Abstract
Objectives
To assess the prognostic value of ventricular arrhythmias (VA) and heart rate variability (HRV) in patients with unstable angina.
Design
Multicentre prospective study.
Setting
17 cardiological centres in Italy.
Patients
543 consecutive patients with unstable angina and preserved left ventricular function (ejection fraction ⩾40%) enrolled in the SPAI (Stratificazione Prognostica dell'Angina Instabile) study.
Methods
Patients underwent 24 h ECG Holter monitoring within 24 h of hospital admission. Tested variables were frequent ventricular extrasystoles (⩾10/h), complex (that is, frequent or repetitive) VA, and bottom quartile values of time‐domain and frequency‐domain HRV variables. Primary end points were in‐hospital and six‐month total and cardiac deaths.
Results
Eight patients died in hospital (1.5%) and 32 (5.9%, 29 cardiac) during follow up. Both complex VA and frequent extrasystoles were strongly predictive of death in hospital and at follow up, even after adjustment for clinical (age, sex, cardiac risk factors and history of myocardial infarction) and laboratory (troponin I, C reactive protein and transient myocardial ischaemia on Holter monitoring) variables. At univariate analysis bottom quartile values of three HRV variables (standard deviation of RR intervals index, low‐frequency amplitude and low to high frequency ratio) were associated with in‐hospital death, and bottom quartile values of most HRV variables predicted six‐month fatal events. At multivariate Cox survival analysis reduced low‐frequency amplitude was consistently found to be independently associated with fatal end points.
Conclusion
In patients with unstable angina with preserved myocardial function, both VA and HRV are independent predictors of in‐hospital and medium‐term mortality, suggesting that these factors should be taken into account in the risk stratification of these patients.
Keywords: unstable angina, ventricular arrhythmias, heart rate variability, prognosis
Ventricular arrhythmias (VA),1,2,3,4,5,6 and impaired cardiac autonomic function, as indicated by depressed heart rate variability (HRV),7,8,9,10,11,12,13 have been shown to predict mortality among patients recovering from acute myocardial infarction (MI).
Despite the recent wave of interest in risk stratification of patients with non‐ST segment elevation acute coronary syndrome,14,15 no study has assessed the prognostic value of VA in patients with unstable angina. HRV was investigated only in small studies that were not sufficiently powered to assess its relationship with survival.16,17,18,19
The SPAI (Stratificazione Prognostica dell'Angina Instabile) study is a prospective, multicentre Italian study designed to investigate the prognostic value of clinical, biohumoral and ECG variables in patients admitted to a coronary care unit with a diagnosis of unstable angina. In this report we focus on the role of VA and HRV in predicting in‐hospital and six‐month survival in this group of patients.
METHODS
Patients
Consecutive patients admitted to coronary care units of participating centres (see appendix) with a clinical diagnosis of unstable angina from December 1997 to December 2001 were recruited in the SPAI study. For patients to be enrolled in the study, however, the diagnosis of unstable angina had to be confirmed during their hospital stay by one or more of the following findings: (1) ischaemic ECG changes during recurrent chest pain; (2) evidence of myocardial ischaemia during exercise ECG stress test or during exercise radionuclide studies or pharmacological echocardiographic stress tests (with either dipyridamole or dobutamine); and (3) documentation of obstructive (> 50%) stenosis in at least one major epicardial artery during coronary angiography. If the diagnosis of unstable angina could not be confirmed by any of the previous clinical and laboratory findings, patients were excluded from the SPAI study.
Unstable angina was defined as new onset angina (< 2 months) ensuing either at low levels of effort or at rest (new onset unstable angina), resting or worsening effort angina occurring in patients with either an old MI or a known history of stable coronary artery disease (worsening unstable angina), and readmission to hospital because of recurrent angina within three months after discharge for an acute MI (post‐MI unstable angina).
An acute MI, according to the definition of the World Health Organization,20 was excluded by monitoring 12‐lead ECG and assaying standard serum cardiac enzymes (creatine kinase and its MB isoform) at admission and after 6, 12 and 24 h. Patients with evidence of reduced left ventricular (LV) function (LV ejection fraction < 40%) on two dimensional echocardiography were excluded from the study.
A standard ECG and a venous blood sample were obtained on admission. Blood was centrifuged, and serum and plasma samples were frozen at −80°C until assayed at a core laboratory. Plasma troponin I was measured by an enzyme immunological assay (Boehringer Mannheim, Mannheim, Germany), with the lowest detection limit of 0.1 ng/ml. Serum C reactive protein was assayed by a high‐sensitivity nephelometric method (Nephelometric 100 Analyzer; Behring, Scoppito, Italy), with the lowest detection limit of 0.05 mg/l. Patients were managed according to local standard protocols.
Holter monitoring
Patients underwent 24 h ECG Holter monitoring within 24 h of admission with two‐channel tape recorders (Oxford Medilog MR45; Oxford Instruments) and monitoring two bipolar chest leads. All Holter monitoring tapes were analysed independently by two expert cardiologists at a core laboratory with the Oxford Medilog Excel 3.0 device.
Transient myocardial ischaemia was diagnosed when one or more episodes of horizontal or downsloping ST segment depression or of ST segment elevation ⩾ 1 mm lasting for ⩾ 1 min were detected.
For each patient the total number of ventricular extrasystoles, couplets and episodes of non‐sustained ventricular tachycardia (NSVT, defined as ⩾ 3 consecutive extrasystoles with a rate > 100 beats/min) were obtained.
HRV was assessed for the entire 24 h in both the time domain and the frequency domain after careful revision and editing of beats in the Oxford V.7.0 HRV analysis package.13 RR interval interpolation was applied in case of extrasystoles. Time‐domain HRV variables were mean RR interval; standard deviation of all RR intervals; standard deviation of the mean RR intervals of all 5 min segments in 24 h; and mean of the standard deviations of RR intervals of all 5 min segments in 24 h (SDNNi). In the frequency domain, HRV was assessed in the range of frequencies of 0–0.5 Hz with a fast Fourier transform spectral analysis algorithm, with a spectral resolution of 0.0005 Hz. Data were analysed in 10 min epochs throughout the 24 h and results from all epochs were averaged to form a composite spectrum. The amplitude of the following frequency‐domain HRV variables was obtained: very low frequency (0.0033–0.04 Hz), low frequency (LF; 0.04–0.15 Hz) and high frequency (HF; 0.15–0.40 Hz). Furthermore, the LF:HF ratio was calculated.
