Abstract
Objective
To investigate the relation between serum high sensitivity (hs) C reactive protein (CRP), proinflammatory cytokine concentrations, proinflammatory to anti‐inflammatory cytokine ratios and long‐term prognosis in patients with non‐ST elevation acute coronary syndrome (NSTEACS).
Design
Prospective follow‐up study for the first six months and then for the first year after admission to hospital.
Setting
Tertiary referral centre.
Patients
80 patients (60 men, 20 women, mean age 60 (SD 10) years) with NSTEACS and moderate to high TIMI (Thrombolysis In Myocardial Infarction) risk scores.
Interventions
Blood samples from patients with NSTEACS were obtained at the time of admission. Serum concentrations of hs‐CRP, (hs) pro‐inflammatory (interleukin (IL) ‐1β, IL‐6, tumour necrosis factor α) and (hs) anti‐inflammatory (IL‐10) cytokines were analysed and proinflammatory to anti‐inflammatory cytokine ratios were calculated by dividing proinflammatory cytokine concentrations by anti‐inflammatory cytokine IL‐10.
Main outcome measure
The primary end point of the study was new coronary events (NCE) defined as the combination of cardiac death, non‐fatal myocardial infarction and recurrent rest angina that required hospitalisation within 12 months of follow up.
Results
During the one‐year follow‐up period, 23 patients (29%) met the NCE criteria. Concentrations of hs‐CRP, IL‐1β and IL‐6 and ratios of IL‐1β:IL‐10 and IL‐6:IL‐10 were significantly higher in patients with NCE than in patients without NCE. In the logistic regression analysis, IL‐6:IL‐10 ratio was the most important predictor for NCE (p = 0.006) with an odds ratio of 2.24 (95% CI 1.26 to 3.97).
Conclusions
Cytokine concentrations and proinflammatory to anti‐inflammatory cytokine ratios may be useful markers for predicting vascular risk in patients with NSTEACS.
Atherosclerosis is an inflammatory process and inflammation has a triggering effect in the thrombotic phase as in all stages of the disease.1,2 Current evidence suggests that high sensitivity (hs) inflammatory markers may predict vascular risk.3,4,5 However, these markers have not been used previously in the risk stratification models of current guidelines for non‐ST elevation acute coronary syndrome (NSTEACS).6 The major vascular risk marker C‐reactive protein (CRP), proinflammatory cytokines such as interleukin (IL) ‐1β, IL‐6, tumour necrosis factor (TNF) α, and the anti‐inflammatory cytokine IL‐10 have been detected in human atherosclerotic plaques.7,8,9,10,11 The balance between proinflammatory and anti‐inflammatory cytokines may reflect the intensity of occult plaque inflammation and the vulnerability to rupture.12 Proinflammatory to anti‐inflammatory cytokine ratios can signal the balance between proinflammatory and anti‐inflammatory forces and may predict vascular risk in NSTEACS. The objective of this study was to investigate the long‐term prognostic value of hs‐inflammatory cytokines and proinflammatory to anti‐inflammatory cytokine ratios in patients with NSTEACS and moderate to high TIMI (Thrombolysis In Myocardial Infarction) risk scores.13
METHODS
Patients
The study group consisted of 80 patients (60 men, 20 women, mean age 60 (SD 10) years) with NSTEACS (Braunwald class IIIB unstable angina pectoris and non‐ST elevation myocardial infarction (MI)) who were admitted to our centre from August 2002 to August 2003. TIMI risk score was calculated and patients with a risk score ⩾ 3 were included to the study. Exclusion criteria were active infection, neoplastic disease, loss of consciousness, pregnancy, chronic renal disease (creatinine > 133 μmol/l), degenerative or rheumatic valvular disease, prior valvular or any other prosthetic material or intracardiac defibrillator replacement, MI or revascularisation in the preceding six months and prior surgical intervention in the preceding six months.
Angiographic analysis and revascularisation procedure
All patients underwent an early invasive strategy after 24–48 h of standard medical treatment. Coronary angiography was performed according to the Judkins technique, and images of the coronary tree were obtained in routine, standardised projections in all patients. Significant coronary artery disease was defined as a stenosis ⩾ 50% in the main coronary artery or in one of its branches. According to the coronary anatomy, angioplasty and coronary stenting were chosen as the primary revascularisation strategies. Patients with unprotected left main disease or diffuse three‐vessel disease were scheduled for urgent coronary artery bypass grafting.
