Abstract
Objectives
To assess non‐invasively the acute effects of cardiac resynchronisation therapy (CRT) on functional mitral regurgitation (MR) at rest and during dynamic exercise.
Methods
21 patients with left ventricular (LV) systolic dysfunction and functional MR at rest, treated with CRT, were studied. Each patient performed a symptom‐limited maximal exercise with continuous two dimensional Doppler echocardiography twice. The first exercise was performed with CRT; the second exercise was performed without CRT. Mitral regurgitant flow volume (RV), effective regurgitant orifice area (ERO) and LV dP/dt were measured at rest and at peak exercise.
Results
CRT mildly reduced resting mitral ERO (mean 8 (SEM 2) v 11 (2) mm2 without CRT, p = 0.02) and RV (13 (3) v 18 (3) ml without CRT, p = 0.03). CRT attenuated the spontaneous increase in mitral ERO and RV during exercise (1 (1) v 9 (2) mm2, p = 0.004 and 1 (1) v 8 (2) ml, p = 0.004, respectively). CRT also significantly increased exercise‐induced changes in LV dP/dt (140 (46) v 479 (112) mm Hg/s, p < 0.001).
Conclusion
Attenuation of functional MR, induced by an increase in LV contractility during dynamic exercise, may contribute to the beneficial clinical outcome of CRT in patients with chronic heart failure and LV asynchrony.
Keywords: cardiac resynchronisation therapy, echocardiography, exercise, mitral regurgitation
Patients with chronic heart failure (CHF) due to left ventricular (LV) systolic dysfunction commonly develop functional mitral regurgitation (MR) at rest.1 Functional MR may worsen when these patients exercise.2,3Worsening functional MR during exercise may negatively affect the long‐term outcome of patients with CHF by further activating neurohormonal systems.
Cardiac resynchronisation therapy (CRT) improves clinical outcome in patients with severe CHF.4,5 The present study was undertaken to assess non‐invasively the acute effects of CRT on functional MR at rest and during dynamic exercise.
PATIENTS AND METHODS
Study population
We screened 39 patients with CHF, functional MR and CRT. Eighteen of these patients were excluded for poor acoustic window (n = 4), sinus or atrioventricular blocks (n = 5), atrial fibrillation (n = 3), wire displacement (n = 2), orthopaedic limitation (n = 2) and improved LV ejection fraction > 45% (n = 2).
The study population comprised the remaining 13 men and eight women (mean age 67 (SEM 2) years) with CHF caused by ischaemic (n = 8) or non‐ischaemic dilated cardiomyopathy (LV end diastolic diameter 69 (4) mm, LV end diastolic volume 249 (19) ml) and reduced LV ejection fraction (22 (1)%). These 21 patients had undergone CRT for a mean duration of 2.3 (1.3) months. All patients were in sinus rhythm and before pacemaker implantation had a left bundle branch block (mean QRS interval 175 (16) ms). The devices were programmed in the DDDR mode with the shortest programmable atrioventricular delay (mean 135 (4) ms) to ensure full and permanent biventricular capture. This also provided, by Doppler echocardiography, the longest filling time and optimised LV end diastolic flow before onset of systole.6 The programmable interventricular delays were not modified, as the nominal settings provided the best resynchronisation modality. In addition to CRT, all patients were treated optimally with loop diuretics, angiotensin converting enzyme inhibitors and β blocking agents, except for two patients who did not tolerate β adrenergic blockade. None of the patients had primary valvular disease, aortic regurgitation or inducible myocardial ischaemia. All patients signed an informed consent form.
Exercise echocardiography
Patients performed symptom‐limited maximal exercise on a semirecumbent bicycle ergometer with workload starting at 25 W and increased by 20 W every 3 min. Blood pressure was measured every 2 min. A 12‐lead ECG was continuously monitored. Patients received their usual drugs on the morning of the study.
Echocardiographic analysis
Continuous two dimensional and Doppler echocardiograms were recorded with a scanner (ATL 5000, Philips) equipped with a 2–4 MHz transducer. All studies were recorded digitally on to a magneto‐optical disk for subsequent analysis.