Clinical follow up
Patients were followed up at each centre three and six months after discharge by outpatient clinical visits or by a telephone interview. In case of death, detailed information about the causes were obtained from clinical records or from the patient's physician or relatives. Death was considered of cardiac origin when it was consequent to acute MI or heart failure or when it occurred suddenly (within 1 h of symptom onset).
In‐hospital death and six‐month death were the primary end points of this study, but in‐hospital and cardiac death were also analysed separately. Furthermore, as a secondary end point, we considered the occurrence of non‐fatal acute MI. Acute MI was diagnosed in case of chest pain > 30 min, with ischaemic ST segment or T wave changes on the ECG and the typical rise of creatine kinase MB.20
Statistical analysis
The following clinical and laboratory variables were regarded as potential risk predictors in statistical analyses: age (dichotomised in ⩾ 70 and < 70 years), sex, family history of coronary artery disease, active smoking, hypertension (blood pressure > 140/90 mm Hg or use of hypertension drugs), hypercholesterolaemia (> 5.7 mmol/l or use of lipaemia drugs), diabetes, previous acute MI, type of unstable angina (new onset or worsening), prolonged duration (> 20 min) of the qualifying angina episode, serum C reactive protein concentrations (dichotomised in concentrations ⩾ 3 or < 3 mg/l), plasma troponin I concentrations (dichotomised in concentrations ⩾ 0.4 or < 0.4 ng/ml), drug treatment started in the coronary care unit, presence of basal ST segment depression (> 0.5 mm) in at least one of the Holter monitor chest leads and detection of transient myocardial ischaemia on Holter monitoring. For the analysis, the few patients with post‐MI unstable angina (table 1) were included in the group with worsening unstable angina. The cut off concentrations of 3 mg/l for C reactive protein and of 0.4 ng/ml for troponin I were chosen because, in preliminary analyses, they were found to be those best for predicting six‐month mortality.
Table 1 Main clinical data of patients with unstable angina included in the study and of patients excluded from the Holter study.
| Study patients (n = 543) | Excluded patients (n = 143) | p Value | |
|---|---|---|---|
| Age (years) | 65.2 (10) | 67.0 (10) | 0.06 |
| Age >70 | 201 (37%) | 61 (43%) | 0.25 |
| Men | 356 (66%) | 86 (60%) | 0.27 |
| CAD risk factors | |||
| Active smoking | 133 (25%) | 28 (20%) | 0.26 |
| Systemic hypertension | 294 (54%) | 65 (45%) | 0.08 |
| Family history of CAD | 221 (41%) | 54 (38%) | 0.59 |
| Hypercholesterolaemia | 258 (48%) | 80 (56%) | 0.09 |
| Diabetes mellitus | 103 (19%) | 33 (23%) | 0.33 |
| Previous AMI | 181 (33%) | 39 (27%) | 0.20 |
| Type of unstable angina | |||
| De novo | 210 (39%) | 52 (36%) | 0.68 |
| Worsening | 309 (57%) | 81 (57%) | 0.97 |
| Post‐AMI | 24 (4%) | 8 (6%) | 0.71 |
| Preadmission angina ⩾20 min | 252 (46%) | 70 (49%) | 0.65 |
| Drug treatment in the coronary care unit* | |||
| β blocking agents | 348 (65%) | 89 (65%) | 0.95 |
| Calcium antagonists | 356 (66%) | 93 (68%) | 0.72 |
| Nitrates | 519 (97%) | 123 (90%) | 0.004 |
| Antiplatelet agents | 513 (96%) | 128 (94%) | 0.64 |
| Heparin/LMWH | 449 (84%) | 96 (71%) | 0.006 |
| ACE inhibitors | 226 (42%) | 67 (49%) | 0.16 |
| Statins | 153 (29%) | 37 (27%) | 0.85 |
| Troponin I >0.4 ng/ml | 168 (32%) | 28 (21%) | 0.022 |
| C reactive protein >3 mg/l | 335 (63%) | 83 (64%) | 0.76 |
| STd >0.5 mm at basal ECG | 86 (16%) | NA | NA |
| TMI on Holter monitoring | 135 (25%) | NA | NA |
Age data are mean (SD).
*Drug treatment, troponin I and C reactive protein available for 537, 528 and 532 patients included in the study and for 136, 130 and 130 patients excluded from the study, respectively.
ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; CAD, coronary artery disease; LMWH, low molecular weight heparin; NA, not applicable; STd, ST segment depression; TMI, transient myocardial ischaemia.
Patients were also divided into groups with or without frequent (⩾ 10/h) ventricular extrasystoles, with or without NSVT episodes, and with or without complex VA (defined as frequent or repetitive extrasystoles—either couplets or NSVT episodes). Lastly, patients were dichotomised according to each HRV parameter into groups with values in the bottom quartile and with values in the three upper quartiles.
Univariate and multivariate logistic regression analyses were applied to assess the association of variables with clinical end points, whereas univariate and multivariate survival Cox regression analyses were applied to assess the association of variables with six‐month mortality and non‐fatal MI. The predictive value of VA and HRV variables at follow up was also adjusted, in a separate model of multivariate Cox regression analysis, for coronary revascularisation procedures (either coronary bypass surgery or percutaneous interventions) performed during follow up. Data for patients lost at follow up were censored at the time of the last visit or contact. Survival curves were constructed by the Kaplan–Meier method and compared by log rank test.
A stepwise backward procedure was applied for multivariate analyses (both for logistic regression and for Cox regression). All considered variables were included in the models and progressively removed, leaving only variables with p ⩽ 0.1 in the final models. The independent predictive value of frequent extrasystoles and of complex VA in multivariate models was assessed separately. Similarly, the independent predictive value was assessed individually for each of the considered HRV variables.
Patients included in the study and those who had undergone Holter monitoring but were excluded (see below) from analyses were compared by unpaired t test or Mann–Whitney U test (as indicated) for continuous variables and by χ2 test for proportions. Data were statistically analysed by SPSS V.12.01 statistical software (SPSS Inc, Chicago, Illinois, USA). Data are reported as mean (SD), unless otherwise indicated. A value of p < 0.05 was always required for significance.