Laboratory analysis
Venous blood samples for hs‐CRP and cytokine analysis were taken on admission. After samples were centrifuged and aliquoted, serum and plasma specimens were frozen and stored at −70°C until analysis. Serum hs‐CRP concentrations were measured with a chemiluminescent enzyme immunometric assay in an IMMULITE One analyser. Cytokine concentrations (IL‐1β, IL‐6, TNFα and IL‐10) were measured in serum with an hs quantitative sandwich enzyme immunoassay (Quantikine HS; R&D Systems Inc, Minneapolis, Minnesota, USA). The minimum detectable doses of human inflammatory and anti‐inflammatory cytokines were as follows: IL‐1β < 0.1 pg/ml, IL‐6 ranging from 0.016–0.110 pg/ml (mean minimum detectable dose 0.09 pg/ml), TNFα ranging from 0.06–0.32 pg/ml (mean minimum detectable dose 0.12 pg/ml) and IL‐10 < 0.5 pg/ml. The intra‐assay and interassay precision of the all cytokine assays in the study was < 10%.
Bedside quantitative measurements of troponin I, creatine kinase MB fraction and myoglobin concentrations were analysed with a Triage Cardiac Panel (Biosite Diagnostics). A haemogram, electrolyte concentrations, glucose, urea, creatinine, uric acid, lipid profile and all other biochemistry measurements were carried out by the analytical unit of the Biochemistry Department of our institution by standard methods.
Follow up
The patients were followed up for events occurring during the first six months and then during the first year. The primary end point of the study was new coronary events (NCE), which were defined as the combination of cardiac death, non‐fatal MI and recurrent proven rest angina that required hospitalisation. Recurrent rest angina that required rehospitalisation was defined as chest pain at rest lasting ⩾ 5 min with new transient ST‐T ECG changes after hospital discharge. The study was approved by the local ethics committee, and all patients gave written informed consent to participate.
Statistical analysis
SPSS V.11.5 software (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis of the study. Results are presented as mean (SD) or as percentages and numbers for categorical data. Normality tests were used for all variables. In the comparison between patients with and those without NCE, continuous variables that were normally distributed were analysed with the two‐tailed t test and unequally distributed variables were analysed with the Mann–Whitney U test. Categorical data and proportions were analysed with the χ2 or Fisher's exact test where appropriate.
hs‐CRP, IL‐1β, TNFα, hs‐IL‐6 and hs‐IL‐10 concentrations were compared between the patients with and those without NCE during the six‐month and one‐year follow up. The ratios of proinflammatory cytokine concentrations to anti‐inflammatory cytokine hs‐IL‐10 concentrations were calculated by dividing proinflammatory cytokine concentrations by hs‐IL‐10 concentrations, and these ratios were compared between the two groups. For inflammatory markers and cytokine ratios, which were significantly higher in patients with NCE, the predictive cut‐off values for determining event development were identified by receiver operating characteristic (ROC) curve analysis with MedCalc V.8.1.0.0 statistical software. Inflammatory markers, cytokine ratios and other risk factors that can be significant for development of NCE were assessed with binary logistic regression analysis by the forward Wald method. The resulting parameters that were evaluated in the model were those that were significantly different between event‐positive and event‐negative groups. A value of p < 0.05 was considered significant.
RESULTS
Coronary angiography showed left main disease in four (5%), single‐vessel disease in 18 (22.5%), two‐vessel disease in 28 (35%) and three‐vessel disease in 22 (27.5%) patients. Four patients (5%) had side branch disease and four patients (5%) had no significant coronary lesions. The revascularisation rate during hospitalisation was 79%. Twenty two patients underwent surgical revascularisation and 41 patients underwent percutaneous coronary intervention.
During the first six‐month follow‐up period, 19 NCE developed: one death (1.3%), 18 recurrent rest angina (22.5%) and, among these 18 patients, three non‐fatal MI (3.8% of all patients). In the second six‐month period, nine more NCE were observed, five of them in patients with NCE in the first six‐month period (one death, one non‐fatal MI and three recurrent angina) and four in previously event‐free patients (three deaths, one non‐fatal MI). By the end of one year, 23 patients had had NCE consisting of five deaths (6.2%), four non‐fatal MIs (5%) and 14 recurrent rest angina (17.5%). Table 1 shows baseline characteristics of the patients with and without NCE during follow up.
