid reduction for improvement of myocardial ischemia, symptom status, and vascular function in patients with coronary artery disease not amenable to intervention. Am J Med 2003114445–453. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann R, Lethen H, Marwick T.et al Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol 199627330–336. [DOI] [PubMed] [Google Scholar]
- 32.Oberman A, Fan P H, Nanda N C.et al Reproducibility of two‐dimensional exercise echocardiography. J Am Coll Cardiol 198914923–928. [DOI] [PubMed] [Google Scholar]
- 33.Wanner C, Krane V, Marz W.et al Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005353238–248. [DOI] [PubMed] [Google Scholar]
- 34.Wrone E M, Hornberger J M, Zehnder J L.et al Randomized trial of folic acid for prevention of cardiovascular events in end‐stage renal disease. J Am Soc Nephrol 200415420–426. [DOI] [PubMed] [Google Scholar]