RESULTS
General results
Overall, 843 patients were included in the SPAI study, 686 of whom (81.4%) underwent Holter monitoring. Overall, 143 patients (20.8%) were excluded from the present study for the following reasons: technical deficiencies of Holter monitor recordings (n = 73); Holter monitoring duration < 18 h (n = 34); persistent supraventricular arrhythmias (n = 10); left bundle branch block (n = 16); pacemaker rhythm (n = 8); and lack of data about clinical outcome (n = 2). Thus, 543 patients formed the final cohort of the present study (64.4% of the original SPAI cohort and 79.1% of those with Holter monitoring). Troponin I and C reactive protein concentrations were available for 528 (97%) and 531 (98%) patients, respectively.
Patients included in the study tended to be younger but had higher troponin I values and more commonly received nitrates than the patients excluded from the study, suggesting a slightly more severe form of unstable angina (table 1).
VA during Holter monitoring
The median number of ventricular extrasystoles in 24 h for the group as a whole was 10 (range 0–40 163); 105 patients (19.3%) had frequent extrasystoles on Holter monitoring. Couplets were found in 142 patients (26.1%; median 0, range 0–769) and NSVT episodes were found in 61 patients (11.2%; median 0, range 0–66). Overall, complex VA (that is, frequent or repetitive extrasystoles) were detected in 188 patients (34.6%).
In‐hospital mortality
Eight (1.5%) patients died in hospital, seven (1.3%) of cardiac causes. All of the patients who died had complex VA during Holter monitoring. Furthermore, only old age, frequent extrasystoles and bottom quartile values of three HRV variables (SDNNi, LF and LF:HF ratio) were significantly predictive of death (table 2). Results were similar for cardiac deaths (data not shown).
Table 2 Variables significantly predictive of in‐hospital death at univariate analysis.
| Odds ratio (95% CI) | p Value | |
|---|---|---|
| Age >70 | 12.3 (1.5 to 10.12) | 0.02 |
| ⩾10 extrasystoles/h | 7.25 (1.69 to 30.9) | 0.007 |
| SDNNi <39 ms | 4.99 (1.18 to 21.1) | 0.029 |
| LF <15.7 ms | 4.94 (1.16 to 20.9) | 0.030 |
| LF:HF <1.12 | 5.14 (1.21 to 21.8) | 0.026 |
Complex ventricular arrhythmias are excluded, as all patients who died had these forms of arrhythmias on Holter monitoring.
HF, high frequency; LF, low frequency; NSVT, non‐sustained ventricular tachycardia; SDNNi, mean of the standard deviations of RR intervals of all 5 min segments in 24 h.
Frequent ventricular extrasystoles and, among HRV variables, SDNNi and LF maintained independent significant association with in‐hospital mortality in multivariate logistic regression models including ⩾ 10 extrasystoles/h as an arrhythmic variable, with only age ⩾ 70 years adding independent prognostic information among all other clinical, ECG and laboratory variables (table 3).
Table 3 Multivariate analysis of in‐hospital death, including frequent ventricular extrasystoles and, separately, significant heart rate variability parameters in the logistic regression models.
| Odds ratio (95% CI) | p Value | |
|---|---|---|
| First model | ||
| Age >70 years | 8.60 (1.02 to 72.3) | 0.047 |
| ⩾10 extrasystoles/h | 5.13 (1.16 to 22.6) | 0.031 |
| SDNNi <39 ms | 4.43 (1.01 to 19.4) | 0.048 |
| Second model | ||
| Age >70 years | 8.04 (0.96 to 68.5) | 0.056 |
| ⩾10 extrasystoles/h | 5.27 (1.19 to 23.3) | 0.029 |
| LF <15.7 ms | 4.49 (1.02 to 19.7) | 0.047 |
| Third model | ||
| Age >70 years | 7.44 (0.86 to 64.3) | 0.068 |
| ⩾10 extrasystoles/h | 4.29 (0.96 to 19.1) | 0.056 |
| LF:HF ratio <1.12 | 2.76 (0.61 to 12.4) | 0.18 |
HF, high frequency; LF, low frequency; SDNNi, mean of the standard deviations of RR intervals of all 5 min segments in 24 h.
As all of the patients who died had complex VA, we evaluated whether HRV variables might help to identify those at the highest risk among patients with complex VA. Among HRV variables, LF best predicted mortality in this subgroup: five of 57 (9.6%) patients with LF amplitude < 15.7 ms died compared with two of 136 (2.2%) patients with LF amplitude ⩾ 15.7 ms (odds ratio 4.72; 95% confidence interval (CI) 1.08 to 20.5, p = 0.038).
Six‐month mortality
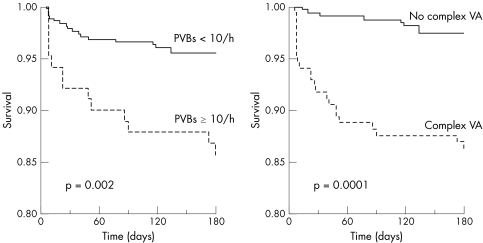
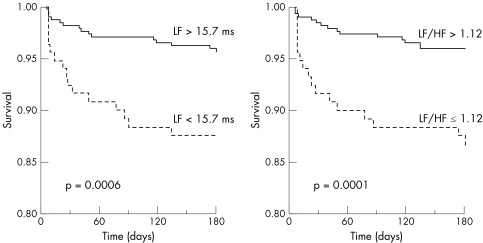
Complete follow up at six months was available for 489 (93.5%) patients. Thirty two (5.9%) patients died, 29 (5.3%) of cardiac causes. Several baseline clinical and laboratory variables were significantly associated with fatal end points at follow up at univariate analysis (table 4). Furthermore, ⩾ 10 extrasystoles/h, complex VA and bottom quartile values of most HRV variables were found to predict total and cardiac deaths (table 5). Figure 1 shows the curves for event‐free survival from cardiac deaths of patients with, compared with those without, frequent extrasystoles or complex VA. Fig 2 shows the curves for patients with LF or LF:HF in the bottom quartile compared with those in the three upper quartiles.