Table 1 Baseline clinical characteristics of the patients with and without new coronary events (NCE) in one year of follow up.
| Variable | NCE− (n = 57) | NCE+ (n = 23) | p Value |
|---|---|---|---|
| Age (years) | 63 (11) | 60 (10) | NS |
| Men/women | 43/14 | 17/6 | NS |
| Hypertension | 41 (71%) | 16 (69%) | NS |
| Dyslipidaemia | 31 (54%) | 14 (61%) | NS |
| Diabetes | 11 (19%) | 4 (17%) | NS |
| Previous MI | 11 (19%) | 5 (21%) | NS |
| Previous PCI | 5 (8.8%) | 0 | NS |
| Previous CABG | 7 (12%) | 3 (13%) | NS |
| Atrial fibrillation | 0 (%0) | 3 (%13) | 0.01 |
| CK‐MB (ng/ml) | 17 (30) | 9.4 (15) | NS |
| Myoglobin (ng/ml) | 196 (174) | 119 (98) | NS |
| Troponin (ng/ml) | 2.3 (7.9) | 0.7 (1.4) | NS |
| Ejection fraction (%)* | 55 (11) | 51 (12) | NS |
| Prior drug treatment | |||
| Statin | 45 (78%) | 21 (91%) | NS |
| Aspirin | 32 (56%) | 14 (61%) | NS |
| ACE inhibitor | 11 (19%) | 3 (13%) | NS |
| β blocker | 15 (26%) | 4 (17%) | NS |
| TIMI score | NS | ||
| 3 | 25 (44%) | 6 (26%) | |
| 4 | 14 (24%) | 8 (34%) | |
| 5 | 12 (21%) | 8 (35%) | |
| 6 | 6 (10.5%) | 1 (4%) |
*Determined by the modified Simpson's method with transthoracic echocardiography.
Data are mean (SD) or number (%).
ACE, angiotensin‐converting enzyme; CABG, coronary artery bypass grafting; CK, creatine kinase; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction.
Demographic and predisposing risk factors, drug treatment before and during hospitalisation, physical examination, TIMI risk score, ST‐T wave changes on ECG and ejection fraction were similar between the two groups. All three patients who had atrial fibrillation had NCE, which was a significantly higher proportion than among the patients with sinus rhythm (p = 0.01). No significant relationship was detected between NCE and angiographic data. NCE did not differ between the patients with and without revascularisation.
Inflammatory markers and cytokine ratios
Mean values of hs‐inflammatory cytokines in the whole study group were as follows: hs‐CRP, 75.1 (103) μmol/l; IL‐1β, 0.83 (1.79) pg/ml; IL‐6, 4.82 (3.48) pg/ml; TNFα, 10.89 (11.86) pg/ml; and IL‐10, 7.97 (6.9) pg/ml. Table 2 gives serum concentrations of hs‐CRP and proinflammatory and anti‐inflammatory cytokines in patients with NCE in the first six months and the entire year of follow up.
Table 2 Inflammatory markers and new coronary events (NCE) in six months and one year of follow up.
| Marker | 0–6 months | p Value | 0–1 year | p Value | ||
|---|---|---|---|---|---|---|
| NCE− (n = 61) | NCE+ (n = 19) | NCE− (n = 57) | NCE+ (n = 23) | |||
| hs‐CRP (μmol/l) | 62 (97) | 115 (106) | 0.01 | 53 (88) | 106 (106) | 0.05 |
| IL‐1β (pg/ml) | 0.6 (1.7) | 1.2 (1.9) | 0.01 | 0.7 (1.7) | 1.1 (1.8) | 0.04 |
| IL‐6 (pg/ml) | 4.4 (3.4) | 5.9 (3.3) | 0.1 | 4.3 (3.4) | 6.0 (0.2) | 0.03 |
| TNFα (pg/ml) | 11 (13) | 8.4 (8.5) | 0.6 | 12 (13) | 8.9 (8.7) | 0.4 |
| IL‐10 (pg/ml) | 8.7 (7.4) | 5.6 (3.8) | 0.08 | 8.5 (7.1) | 6.5 (6.2) | 0.1 |
Data are presented as mean (SD).
hs‐CRP, high sensitivity C‐reactive protein; IL, interleukin; TNF, tumour necrosis factor.