LV volumes and LV ejection fraction were calculated by Simpson's biplane method. The aortic pulsed wave Doppler time–velocity integral was recorded in the apical five‐chamber view. The proximal flow convergence technique has been validated as a quantitative Doppler method to calculate regurgitant flow volume (RV) and effective regurgitant orifice area (ERO) at rest7 and during exercise.2 The proximal flow convergence region was imaged and expanded in the apical four‐chamber view. The aliasing velocity Vr was carefully adjusted (20–40 cm/s) and was unchanged during exercise. The radius r of the hemispheric proximal flow convergence was measured in mid‐systole. Maximum regurgitant velocity and the regurgitant time–velocity integral (RTVI) were obtained from continuous wave Doppler echocardiography. The instantaneous regurgitant flow is measured as 2π × r2 × Vr. The following parameters are calculated: ERO = RV/maximum regurgitant velocity; and RV = ERO × RTVI. RV was also measured by the echocardiographic Doppler method.7 LV contractility was assessed by LV dP/dt from continuous wave Doppler recording from the MR.8 Systolic pulmonary artery pressure was derived from Doppler echocardiographic measurement of the tricuspid regurgitation velocity.
Interventricular asynchrony was assessed at rest by using standard pulsed Doppler echocardiography. Aortic ejection delay was defined as the delay between the QRS onset and the onset of the systolic aortic flow. Pulmonary ejection delay was defined as the delay between the QRS onset and the onset of systolic pulmonary flow recorded in the parasternal view. Interventricular asynchrony was calculated as the difference between the aortic and the pulmonary ejection delays. Pulsed tissue Doppler imaging was used to evaluate intraventricular synchronism at rest as previously described.9 The explored areas were the basal segments of the septum, lateral wall, inferior wall and anterior wall. Parietal delay was measured between the QRS onset and the onset of the S wave. Intraventricular asynchrony was calculated as the difference between the earliest and the most delayed site.
All measurements were taken with and without CRT. At least three cardiac cycles were used for each measurement and averaged. Absolute interobserver differences for RV were 1.8 (1) ml at rest and 2 (3) ml during exercise.
Study protocol
All patients were allowed a resting period of 6 h between symptom‐limited maximal exercises. Patients exercised first with CRT and again at least 6 h after CRT had been discontinued.
Statistical analysis
Continuous variables are expressed as mean (SEM). Changes at rest and during exercise at both device settings were compared by a paired non‐parametric test (Wilcoxon test). The correlation between RV obtained by the proximal isovelocity surface area method and RV calculated by the echocardiographic Doppler method was assessed by the regression analysis of Spearman. Significance was accepted at p < 0.05.
RESULTS
Table 1 summarises haemodynamic and echocardiographic data.
Table 1 Haemodynamic and echocardiographic of patients with and without CRT.
| With CRT | Without CRT | |||
|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | |
| Heart rate (beats/min) | 66 (30) | 93 (4)** | 67 (3) | 96 (4)** |
| Systolic blood pressure (mm Hg) | 115 (4) | 127 (4)** | 111 (3) | 118 (6) |
| LV ejection fraction (%) | 22 (1) | 26 (2)* | 22 (1) | 22 (2) |
| LV dP/dt (mm Hg/s) | 590 (39) | 1069 (118)** | 432 (46) | 572 (62)** |
| LVOT VTI (cm) | 11 (0.3) | 13 (0.7)* | 12 (0.6) | 11 (0.8) |
| Mitral ERO (mm2) | 8 (2) | 9 (2) | 11 (2) | 19 (3)** |
| Mitral regurgitant volume (ml) | 13 (3) | 14 (3) | 18 (3) | 26 (3)** |
| Maximum MR velocity (cm/s) | 452 (16) | 467 (11)** | 442 (15) | 418 (13)** |
| Transtricuspid gradient (mm Hg) | 27 (2) | 49 (4)** | 28 (2) | 52 (4)** |
Data are mean (SEM).
*p<0.05, **p<0.01 peak exercise v resting condition.
CRT, cardiac resynchronisation therapy; ERO, effective regurgitant orifice area; LV, left ventricular; LVOT VTI, left ventricular outflow tract velocity–time integral; MR, mitral regurgitation.
Resting conditions
CRT significantly reduced interventricular and left intraventricular delays (19 (4) v 52 (11) ms, p < 0.001 and 64 (10) v 113 (17) ms, p < 0.001, respectively). Resting heart rate and systolic blood pressure were unaffected by CRT. Similarly, resting LV ejection fraction remained unchanged (22 (1) v 22 (1)%, NS). However, CRT increased LV dP/dt from 432 (46) to 590 (39) mm Hg/s (p < 0.001). Averaged maximum MR velocity also increased with CRT (442 (15) v 452 (16) cm/s, p = 0.003). Resting mitral ERO and RV were mildly reduced by CRT (8 (2) v 11 (2) mm2, p = 0.02 and 13 (3) v 18 (3) ml, p = 0.03 respectively).