Table 4 Univariate association of clinical and laboratory variables with fatal events at six‐month follow up.
| Total deaths | p Value | Cardiac deaths | p Value | |
|---|---|---|---|---|
| Men | 0.68 (0.34 to 1.36) | 0.27 | 0.75 (0.36 to 1.53) | 0.75 |
| Age >70 years | 8.14 (3.35 to 19.8) | 0.00001 | 9.01 (3.44 to 23.06) | 0.00001 |
| Active smoking | 0.32 (0.10 to 1.03) | 0.06 | 0.23 (0.05 to 0.96) | 0.013 |
| Hypertension | 1.00 (0.49 to 2.03) | 0.99 | 0.91 (0.43 to 1.92) | 0.91 |
| Family history of CAD | 1.14 (0.59 to 2.29) | 0.71 | 1.35 (0.55 to 2.84) | 0.40 |
| Hypercholesterolaemia | 0.73 (0.36 to 1.49) | 0.73 | 0.65 (0.31 to 1.40) | 0.27 |
| Diabetes mellitus | 3.77 (1.86 to 7.65) | 0.0002 | 4.57 (2.18 to 9.59) | 0.0001 |
| Previous MI | 2.69 (1.34 to 5.40) | 0.006 | 2.96 (1.41 to 6.19) | 0.004 |
| Worsening unstable angina | 3.47 (1.34 to 9.02) | 0.011 | 5.57 (1.69 to 18.4) | 0.005 |
| Angina >20 min | 1.31 (0.65 to 2.82) | 0.45 | 1.42 (0.68 to 2.95) | 0.35 |
| β blocking agents | 0.65 (0.32 to 1.31) | 0.23 | 0.62 (0.29 to 1.30) | 0.20 |
| Calcium antagonists | 1.41 (0.63 to 3.14) | 0.41 | 1.47 (0.62 to 3.46 | 0.38 |
| ACE inhibitors | 1.20 (0.59 to 2.44) | 0.23 | 1.09 (0.52 to 2.31) | 0.81 |
| Basal ST changes | 1.81 (0.81 to 4.03) | 0.15 | 2.07 (0.92 to 4.68) | 0.08 |
| Troponin I >0.4 ng/ml | 2.01 (0.99 to 4.06) | 0.052 | 2.24 (1.22 to 4.11) | 0.009 |
| C reactive protein >3 mg/l | 2.60 (1.07 to 6.34) | 0.036 | 2.71 (1.32 to 5.56) | 0.007 |
| TMI on Holter monitoring | 2.72 (1.36 to 5.45) | 0.005 | 2.50 (1.20 to 5.20) | 0.014 |
ACE, angiotensin converting enzyme; CAD, coronary artery disease; MI, myocardial infarction; TMI, transient myocardial ischaemia. Data are presented as relative risk with 95% confidence intervals.
Table 5 Association of ventricular arrhythmias and heart rate variability parameters with six‐month fatal events according to univariate Cox regression analysis.
| Total number | Total deaths | Cardiac deaths | |||||
|---|---|---|---|---|---|---|---|
| Number | RR (95% CI) | p Value | Number | RR (95% CI) | p Value | ||
| Extrasystoles | |||||||
| ⩾10/h | 105 | 14 (13.3) | 3.5 | 0.000 | 13 (12.4) | 3.66 | 0.001 |
| <10/h | 428 | 18 (4.1) | (1.74 to 7.03) | 16 (3.6) | (1.76 to 7.60) | ||
| Non‐sustained ventricular tachycardia | |||||||
| Yes | 61 | 5 (8.2) | 1.47 | 0.43 | 5 (8.2) | 1.65 | 0.31 |
| No | 482 | 27 (8.2) | (0.57 to 3.82) | 24 (5.2) | (0.63 to 4.30) | ||
| Complex ventricular arrhythmias | |||||||
| Yes | 188 | 24 (12.8) | 6.14 | 0.00001 | 23 (12.2) | 7.84 | 0.00001 |
| No | 355 | 8 (2.2) | (2.76 to 13.66) | 6 (1.7) | (3.19 to 19.3) | ||
| RR interval <833 ms | |||||||
| Yes | 135 | 13 (9.6) | 2.06 | 0.044 | 12 (8.8) | 2.12 | 0.046 |
| No | 408 | 19 (4.7) | (1.02 to 4.18) | 17 (4.2) | (1.01 to 4.44) | ||
| SDNN <80 ms | |||||||
| Yes | 135 | 10 (7.4) | 1.56 | 0.23 | 10 (7.4) | 1.82 | 0.12 |
| No | 408 | 22 (5.4) | (0.75–3.24) | 19 (4.7) | (0.86 to 3.86) | ||
| SDANN <62 ms | |||||||
| Yes | 135 | 11 (8.1) | 1.84 | 0.09 | 11 (8.1) | 1.97 | 0.077 |
| No | 408 | 21 (5.1) | (0.90 to 3.74) | 18 (4.4) | (0.93 to 4.16) | ||
| SDNNi <39 ms | |||||||
| Yes | 135 | 16 (11.8) | 3.00 | 0.002 | 15 (11.1) | 3.21 | 0.002 |
| No | 408 | 16 (3.9) | (1.50 to 5.99) | 14 (3.4) | (1.55 to 6.54) | ||
| Very low‐frequency amplitude <31 ms | |||||||
| Yes | 138 | 16 (11.6) | 3.07 | 0.002 | 15 (10.9) | 3.28 | 0.001 |
| No | 405 | 16 (3.9) | (1.53 to 6.14) | 14 (3.5) | (1.58 to 6.80) | ||
| LF <15.7 ms | |||||||
| Yes | 136 | 16 (11.8) | 3.09 | 0.001 | 15 (10.9) | 3.31 | 0.001 |
| No | 407 | 16 (3.9) | (1.54 to 6.17) | 14 (3.5) | (1.60 to 6.85) | ||
| HF <11.2 ms | |||||||
| Yes | 135 | 12 (8.9) | 1.80 | 0.11 | 12 (8.9) | 2.12 | 0.046 |
| No | 408 | 20 (4.9) | (0.88 to 3.69) | 17 (4.2) | (1.01 to 4.44) | ||
| LF:HF <1.12 | |||||||
| Yes | 138 | 17 (12.3) | 3.57 | 0.0003 | 16 (14.6) | 3.87 | 0.0003 |
| No | 405 | 15 (3.7) | (1.78 to 7.15) | 13 (3.2) | (1.86 to 8.04) | ||
HF, high frequency; LF, low frequency; RR, relative risk; SDANN, standard deviation of the mean RR intervals of all 5 min segments in 24 h; SDNN, standard deviation of all RR intervals; SDNNI, mean of the standard deviations of RR intervals of all 5 min segments in 24 h.