Serum concentrations of hs‐CRP were higher in patients with NCE during the first and second follow up periods. Like hs‐CRP, IL‐1β concentrations were significantly higher in patients with NCE than in patients without NCE in both the first and the second follow‐up periods. However, IL‐6 concentrations were significantly higher in patients with NCE during the one‐year follow‐up period (table 2). Although IL‐10 and TNFα concentrations did not differ significantly between patients with and without events, serum IL‐10 concentrations were lower in patients with NCE; this difference approached significance during the first six‐month follow up.
In the analysis of the relation between the ratio of proinflammatory to anti‐inflammatory cytokines and NCE, the ratios of IL‐1β:IL‐10 and IL‐6:IL‐10 were significantly higher in patients with NCE than in event‐free patients (table 3).
Table 3 Proinflammatory to anti‐inflammatory cytokine ratios in patients with and without new coronary events.
| Cytokine ratio | 0–6 months | p Value | 0–1 year | p Value | ||
|---|---|---|---|---|---|---|
| NCE− (n = 61) | NCE+ (n = 19) | NCE− (n = 57) | NCE+ (n = 23) | |||
| IL‐1β:IL‐10 | 0.14 (0.4) | 0.28 (0.46) | 0.006 | 0.14 (0.41) | 0.23 (0.42) | 0.03 |
| IL‐6:IL‐10 | 0.75 (0.76) | 1.44 (1.12) | 0.01 | 0.73 (0.74) | 1.38 (1.08) | 0.007 |
| TNFα:IL‐10 | 1.85 (2.25) | 2.0 (1.6) | 0.4 | 1.93 (2.3) | 1.78 (1.8) | 0.7 |
Data are mean (SD).
IL, interleukin; TNF, tumour necrosis factor.
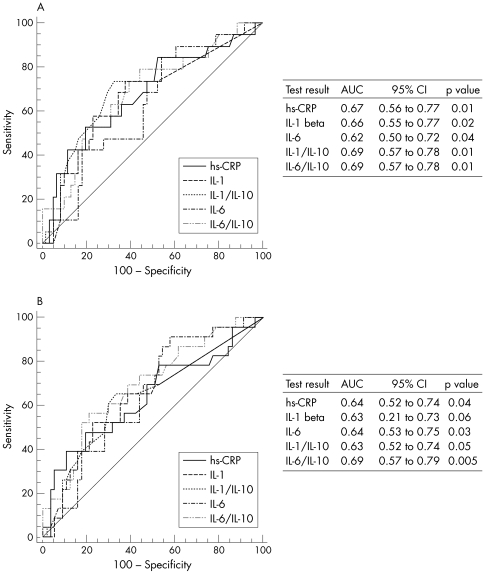
We used ROC curve analysis to identify the predictive cut‐off values for inflammatory risk factors, which were significantly higher in patients with NCE. Inflammatory risk factors and their predictive cut‐off values for two follow‐up periods were as follows: hs‐CRP > 78 μmol/l for the first and > 121 μmol/l for the second follow‐up period, IL‐1β > 0.12 pg/ml for the first and > 0.39 pg/ml for the second follow‐up period, IL‐6 > 2.5 pg/ml for the first and > 2.3 pg/ml for the second follow‐up period, IL‐1β:IL‐10 > 0.02 and IL‐6:IL‐10 > 1.18 for both the first and the second follow up periods. Figure 1 shows ROC curves and area under the curve for each marker. Table 4 shows the relation between these inflammatory risk factors and NCE in six‐month and one‐year follow‐up periods. Table 5 gives the prognostic value of specific markers for predicting NCE during the two follow‐up periods.
Figure 1 Receiver operating characteristic curves of inflammatory markers and area under the curve during (A) the first six months and (B) the first year of follow up. CI, confidence interval; hs‐CRP, high sensitivity C‐reactive protein; IL, interleukin.