Exercise‐induced changes
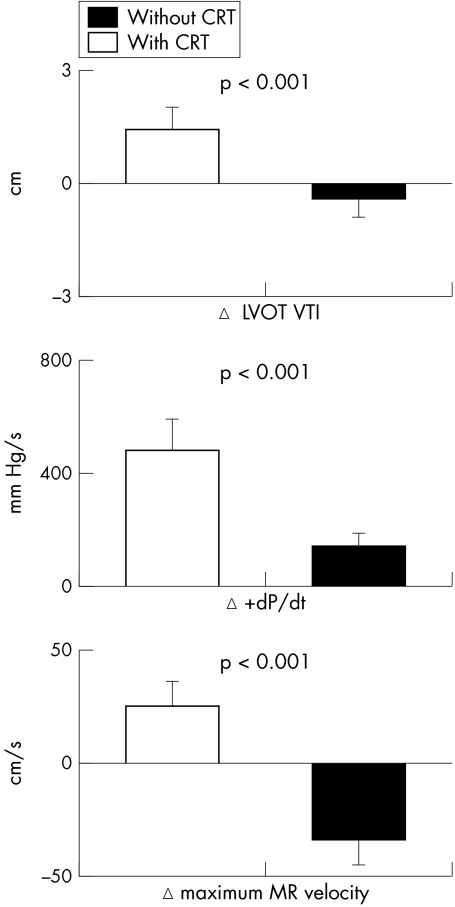
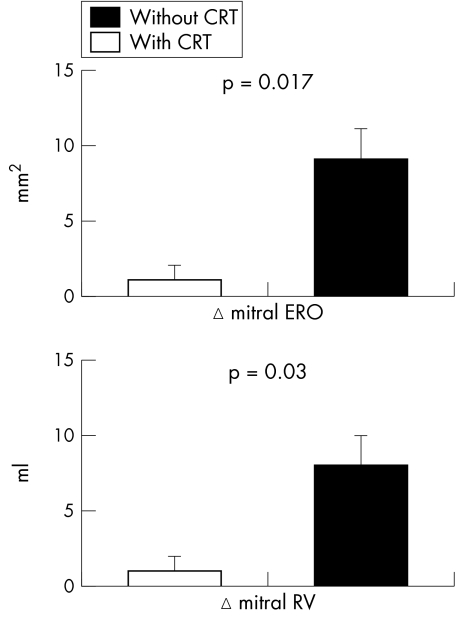
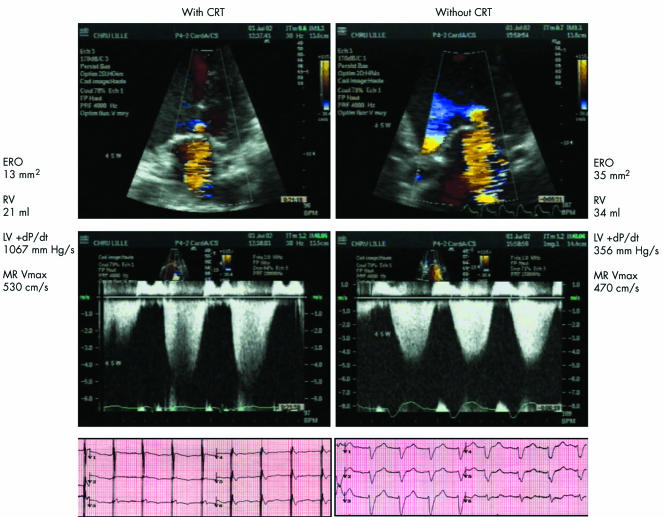
Continuous ECG monitoring documented effective CRT at all heart rates. The increase in heart rate during dynamic exercise was similar with and without CRT (30 (4) and 31 (3) beats/min). The rise in systolic blood pressure was greater with CRT than without CRT (14 (4) v 10 (4) mm Hg, p < 0.001). Exercise duration with CRT and without CRT was similar (5.8 (0.6) v 5.5 (0.6) min). CRT improved LV ejection fraction (p = 0.007) and reduced slightly the increase in pulmonary artery pressure during exercise (p = 0.001). Forward stroke volume assessed by the left ventricular outflow tract velocity–time integral increased during dynamic exercise with CRT and decreased during exercise without CRT (1.4 (0.6) v −0.4 (0.5) cm, p < 0.001). CRT substantially enhanced the change in LV dP/dt during dynamic exercise compared with exercise performed without CRT (479 (112) v 140 (46) mm Hg/s, p < 0.001). Furthermore, mean MR maximum velocity increased during exercise with CRT and decreased without CRT (25 (11) v −34 (11) cm/s, p = 0.001) (fig 1). CRT significantly reduced changes in mitral ERO and RV during dynamic exercise compared with exercise without CRT (1 (1) v 9 (2) mm2, p = 0.004 and 1 (1) v 8 (2) ml, p = 0.004, respectively) (fig 2). In the 15 patients with moderate MR (RV below the median value of 15 ml), CRT had similar effects on changes in ERO and RV (2 (1) v 8 (2) mm2, p = 0.017 and 3 (1) v 9 (2) ml, p = 0.03, respectively), as well as in the six patients with more severe MR (−2 (4) v 10 (4) mm2, p = 0.02 and −4 (4) v 6 (4) ml, p = 0.02, respectively). Figure 3 shows an example of the proximal flow convergence zone and the envelope of the MR jet obtained at maximal exercise with and without CRT. The correlation between mitral RV calculated by the pulsed Doppler quantitative method and mitral RV obtained by the proximal isovelocity surface area method was closer during exercise than at rest (r = 0.84, p < 0.0001 and r = 0.73, p = 0.0003, respectively).
Figure 1 Cardiac resynchronisation therapy (CRT) enhanced forward stroke volume (left ventricular outflow tract velocity–time integral (LVOT VTI)) and myocardial contractility during dynamic exercise as shown by a significant increase in left ventricular dP/dt and maximum mitral regurgitation velocity. Data are mean (SEM).
Figure 2 Cardiac resynchronisation therapy (CRT) attenuated the increase in mitral regurgitant flow volume (RV) and effective regurgitant orifice area (ERO) during dynamic exercise relative to exercise performed without CRT. Data are mean (SEM).
