Abstract
Objective
To determine the prevalence of chronic oral anticoagulant drug treatment (COA) among patients with acute myocardial infarction (AMI) and its impact on management and outcome.
Methods
All patients with ST segment elevation AMI on the RICO (a French regional survey for AMI) database were included in this analysis. COA was defined as continuous use ⩾ 48 hours before AMI.
Results
Among the 2112 patients with ST elevation myocardial infarction (STEMI), 93 (4%) patients were receiving COA. These patients were older and more likely to have a history of hypertension, diabetes and prior myocardial infarction than patients without COA. In addition, fewer patients who received COA underwent reperfusion therapy or received an antiplatelet agent (aspirin/thienopyridines). Moreover, patients receiving COA experienced a higher incidence of in‐hospital major adverse events (death, recurrent myocardial infarction or major bleeding, p = 0.005). Multivariate analysis showed that only ejection fraction, current smoking and multiple vessel disease, but not COA, were independent predictive factors for major adverse events. In contrast, COA was an independent predictive factor for heart failure when adjusted for age, diabetes, creatinine clearance, reperfusion, heparin and glycoprotein IIb/IIIa inhibitors (odds ratio 2.06, CI 95% 1.23 to 3.43, p = 0.005).
Conclusion
In this population based registry, patients with STEMI with prior use of COA constituted a fairly large group (4%) with an overall higher baseline risk profile than that of patients without COA. Fewer in the COA group received reperfusion therapy or aggressive antithrombotic treatment and they experienced more adverse in‐hospital outcomes. Thus, further studies are warranted to develop specific management strategies for this high risk group.
Keywords: acute myocardial infarction, chronic oral anticoagulation, management, outcome
Chronic oral anticoagulant drug treatment (COA), particularly with vitamin K antagonists, is standard for specific cardiovascular indications such as mechanical valvular prostheses, pulmonary embolism or prevention of stroke in atrial fibrillation. Vitamin K antagonists inhibit the synthesis of the vitamin K dependent coagulation proteins, prothrombin and factors VII, IX and X.1 Patients receiving COA, however, need close medical care and careful international normalised ratio (INR) monitoring due to the high risk of fluctuations in anticoagulation efficiency related to food intake, drug interactions and other processes.
Practice guidelines have been developed for the use of antithrombotic and antiplatelet drugs in acute coronary syndromes,2,3 which resulted in more aggressive use of antiplatelet and anticoagulant drugs in acute myocardial infarction (AMI). These guidelines are, however, based on data from clinical trials performed in highly selected patient populations. As patients under COA have generally been excluded from randomised studies, there is little information about their optimal management in AMI. In these patients, the risk of interaction of the oral anticoagulants with drugs used in the acute phase for reperfusion therapy such as thrombolytics, antiplatelet/glycoprotein IIb/IIIa inhibitors or aspirin needs to be taken into account. Actually, the most common complication of drug interactions is bleeding.4 The impact of chronic use of oral anticoagulants on the presentation and outcomes of AMI is largely unknown.
The objective of this study on a population based registry was to determine the prevalence of COA among patients with AMI and its impact on management and outcome.
METHODS
Patients
Briefly, since 1 January 2001, the RICO survey has been collecting in‐hospital data from patients hospitalised with AMI in the six public and private hospitals of Côte‐d'Or, a French region with a population of about 500 000 inhabitants. These hospitals house all of the emergency departments (three units) and coronary care units (three units) in the region.
Data were collected at each site by a study coordinator trained in completing the core record form and in extracting data from medical records on a standardised case report form. Cases were ascertained by prospective collection of consecutive admissions. Standardised definitions for myocardial infarction and of patient related variables and clinical outcomes were used. Patients were enrolled in the registry if they were ⩾ 18 years of age and were admitted to participating hospitals within 24 hours of the onset of symptoms with a suspected diagnosis of myocardial infarction. AMI was diagnosed according to European Society of Cardiology and American College of Cardiology criteria. Patients presenting with ST segment elevation, a new or presumed new left bundle branch block or a documented new Q wave on their ECG were included in this analysis.5 The present study complied with the Declaration of Helsinki and was approved by the ethics committee of the University Hospital of Dijon. Each patient gave written consent before participation.