Figure 1 Cardiac death‐free survival according to the presence or absence of frequent ventricular extrasystoles (left) and the presence or absence of complex ventricular arrhythmias (VA; right) on 24 h ECG Holter monitoring of patients with unstable angina.
Figure 2 Cardiac death‐free survival according to low‐frequency (LF) amplitude values in the bottom versus the three upper quartiles (left) and ratio of low frequency to high frequency (LF:HF) in the bottom versus the three upper quartiles (right) on frequency‐domain heart rate variability analysis for patients with unstable angina.
On multivariate Cox survival analysis, ⩾ 10 extrasystoles/h and complex VA, separately, maintained a significant association with total and cardiac mortality. Among HRV variables, LF only was found to be independently associated with fatal end points (table 6), with low LF:HF ratio being of independent significance for cardiac death in the multivariate model including complex VA (relative risk (RR) 2.36, 95% CI 1.08 to 5.16, p = 0.03) and of borderline significance in the model including ⩾ 10 extrasystoles/h (RR 1.97, 95% CI 0.88 to 4.40, p = 0.1). Other variables independently predictive of fatal end points were old age, diabetes and (for cardiac deaths only) basal persistent ST depression on the Holter monitor ECG (table 6), but not a history of MI.
Table 6 Results of multivariate Cox survival analysis of fatal end points at six‐month follow up.
| Total deaths | Cardiac deaths | |||
|---|---|---|---|---|
| RR (95% CI) | p Value | RR (95% CI) | p Value | |
| Model with ⩾10 extrasystoles/h | ||||
| Age >70 years | 8.61 (3.24 to 22.9) | 0.00002 | 10.96 (3.63 to 33.0) | 0.00002 |
| Diabetes | 4.29 (2.06 to 8.93) | 0.0001 | 6.39 (2.83 to 14.5) | 0.00001 |
| Worsening UA | 2.34 (0.89 to 6.14) | 0.085 | 3.90 (1.15 to 13.1) | 0.028 |
| Persistent ST depression | NA | NA | 3.07 (1.24 to 7.59) | 0.015 |
| Troponin I >0.4 ng/ml | NA | NA | 2.18 (0.97 to 4.64) | 0.06 |
| ⩾10 extrasystoles/h | 2.52 (1.22 to 5.21) | 0.012 | 2.40 (1.10 to 5.20) | 0.027 |
| LF <15.7 ms | 2.14 (1.03 to 4.44) | 0.04 | 2.29 (1.00 to 5.28) | 0.05 |
| Model with complex VA | ||||
| Age >70 years | 8.27 (3.13 to 21.9) | 0.00002 | 9.38 (3.18 to 27.7) | 0.0001 |
| Diabetes | 4.44 (2.14 to 9.24) | 0.0001 | 6.31 (2.87 to 13.9) | 0.00001 |
| Worsening UA | 2.30 (0.88 to 6.00) | 0.09 | 3.53 (1.06 to 11.8) | 0.04 |
| Persistent ST depression | NA | NA | 3.17 (1.30 to 7.72) | 0.011 |
| Complex VA | 4.99 (2.13 to 11.7) | 0.0002 | 6.38 (2.38 to 17.1) | 0.0002 |
| LF <15.7 ms | 2.23 (1.08 to 4.60) | 0.03 | 2.90 (1.30 to 6.46) | 0.009 |
LF, low frequency; NA, not applicable; RR, relative risk; UA, unstable angina; VA, ventricular arrhythmias.
During the whole period of the study 257 patients (47.3%) underwent coronary revascularisation (142 percutaneous coronary interventions, 112 coronary artery bypass surgery, 3 both). Frequent extrasystoles, complex VA and bottom quartile LF amplitude maintained a significant association with total (RR 3.60, 95% CI 1.72 to 7.52, p = 0.001; RR 6.50, 95% CI 2.77 to 15.2, p = 0.00002; RR 2.86, 95% CI 1.38 to 5.92, p = 0.005, respectively) and cardiac (RR 3.81, 95% CI 1.75 to 8.29, p = 0.001; RR 8.69, 95% CI 3.27 to 23.0, p = 0.00001; RR 3.06, 95% CI 1.42 to 6.60, p = 0.004, respectively) mortality even after adjustment for coronary interventions.
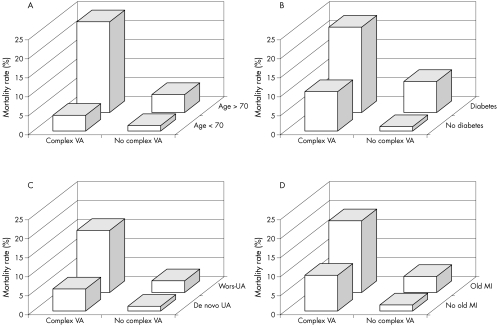
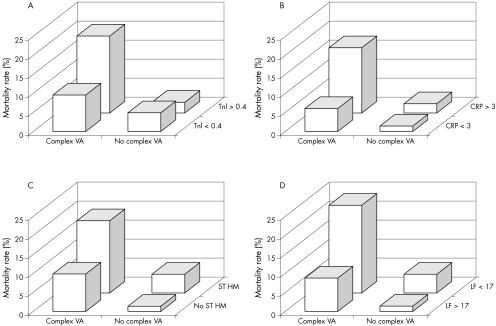
Figures 3 and 4 illustrate the incremental prognostic value of complex VA combined with established clinical and laboratory predictors of risk and with LF value. Table 7 shows sensitivity, specificity and positive (PPV) and negative (NPV) predictive values for total mortality of the most important arrhythmic and HRV variables. Individual variables had high NPV but low PPV. Combining complex VA with low HRV variables resulted in a significant but limited improvement in PPV (25.4% for complex VA and low LF:HF ratio), with only a mild reduction in NPV.
Figure 3 Crude six‐month mortality according to complex ventricular arrhythmias (VA) in groups of patients with presence or absence of established clinical risk predictors. (A) Age, (B) diabetes, (C) type of unstable angina (UA) and (D) previous myocardial infarction (MI). Wors, worsening; p < 0.001 for data in each graph.