Table 4 Relation between inflammatory risk factors and new coronary events (NCE) during six months and one year of follow up.
| Risk factor | 0–6 months | p Value | Risk factor | 0–1 year | p Value | ||
|---|---|---|---|---|---|---|---|
| NCE− (n = 61) | NCE+ (n = 19) | NCE− (n = 57) | NCE+ (n = 23) | ||||
| CRP >78 μmol/l | 12 (20%) | 10 (53%) | 0.005 | CRP 121 μmol/l | 6(11%) | 9(40%) | 0.003 |
| IL‐1β >0.12 pg/ml | 23 (38%) | 14 (74%) | 0.006 | IL‐1β >0.39 pg/ml | 14(25%) | 12(53%) | 0.01 |
| IL‐6 >2.5 pg/ml | 36 (56%) | 16 (85%) | 0.02 | IL‐6 >2.3 pg/ml | 33(58%) | 21(92%) | 0.004 |
| IL‐6:IL‐10 >1.18 | 14 (23%) | 11 (58%) | 0,004 | IL‐6:IL‐10 >1.18 | 12(21%) | 13(57%) | 0.002 |
| IL‐1β:IL‐10 >0.02 | 24 (40%) | 14 (74%) | 0.009 | IL‐1β:IL‐10 >0.02 | 23(40%) | 15(65%) | 0.04 |
CRP, C‐reactive protein; IL, interleukin.
Table 5 Prognostic value of specific markers for predicting new coronary events (NCE) at six months and one year.
| Risk factor | NCE 0–6 months | Risk factor | NCE 0–1 year | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | Sens | Spec | PPV | NPV | ||
| CRP >78μmol/l | 52% | 80% | 45% | 84% | CRP >121 μmol/l | 39% | 89% | 60% | 78% |
| IL‐1β >0.12 pg/ml | 73% | 62% | 37% | 88% | IL‐1β >0.39 pg/ml | 52% | 75% | 46% | 79% |
| IL‐6 >2.5 pg/ml | 84% | 40% | 30% | 89% | IL‐6 >2.3 pg/ml | 91% | 42% | 38% | 92% |
| IL‐6:IL‐10 >1.18 | 58% | 77% | 44% | 85% | IL‐6:IL‐10 >1.18 | 56% | 78% | 52% | 81% |
| IL‐1β:IL‐10 >0.02 | 73% | 60% | 36% | 88% | IL‐1β:IL‐10 >0.02 | 65% | 59% | 38% | 80% |
CRP, C‐reactive protein; IL, interleukin; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Although IL‐6 > 2.5 pg/ml was more sensitive in predicting NCE at six (84%) and 12 (91%) months, its specificity and positive predictive value were low. When we considered IL‐6:IL‐10 > 1.18, sensitivity decreased but specificity and the positive predictive value at six (44%) and 12 months (52%) were increased. In both the first six months and one year of follow up, the IL‐6:IL‐10 ratio had the greatest area under the curve, and this finding suggested that it may be the most important predictor of NCE in this study population.
In the multivariate analysis adjusted for hs‐CRP, IL‐1β, IL‐6, IL‐10, IL‐1β:IL‐10 ratio and IL‐6:IL‐10 ratio, the IL‐6:IL‐10 ratio was the only significant predictor of NCE during the first six months (OR 2.2, 95% CI 1.2 to 3.9, p = 0.007) and one year of follow up (OR 2.2, 95% CI 1.2 to 3.9, p = 0.006).
DISCUSSION
Coronary atherosclerotic plaque disruption or erosion with consequent thrombosis is the major cause of NSTEACS.14,15 Inflammatory mediators have a major potential role in this process, as in all stages of atherosclerosis. They are the “molecular soldiers” of the oxidative modifiers of lipoproteins in vulnerable plaque, which have been described as “molecular Trojan horses”.16,17 As inflammation and thrombosis are intertwined vascular pathologies in the natural history of atherosclerosis, hs measurements of inflammatory markers can more accurately predict NCE as shown for hs‐CRP in several previous trials.18
In the present study serum concentrations of hs‐CRP were higher in patients with NCE. This is in agreement with many large prospective randomised trials supporting the predictive value of this marker for risk stratification in NSTEACS.