Data collection
Demographic data, cardiovascular risk factors and history, as well as on‐admission data, were reviewed. Left ventricle ejection fraction was measured by echocardiography. The delays and use of reperfusion (lysis and percutaneous coronary intervention (PCI)) were determined for patients eligible for reperfusion (that is, admitted within 12 hours of symptom onset). Detailed use of all antithrombotics such as antiplatelet agents (aspirin and thienopyridines) and heparins (low molecular weight and unfractionated heparin) administered during the first 48 hours after hospital admission were collected. In‐hospital events—recurrent myocardial infarction, ventricular arrhythmia (ventricular tachycardia or fibrillation), stroke, cardiogenic shock, heart failure (defined by Killip class > I) and death—were recorded. Cardiogenic shock was defined as systolic blood pressure < 90 mm Hg persisting for > 1 hour despite fluid challenge, associated with clinical signs of hypoperfusion.6 Major bleeding was defined as important or life‐threatening blood loss with substantial haemodynamic compromise requiring transfusion.7
Group definition and analysis
COA was defined as the continuous use of COA for at least 30 days before admission. The INR was measured during the first six hours after admission. The COA indication and type of COA were also recorded.
Statistical analysis
Continuous data were expressed as median and interquartile range and dichotomous data, as percentages. Continuous variables were analysed by a Kolmogorov–Smirnov test for normality. The two groups were compared either by the unpaired Student's t test or by the non‐parametric Mann–Whitney U test as appropriate. Categorical data were analysed by the χ2 test. Multiple logistic regression models were chosen to assess the relationships between variables and the occurrence of adverse events. Models were built by selecting the variables that were prognostic for adverse hospital outcome in multiple regression analyses. The first model (model 1) included baseline characteristics (log(age), sex, diabetes mellitus, heart failure, abnormal creatinine clearance (< 30 ml/min) and prior COA), as well as reperfusion procedures and short term drug treatments (during the first 48 hours; heparins, aspirin, glycoprotein IIb/IIIa inhibitors, lysis and primary PCI) as predictors of major bleeding in a backward stepwise regression analysis. In model 2, log(age), diabetes, altered creatinine clearance and the use of heparins, aspirin, glycoprotein IIb/IIIa inhibitors, lysis, primary PCI and prior COA were tested in a multivariate analysis as predictive factors for heart failure (Killip class > I) during in‐hospital stay. For model 3, log(age), sex, diabetes, altered creatinine clearance, heart failure, lysis and prior COA were tested as predictors of the use of coronary angiography in patients eligible for reperfusion (time to admission < 12 hours). The significance level required for inclusion in multivariate analysis was 0.20. Backward stepwise regression analyses were then performed to test for independent predictors of adverse events. In this analysis, an α value of 5% for the significance level was required to account for the increase in the overall type I error due to multiple testing. The Wald test was performed to test for significance. Results are expressed as the odds ratio with 95% confidence interval (CI). All tests were two sided.
RESULTS
Baseline characteristics
Among the 2112 patients with ST elevation myocardial infarction (STEMI) in the survey, 93 (4%) had received COA (table 1). These patients were older than patients not treated with COA and were more likely to have a history of myocardial infarction, stroke or peripheral arterial disease. The median delay (interquartile range) from stroke and myocardial infarction was 24 (10–57) months. This group also had higher rate of risk factors such as hypertension and diabetes mellitus.