Figure 4 Crude six‐month mortality according to complex ventricular arrhythmias (VA) in groups of patients with presence or absence of established prognostic laboratory variables. (A) Troponin I (TnI), (B) C reactive protein (CRP), (C) transient myocardial ischaemia on Holter monitoring (ST‐HM), and (D) low‐frequency (LF) amplitude. p < 0.001 for data in each graph.
Table 7 Sensitivity, specificity, positive predictive value and negative predictive value for all cause mortality in the study group.
| Sens (%) | Spec (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| ⩾10 extrasystoles/h | 43.7 | 81.8 | 13.3 | 95.8 |
| Complex VA | 75.0 | 67.9 | 12.8 | 97.7 |
| LF amplitude <15.7 ms | 50.0 | 76.5 | 11.8 | 96.1 |
| LF:HF ratio <1.12 | 53.1 | 76.3 | 12.3 | 96.3 |
| Complex VA and low LF | 37.5 | 92.2 | 23.1 | 95.6 |
| Complex VA and low LF:HF | 43.7 | 92.0 | 25.4 | 94.4 |
HF, high frequency; LF, low frequency; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; VA, ventricular arrhythmias.
Non‐fatal acute MI
There were 8 (1.5%) non‐fatal acute MIs during hospital stay and 23 (4.7%) from discharge to the six‐month follow up. Neither VA nor reduced HRV was significantly associated with the occurrence of non‐fatal MI, both in hospital (data not shown) and at the six‐month follow up (table 8).
Table 8 Relationship of major potential prognostic variables with non‐fatal acute myocardial infarction at six‐month follow up (univariate Cox regression).
| RR (95% CI) | p Value | |
|---|---|---|
| Age >70 years | 0.80 (0.32 to 2.0) | 0.64 |
| Men | 1.33 (0.22 to 4.0) | 0.42 |
| Active smoking | 2.49 (1.06 to 5.76) | 0.033 |
| Hypertension | 1.13 (0.49 to 2.61) | 0.78 |
| Family history of CAD | 1.15 (0.49 to 2.69) | 0.75 |
| Hypercholesterolaemia | 1.14 (0.47 to 2.76) | 0.77 |
| Diabetes mellitus | 1.40 (0.50 to 3.91) | 0.52 |
| Previous AMI | 0.96 (0.38 to 2.45) | 0.94 |
| No de novo unstable angina | 0.74 (0.32 to 1.71) | 0.48 |
| Angina >20 min | 0.92 (0.39 to 2.15) | 0.85 |
| β blocking agents | 1.02 (0.43 to 2.40) | 0.97 |
| Calcium antagonists | 0.62 (0.27 to 1.42) | 0.26 |
| ACE inhibitors | 0.79 (0.34 to 1.67) | 0.79 |
| Basal ST changes | 0.52 (0.12 to 2.22) | 0.38 |
| Troponin I >0.4 ng/ml | 2.17 (0.94 to 5.0) | 0.069 |
| C reactive protein >3 mg/l | 2.20 (0.82 to 5.92) | 0.12 |
| TMI on Holter monitoring | 1.66 (0.71 to 3.9) | 0.24 |
| ⩾10 extrasystoles/h | 0.42 (0.10 to 1.78) | 0.24 |
| Complex VA | 0.71 (0.28 to 1.80) | 0.47 |
| LF amplitude <15.7 ms | 0.64 (0.22 to 1.88 | 0.42 |
| HF amplitude <11.2 ms | 0.28 (0.07 to 1.19) | 0.086 |
| LF:HF ratio <1.12 | 0.91 (0.34 to 2.45) | 0.85 |
ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; CAD, coronary artery disease; HF, high frequency; LF, low frequency; RR, relative risk; TMI, transient myocardial ischaemia; VA, ventricular arrhythmias.
DISCUSSION
This study, to the best of our knowledge, is the first to show in a large population of patients that both VA and low HRV, as detected on Holter monitoring started within 24 h after admission for unstable angina, are predictors of in‐hospital and medium‐term mortality in patients with unstable angina and preserved LV function. Of note, only patients with complex VA died in hospital, and in multivariate models frequent extrasystoles and two HRV parameters (SDNNi and LF) maintained an independent association with in‐hospital mortality, despite the low incidence of deaths. Only old age added independent prognostic information to VA and HRV parameters for in‐hospital death.
Frequent or complex VA on Holter monitoring started within 24 h after admission for unstable angina was also a strong predictor of total and cardiac mortality at six‐month follow up, and most HRV variables had a significant univariate association with fatal events. Notably, frequent extrasystoles, complex VA and bottom quartile values of LF amplitude were found to be predictors of six‐month mortality independent of several common prognostic indicators, including transient myocardial ischaemia, C reactive protein and troponin I, which were not independently associated with mortality in this study when VA and HRV were taken into account. Combination of laboratory prognostic variables showed that the subgroup of patients with both complex VA and low HRV parameters had the highest risk of death (fig 3). In contrast with the high specificity and NPV, however, PPV remained insufficiently low, being at best 25.4% when complex VA and low LF:HF ratio were combined. The low PPV, however, is a limitation that has consistently been shown to concern all prognostic variables in patients with acute coronary syndromes.1,2,3,4,5,6,7,8,9,10,11,12,13,16,17,18,19
The group of patients included in this study had preserved LV function (ejection fraction ⩾40%). VA and HRV were predictive of death independently of a history of MI, which was documented for only 33% of patients. In contrast to mortality, no significant association was found in this cohort of patients with unstable angina between VA or reduced HRV and the occurrence of non‐fatal acute MI in the in‐hospital phase and at the six‐month follow up.
Comparison with previous studies
The predictive prognostic value of predischarge VA and HRV was consistently observed in most studies of patients recovering from acute MI,1,2,3,4,5,6,7,8,9,10,11,12,13 but our study is the first to show an association between VA and mortality also in patients with unstable angina.
HRV was previously investigated in studies of patients with unstable angina, showing a significant association with composite cardiac end points,16,17,18,19 but the limited number of patients recruited precluded the assessment of the predictive value of HRV for total and cardiac mortality.