Figure 3 Colour flow (top) and continuous wave (middle) Doppler echocardiographic recordings with corresponding ECG tracings (bottom) in a representative patient at maximal exercise with (left) and without (right) cardiac resynchronisation therapy. Left ventricular (LV) dP/dt and maximum mitral regurgitation velocity (MR Vmax) were reduced without CRT. ERO, effective regurgitant orifice area; RV, regurgitant flow volume.
DISCUSSION
The present data clearly indicate that acute CRT attenuates functional MR volume during dynamic exercise in patients with LV dilatation and asynchrony.
Several mechanisms lead to functional MR in patients with dilated and poorly contracting left ventricles. These mechanisms include mitral annulus dilatation, apical displacement of the papillary muscles and reduced transmitral force closure secondary to LV systolic dysfunction.10,11 LV asynchrony further reduces LV systolic performance and thereby exacerbates functional MR in patients with dilated left ventricles. Conversely, by producing a more efficient LV contraction, CRT may increase transmitral force closure and thereby reduce the severity of functional MR.12 In our patients, CRT increased LV contractility twofold during dynamic exercise. This twofold increase in LV contractility presumably greatly increased transmitral force closure. Moreover, the increase in MR maximum velocity during exercise with CRT reflects an increase in transmitral pressure gradient resulting in a decrease in ERO.
Although acute CRT increased the forward stroke volume and thereby cardiac output during dynamic exercise, it did not affect the duration of symptom‐limited maximal exercise. The dissociation between maximum functional capacity and resting LV performance is well documented in patients with CHF.13 This dissociation is also observed during short term administration of positive inotropic agents such as dobutamine and of vasodilators such as angiotensin converting enzyme inhibitors.14,15 Acute interventions are unlikely to reverse the alterations in skeletal muscle metabolism, mass and vasculature that primarily limit peak functional capacity in ambulatory patients with CHF.
Surprisingly, biventricular pacing did not significantly affect pulmonary pressures during dynamic exercise in our series. Multiple intricate factors at rest and during exercise, however, account for the variability of the degree of pulmonary hypertension in patients with LV systolic dysfunction. In LV systolic dysfunction, pulmonary pressures depend not only on the severity of MR but also on LV diastolic function,16 pulmonary vascular resistance, loading conditions and right ventricular function.