Previous studies reported that proinflammatory cytokine concentrations were increased and anti‐inflammatory cytokine concentrations were decreased in patients with NSTEACS.19,20,21,22,23 In recent years, a new concept emerged of an imbalance between proinflammatory and anti‐inflammatory factors, in favour of the proinflammatory factors, that results in rupture of atherosclerotic plaque.24,25 Nevertheless, no data are available on the long‐term prognostic value of proinflammatory to anti‐inflammatory cytokine ratios in patients with NSTEACS. Most of the trials with inflammatory cytokines in NSTEACS were comparative trials between patients with stable angina and healthy controls.19,20,21,25 In addition, hs cytokine assay methods were not used in most of these studies and more sensitive methods possibly would have provided more detailed prognostic information.20,21,22,23
The present study examined the predictive values of proinflammatory and anti‐inflammatory cytokines and cytokine ratios for determining the long‐term prognosis in patients with NSTEACS and moderate to high TIMI risk score. All patients included in the study underwent an early invasive strategy according to current concepts and received optimal drug treatment consisting of aspirin, β blockers, angiotensin‐converting enzyme inhibitors, statins and clopidogrel after revascularisation. Owing to the selection criteria at the beginning of the study, aggressive treatment of well‐established atherosclerotic risk factors and early invasive treatment of the patients, none of the classic risk factors (diabetes, history of coronary artery disease, troponin concentration and TIMI score) had a predictive value for NCE.
On the other hand, in this selected group of patients, IL‐1β and IL‐6 concentrations were powerful predictors of NCE, but TNFα concentrations and NCE were not related. However, it has been postulated that the short plasma half life (6–20 min after intravenous injection) of TNFα may limit its potential clinical utility as a screening tool.19,26,27 The delay between obtaining and studying the samples may have been too long for prognostic usefulness as mentioned in the other TNFα studies.19,26 Although we have not found any significant relationship between IL‐10 concentrations and primary outcome, IL‐10 concentrations were lower in patients who had events. These results suggest that the risk for development of NCE is higher in patients with higher proinflammatory cytokine concentrations and lower anti‐inflammatory cytokine concentrations.
When we combined these markers as proinflammatory to anti‐inflammatory cytokine ratios (as IL‐1β:IL‐10 and IL‐6:IL‐10), we observed a very strong relation between them and the development of NCE compared with individual concentrations of each marker. In particular, the IL‐6:IL‐10 ratio was the most powerful predictor of the development of NCE during both the first six months and the one‐year follow‐up period. This finding suggests that cytokine concentrations and especially proinflammatory to anti‐inflammatory cytokine ratios can be used for further risk assessment in patients already evaluated by classic risk estimation models.
This idea was supported by a recent study that evaluated the in‐hospital prognostic utility of the IL‐18:IL‐10 ratio in patients with ST elevation acute coronary syndrome and NSTEACS.28 Our study is the first to investigate the long‐term prognostic effectiveness of proinflammatory to anti‐inflammatory cytokine ratios in patients with moderate to high TIMI score and NSTEACS.
Unfortunately, although all patients underwent an invasive strategy and received optimal drug treatment in our study, NCE could not be prevented in patients with an imbalance between proinflammatory and anti‐inflammatory cytokines. This finding may have two clinical implications: firstly, these patients should be followed up more closely than patients with lower concentrations of inflammatory markers; secondly, current treatment strategies are not sufficient to protect these patients.
Limitations of the study
Our study was not a multicentre study and the number of patients in the study was relatively low. In addition, technical difficulties, which most of the other cytokine trials also experienced, such as aliquoting of serum and plasma, freezing methods and the time between sampling and assessing results, were limiting factors in our study. The positive predictive value of the presented markers ranged between 30–60%; thus, any intervention based on the presented data will overtreat one to two in three patients. Also, c statistics for cut‐off values of our markers are in a relatively low range.
Conclusions
Cytokine concentrations and proinflammatory to anti‐inflammatory cytokine ratios, especially IL‐6:IL‐10, may be useful markers for predicting the development of NCE in NSTEACS.