In our study several HRV variables were significantly associated with six‐month mortality, although only a frequency‐domain variable, LF, was independently associated with both in‐hospital and six‐month mortality in multivariate survival analyses. Notably, a low LF:HF ratio was also independently associated with cardiac death when complex VA were taken into account (table 6) and its combination with complex VA resulted in the highest PPV for mortality (table 7). Thus, our findings complement the observations of the prognostic value of low LF amplitude and LF:HF ratio observed in other populations of patients.8,10,13,21,22
Pathophysiological mechanisms
The reasons why non‐sustained VA and impaired HRV are strongly predictive of mortality in patients with unstable angina are speculative. They may reflect impaired LV function,23,24,25,26 but LV ejection fraction was normal or only mildly reduced in our cohort of patients, 39% of whom had a history of de novo unstable angina and 67% of whom had no history of MI, thus strongly limiting the possible role of impaired LV function.
HRV is also reduced in patients with diabetes,27,28 which was a strong predictor of death in our study, but decreased LF amplitude was associated with fatal end points also independently of diabetes.
The relationship between depressed HRV and mortality is also difficult to ascertain as the exact physiological mechanisms responsible for the various HRV components are still incompletely known.29,30 Decreased values of HRV variables, including LF, may reflect reduced vagal tone or predominant sympathetic influence to the heart.31,32 The presence of frequent or complex non‐sustained VA in the context of sympathovagal imbalance can increase the susceptibility to fatal VA, in particular during myocardial ischaemia.33,34 On the other hand, VA and depressed HRV are unlikely to be associated with the triggers of acute MI, as they were not predictive of non‐fatal MI in hospital or at six‐month follow up.
Limitations of the study
A significant number of patients (47%) during follow up underwent percutaneous or surgical revascularisation. Both VA and low LF amplitude, however, continued to be significantly associated with death after adjustment for revascularisation procedures.
The SPAI study was designed to assess the predictive value of prognostic variables in patients with unstable angina with sufficiently preserved LV myocardial function. Although all SPAI patients had LV ejection fraction ⩾ 40% on admission, however, the exact value was not obtained. Thus, we cannot exclude some influence of LV function on clinical outcome among our patients, although it is unlikely, as the effect on mortality of differences in LV ejection fraction for values ⩾ 40% is well known to be negligible in patients with coronary artery disease.35,36
Conclusions
This study shows that, in patients with unstable angina, early assessment of VA and of cardiac autonomic function by ECG monitoring adds incremental prognostic information above that provided by established clinical and laboratory markers of risk; indeed, these variables can identify patients at extremely low risk and those at high risk of in‐hospital and six‐month death, and therefore should be considered in risk stratification of patients with non‐ST segment elevation unstable angina.
ACKNOWLEDGEMENTS
We thank Helen Raiswell BSc for the design and administration of the SPAI database.
Abbreviations
HF - high frequency
HRV - heart rate variability
LF - low frequency
LV - left ventricular
MI - myocardial infarction
NPV - negative predictive value
NSVT - non‐sustained ventricular tachycardia
PPV - positive predictive value
RR - relative risk
SDNNi - mean of the standard deviations of RR intervals of all 5 min segments in 24 h
SPAI - Stratificazione Prognostica dell'Angina Instabile
VA - ventricular arrhythmias
Appendix
List of participant centres in the SPAI study: Ancona, Azienda Ospedaliera “G M Lancisi” (Purcaro A, Perna G, Costantini C, Moretti S); Aosta, Ospedale Civile (De Marchi M, Leone G, Giudice M); Bologna, Policlinico Universitario “Sant'Orsola” (Bugiardini R, Manfrini O, Pizzi C); Brescia, Policlinico Universitario Ospedale Civili (Dei Cas L, Metra M, Gaiti M, Fiorini C); Castelnuovo Garfagnana, Ospedale “Santa Croce” (Bernardi D, Volterrani C); Firenze, Ospedale Santa Maria Nuova (Marchi F, Ciriello G, Crisano S); Frascati, Ospedale San Sebastiano Martire (Giorgi G, Ciavolella M, Sarli G); Genova, Policlinico Universitario “San Martino” DI MI, (Brunelli C, Spallarossa P, Ferraris F); Livorno, Ospedale Riuniti (Magini G, Galli M, Di Giorgio A); Padova, Policlinico Universitario Giustinianeo (Iliceto S, Nava A, Maddalena F, Babuin L, Pedrocco A); Roma, Policlinico Universitario A Gemelli (Maseri A, Crea F, Cianflone D, Rebuzzi AG, Angeloni G, Lanza GA, Sestito A); Roma, Ospedale San Filippo Neri (Santini M, Tubaro M); Roma; Ospedale Santo Spirito (Ceci V, Aspromonte N, Leone F); San Giovanni Rotondo, Ospedale “Casa Sollievo della Sofferenza” (Fanelli R, Cianfrone N, Santoro T); Teramo, Ospedale Civile “G Mazzini” (Iacovoni F, Delle Monache S, Iacovoni A); Torino, Ospedale “Le Molinette” (Trevi GP, Pistono M, Bianchi F, Bergerone S); Verona, Ospedale Borgotrento (Zardini P, Destro G, Brighetti G, Zorzi A); Viareggio, Ospedale Civile (Pesola A, Comella A).
Footnotes
The SPAI study was supported financially by the Regione Calabria and by the Fondazione per il Cuore ONLUS.