Study limitations
Although the symptom‐limited maximal exercises were separated by at least 6 h, patients with CHF were asked to perform two maximal exercises on the same day. The possibility that the second exercise was affected by residual fatigue cannot be excluded and may have biased our study towards CRT. Assessment of myocardial asynchrony during exercise may permit better characterisation of how myocardial asynchrony contributes to worsening functional MR during dynamic exercise. Concomitant quantification of functional MR and myocardial asynchrony, however, was technically impossible during exercise. CRT cannot be evaluated echocardiographically in a double‐blind fashion. However, the disparate effects of CRT on echocardiographic Doppler parameters indirectly attest to our unbiased approach. Lastly, this is a descriptive study with a limited number of patients. More patients have to be enrolled and followed up to try to correlate the extent of the MR exercise‐induced response with the clinical response.
Conclusion
In the short term, CRT attenuates functional MR during dynamic exercise in patients with LV dilatation and asynchrony. Attenuation of functional MR during dynamic exercise may be in part responsible for the beneficial effect of long‐term CRT on the clinical outcome of patients with CHF.5
Abbreviations
CHF - chronic heart failure
CRT - cardiac resynchronisation therapy
ERO - effective regurgitant orifice area
LV - left ventricular
MR - mitral regurgitation
RTVI - regurgitant time–velocity integral
RV - regurgitant flow volume
References
- 1.Selzer A, Katayama F. Mitral regurgitation: clinical patterns, pathophysiology and natural history. Medicine 197251337–366. [PubMed] [Google Scholar]
- 2.Lebrun F, Lancellotti P, Pierard L. Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol 2001381685–1692. [DOI] [PubMed] [Google Scholar]
- 3.Lapu‐Bula R, Robert A, Van Craeynest D.et al Contribution of exercise‐induced mitral regurgitation to exercise stroke volume and exercise capacity in patients with left ventricular dysfunction. Circulation 20021061342–1348. [DOI] [PubMed] [Google Scholar]
- 4.Cazeau S, Leclercq C, Lavergne T.et al Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001344873–880. [DOI] [PubMed] [Google Scholar]
- 5.Cleland J, Daubert J, Erdmann E.et al The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 20053521539–1549. [DOI] [PubMed] [Google Scholar]
- 6.Kindermann M, Frohlig G, Doerr T.et al Optimizing the AV delay in DDD pacemaker patients with high degree AV block: mitral valve Doppler versus impedance cardiography. Pacing Clin Electrophysiol 1997202453–2462. [DOI] [PubMed] [Google Scholar]
- 7.Enriquez‐Sarano M, Miller F, Hayes S.et al Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol 199525703–709. [DOI] [PubMed] [Google Scholar]
- 8.Bargiggia G, Bertucci C, Recusani F.et al A new method for estimating left ventricular dP/dT by continuous wave Doppler‐echocardiography. Circulation 1989801287–1292. [DOI] [PubMed] [Google Scholar]
- 9.Bader H, Garrigue S, Lafitte S.et al Intra‐left ventricular electromechanical asynchrony: a new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 200443248–256. [DOI] [PubMed] [Google Scholar]
- 10.He S, Fontaine A, Schwammenthal E.et al Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation 1997961826–1834. [DOI] [PubMed] [Google Scholar]
- 11.Yiu S, Enriquez‐Sarano M, Tribouilloy C.et al Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction. Circulation 20001021400–1406. [DOI] [PubMed] [Google Scholar]
- 12.Breithardt O, Sinha A, Schwammenthal E.et al Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol 200341765–770. [DOI] [PubMed] [Google Scholar]
- 13.Benge W, Litchfield R L, Marcus M L. Exercise capacity in patients with severe left ventricular dysfunction. Circulation 198061955–959. [DOI] [PubMed] [Google Scholar]
- 14.Maskin C S, Forman R, Sonnenblick E H.et al Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol 198351177–182. [DOI] [PubMed] [Google Scholar]
- 15.Kugler J, Maskin C, Frishman W H.et al Regional and systemic metabolic effects of angiotensin‐converting enzyme inhibition during exercise in patients with severe heart failure. Circulation 1982661256–1261. [DOI] [PubMed] [Google Scholar]
- 16.Enriquez‐Sarano M, Rossi A, Seward J B.et al Determinants of pulmonary hypertension in left ventricular dysfunction. J Am Coll Cardiol 199729153–159. [DOI] [PubMed] [Google Scholar]