Abbreviations
CRP - C‐reactive protein
hs - high sensitivity
IL - interleukin
MI - myocardial infarction
NCE - new coronary events
NSTEACS - non‐ST elevation acute coronary syndrome
ROC - receiver operating characteristic
TIMI - Thrombolysis In Myocardial Infarction
TNFα - tumour necrosis factor α
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Simon D I. Inflammation and thrombosis: the clot thickens. Circulation 20011031718–1720. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Whincup P, Walker M.et al Low grade inflammation and coronary heart disease: prospective study and updated meta‐analyses. BMJ 2000321199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake G J, Ridker P M. C‐reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol 200341(4 Suppl S)37S–42S. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker P M, Maseri A. Inflammation and atherosclerosis. Circulation 20021051135–1143. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E, Antman E M, Beasley J W.et al ACC/AHA 2002 guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). Circulation 20021061893–1900. [DOI] [PubMed] [Google Scholar]
- 7.Torzewski M, Rist C, Mortensen R F.et al C‐reactive protein in the arterial intima: role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000202094–2099. [DOI] [PubMed] [Google Scholar]
- 8.Galea J, Armstrong J, Gadsdon P.et al Interleukin‐1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 1996161000–1006. [DOI] [PubMed] [Google Scholar]
- 9.Barath P, Fishbein M C, Cao J.et al Detection and localization of TNF in human atheroma. Am J Cardiol 199065297–302. [DOI] [PubMed] [Google Scholar]
- 10.Rus H G, Vlaicu R, Niculescu F. Interleukin‐6 and interleukin‐8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 1996127263–271. [DOI] [PubMed] [Google Scholar]
- 11.Mallat Z, Heymes C, Ohan J.et al Expression of interleukin‐10 in advanced human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 199919611–616. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Allergy 20032257–265. [DOI] [PubMed] [Google Scholar]
- 13.Antman E M, Cohen M, Bernink P J.et al The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000284835–842. [DOI] [PubMed] [Google Scholar]
- 14.Corti R, Fuster V, Badimon J J. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol 2003417S–14S. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V, Badimon L, Badimon J.et al The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 1992326242–250. [DOI] [PubMed] [Google Scholar]
- 16.Hajjar D P, Haberland M E. Lipoprotein trafficking in vascular cells: molecular Trojan horses and cellular saboteurs. J Biol Chem 199727222975–22978. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti R, Gotto A M, Jr, Hajjar D P. Inflammation in atherosclerosis and implications for therapy. Circulation 2004109(23 Suppl 1)III20–III26. [DOI] [PubMed] [Google Scholar]
- 18.Rifai N, Ridker P M. High‐sensitivity C‐reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 200147403–411. [PubMed] [Google Scholar]
- 19.Simon A D, Yazdani S, Wang W.et al Circulating levels of IL‐1beta, a prothrombotic cytokine, are elevated in unstable angina versus stable angina. J Thromb Thrombolysis 20009217–222. [DOI] [PubMed] [Google Scholar]
- 20.Smith D A, Irving S D, Sheldon J.et al Serum levels of the antiinflammatory cytokine interleukin‐10 are decreased in patients with unstable angina. Circulation 2001104746–749. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita H, Shimada K, Seki E.et al Concentrations of interleukins, interferon, and C‐reactive protein in stable and unstable angina pectoris. Am J Cardiol 200391133–136. [DOI] [PubMed] [Google Scholar]
- 22.Koukkunen H, Penttila K, Kemppainen A.et al C‐reactive protein, fibrinogen, interleukin‐6 and tumour necrosis factor‐alpha in the prognostic classification of unstable angina pectoris. Ann Med 20013337–47. [DOI] [PubMed] [Google Scholar]
- 23.Lindmark E, Diderholm E, Wallentin L.et al Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA 20012862107–2113. [DOI] [PubMed] [Google Scholar]
- 24.Alam S E, Nasser S S, Fernainy K E.et al Cytokine imbalance in acute coronary syndrome. Curr Opin Pharmacol 20044166–170. [DOI] [PubMed] [Google Scholar]
- 25.Waehre T, Halvorsen B, Damas J K.et al Inflammatory imbalance between IL‐10 and TNFalpha in unstable angina potential plaque stabilizing effects of IL‐10. Eur J Clin Invest 200232803–810. [DOI] [PubMed] [Google Scholar]
- 26.Blake G J, Ridker P M. Novel clinical markers for vascular wall inflammation. Circ Res 200189763–771. [DOI] [PubMed] [Google Scholar]
- 27.Blake G J, Ridker P M. Tumour necrosis factor‐alpha, inflammatory biomarkers, and atherogenesis. Eur Heart J 200223345–347. [DOI] [PubMed] [Google Scholar]
- 28.Chalikias G K, Tziakas D N, Kaski J C.et al Interleukin‐18: interleukin‐10 ratio and in‐hospital adverse events in patients with acute coronary syndrome. Atherosclerosis 2005182135–143. [DOI] [PubMed] [Google Scholar]