References
- 1.Ruberman W, Weinblatt E, Goldberg J D.et al Ventricular premature complexes and sudden death after myocardial infarction. Circulation 198164297–305. [DOI] [PubMed] [Google Scholar]
- 2.Bigger J T, Weld F M, Rolnitzki L M. Prevalence, characteristics and significance of ventricular tachycardia (three or more complexes) detected with ambulatory electrocardiographic recording in the late hospital phase of acute myocardial infarction. Am J Cardiol 198148815–823. [DOI] [PubMed] [Google Scholar]
- 3.Mukharji J, Rude R E, Poole W K.et al Risk factors for sudden death after acute myocardial infarction: two‐year follow‐up. Am J Cardiol 19845431–36. [DOI] [PubMed] [Google Scholar]
- 4.Kostis J B, Friedman L M, Goldstein S, for the BHATB Study Group et al Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. J Am Coll Cardiol 198710231–242. [DOI] [PubMed] [Google Scholar]
- 5.Farrell T G, Bashir Y, Cripps T.et al Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal‐averaged electrocardiogram J Am Coll Cardiol 199118687–697. [DOI] [PubMed] [Google Scholar]
- 6.Maggioni A P, Zuanetti G, Franzosi M G.et al, on behalf of GISSI‐2 Investigators. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI‐2 results. Circulation 199387312–322. [DOI] [PubMed] [Google Scholar]
- 7.Kleiger R E, Miller J P, Bigger J T, The Multicenter Postinfarction Research Group et al Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 198759256–262. [DOI] [PubMed] [Google Scholar]
- 8.Bigger J T, Fleiss J L, Steinman R C.et al Frequency‐domain measures of heart period variability and mortality after myocardial infarction. Circulation 199285164–171. [DOI] [PubMed] [Google Scholar]
- 9.Hartikainen J E K, Malik M, Staunton A.et al Distinction between arrhythmic and nonarrhythmic death after acute myocardial infarction based on heart rate variability, signal‐averaged electrocardiogram, ventricular arrhythmias and left ventricular ejection fraction. J Am Coll Cardiol 199628296–304. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Mironow D, Armstrong P W, for the GUSTO ECG Substudy Investigators: Heart rate variability assessment early after acute myocardial infarction: pathophysiological and prognostic correlates et alCirculation 1996931388–1395. [DOI] [PubMed] [Google Scholar]
- 11.Zuanetti G, Neils J M M, Latini R.et al on behalf of GISSI‐2 Investigators. Prognostic significance of heart rate variability in post‐myocardial infarction patients in the fibrinolytic era: the GISSI 2 results, Circulation 199694432–436. [DOI] [PubMed] [Google Scholar]
- 12.La Rovere M T, Bigger J T, Jr, Marcus F I.et al Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators. Lancet 1998351478–484. [DOI] [PubMed] [Google Scholar]
- 13.Lanza G A, Guido V, Galeazzi M M.et al Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol 1998821323–1328. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Antman E M, Beasley J W.et al ACC/AHA guidelines for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 200036970–1062. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand M E, Simoons M L, Fox K A.et al Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation Eur Heart J 2002231809–1840. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Sopher S M, Leatham E.et al Heart rate variability depression in patients with unstable angina. Am Heart J 1995130772–779. [DOI] [PubMed] [Google Scholar]
- 17.Lanza G A, Pedrotti P, Rebuzzi A G.et al Usefulness of the addition of heart rate variability to Holter monitoring in predicting in‐hospital cardiac events in patients with unstable angina pectoris. Am J Cardiol 199780263–267. [DOI] [PubMed] [Google Scholar]
- 18.Kennon S, Price C P, Mills P G.et al Cumulative risk assessment in unstable angina: clinical, electrocardiographic, autonomic, and biochemical markers. Heart 20038936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manfrini O, Pizzi C, Trere D.et al Parasympathetic failure and risk of subsequent coronary events in unstable angina and non‐ST‐segment elevation myocardial infarction. Eur Heart J 2003241560–1566. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on Standardization of Clinical Nomenclature. Nomenclature and criteria for diagnosis of ischemic heart disease. Circulation 197959607–609. [DOI] [PubMed] [Google Scholar]
- 21.Van de Borne P, Montano N, Pagani M.et al Absence of low‐frequency variability of sympathetic nerve activity in severe heart failure. Circulation 1997951449–1454. [DOI] [PubMed] [Google Scholar]
- 22.La Rovere M T, Pinna G D, Maestri R.et al Short‐term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003107565–570. [DOI] [PubMed] [Google Scholar]
- 23.Bigger J T, Jr, Fleiss J L, Kleiger R.et al The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation 198469250–258. [DOI] [PubMed] [Google Scholar]
- 24.Schulze R A, Jr, Humphries J O, Griffith L S.et al Left ventricular and coronary angiographic anatomy: relationship to ventricular irritability in the late hospital phase of acute myocardial infarction. Circulation 197755839–843. [DOI] [PubMed] [Google Scholar]
- 25.Casolo G C, Stroder, Sulla A.et al Heart rate variability and functional severity of congestive heart failure secondary to coronary artery disease. Eur Heart J 199516360–367. [DOI] [PubMed] [Google Scholar]
- 26.Nolan J, Flapan A D, Capewell S.et al Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J 199267482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien I A, O'Hare J P, Lewin I G.et al The prevalence of autonomic neuropathy in insulin‐dependent diabetes mellitus: a controlled study based on heart rate variability. Q J Med 198661957–967. [PubMed] [Google Scholar]
- 28.Nolan J, Flapan A D, Goodfield N E.et al Measurement of parasympathetic activity from 24‐hour ambulatory electrocardiograms and its reproducibility and sensitivity in normal subjects, patients with symptomatic myocardial ischemia, and patients with diabetes mellitus. Am J Cardiol 199677154–158. [DOI] [PubMed] [Google Scholar]
- 29.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996931043–1065. [PubMed] [Google Scholar]
- 30.Tsuji H, Venditti F J, Jr, Manders E S.et al Determinants of heart rate variability. J Am Coll Cardiol 1996281539–1546. [DOI] [PubMed] [Google Scholar]
- 31.Chess G F, Tam R M K, Calaresu F R. Influences of cardiac neural inputs on rhythmic variations of heart rate period in the cat. Am J Physiol 1975228775–780. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith R L, Bigger J T, Jr, Steinman R C.et al Comparison of 24‐hour parasympathetic activity in endurance‐trained and untrained young men. J Am Coll Cardiol 199220552–558. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz P J, Vanoli E, Stramba‐Badiale M.et al Autonomic mechanisms and sudden death: new insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 198878969–979. [DOI] [PubMed] [Google Scholar]
- 34.Kent K M, Smith E R, Redwood D R.et al Electrical stability of acutely ischemic myocardium: influences of heart rate and vagal stimulation. Circulation 197347291–298. [DOI] [PubMed] [Google Scholar]
- 35.The Multicenter Postinfarction Research Group Risk stratification and survival after myocardial infarction. N Engl J Med 1983309331–336. [DOI] [PubMed] [Google Scholar]
- 36.Solomon S D, Zelenkofske S, McMurray J J V, the Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators et al Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 20053522581–2588. [DOI] [PubMed] [Google Scholar]