Table 1 Demographic data, risk factors, cardiovascular history and presenting characteristics of patients with and without chronic oral anticoagulant drug treatment (COA).
| No COA group (n = 2019 (96%)) | COA group (n = 93 (4%)) | p Value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 66 (52–76) | 76 (71–83) | <0.001 |
| Men | 1469 (73%) | 60 (65%) | 0.105 |
| Risk factors | |||
| Hypertension | 949 (47%) | 60 (65%) | 0.001 |
| Diabetes mellitus | 390 (19%) | 34 (37%) | <0.001 |
| BMI (kg/m2) | 26.2 (23.8–29.1) | 25.8 (23.5–28.8) | 0.559 |
| Hypercholesterolaemia | 804 (40%) | 46 (49%) | 0.081 |
| Current smoking | 664 (33%) | 12 (13%) | <0.001 |
| Cardiovascular history | |||
| MI | 226 (11%) | 31 (33%) | <0.001 |
| Stroke | 93 (5%) | 12 (13%) | <0.001 |
| PAD | 138 (7%) | 12 (13%) | 0.043 |
| Presenting characteristics | |||
| TIMI score | 3 (2–5) | 6 (4–7) | <0.001 |
| Killip >I | 418 (21%) | 37 (40%) | <0.001 |
| DBP (mm Hg) | 80 (69–90) | 80 (70–90) | 0.577 |
| SBP (mm Hg) | 133 (117–153) | 131 (120–153) | 0.915 |
| HR (beats/min) | 78 (65–90) | 78 (66–94) | 0.515 |
| Anterior MI | 875 (43%) | 45 (48%) | 0.394 |
| LVEF (%) | 52 (43–62) | 45 (35–52) | <0.001 |
| Time to admission (min) | 209 (104–580) | 250 (120–607) | 0.497 |
Data are number (%) or median (interquartile range).
BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIMI, thrombolysis in myocardial infarction.
In patients who received COA, the median INR on admission was 2.12 (table 2), but only 44% had an INR between 2 and 3. Atrial fibrillation or venous thromboembolism accounted for most COA indications (table 2). The remaining indications for COA were valve disease, thrombophilia, altered left ventricular ejection fraction or other. Most of the patients receiving COA (80%) were treated with long half life vitamin K antagonists such as fluindione or warfarin. Clinical data on admission (table 1) showed no difference in myocardial infarction location, admission blood pressure and heart rate, and time to admission, but higher Killip class and lower left ventricular ejection fraction in the COA group than in the non‐COA group.
Table 2 Indications and type of treatment of patients treated with chronic anticoagulant drugs (n = 84).
| INR at admission | 2.12 (1.68–2.70) |
| 2<INR<3 (therapeutic range) | 44 (52%) |
| Indication | |
| Valve disease | 2 (2%) |
| Atrial fibrillation | 32 (38%) |
| Valve disease and atrial fibrillation | 7 (8%) |
| Pulmonary embolism, venous thrombosis, or both | 21 (25%) |
| Thrombophilia (factor V Leiden mutation) | 2 (2%) |
| Altered LVEF (<35%) | 10 (12%) |
| Others or unknown | 8 (10%) |
| Drug | |
| Warfarin | 2 (2%) |
| Fluindione | 63 (73%) |
| Acenocoumarol | 13 (15%) |
Data are number (%) or median (interquartile range).
INR, international normalised ratio; LVEF, left ventricular ejection fraction.
Short term treatments and reperfusion procedures
With regard to the reperfusion procedures used during the acute phase of myocardial infarction, there were some important differences between the two groups (table 3). Among patients eligible for reperfusion therapy, the use of thrombolysis was far lower in patients receiving COA, although the time to lysis was similar. Patients receiving COA less commonly underwent coronary angiography but only slightly more commonly underwent primary PCI. The lower use of lysis in this group seems to be linked to a reduction in rescue PCI. The delays to PCI were similar for the two groups. Overall, many more patients without COA received any reperfusion therapy (that is, thrombolysis or primary PCI) (69% v 47%, p < 0.001).
Table 3 Reperfusion procedures in patients with time to admission <12 hours.
| No COA group (n = 1492 (96%)) | COA group (n = 66 (4%)) | p Value | |
|---|---|---|---|
| Time to admission (min) | 150 (90–270) | 173 (94–295) | 0.277 |
| Lysis | 634 (42%) | 6 (9%) | <0.001 |
| Time to lysis | 150 (100–240) | 208 (164–210) | 0.484 |
| Coronary angiography | 1395 (93%) | 49 (74%) | <0.001 |
| PCI | 1046 (70%) | 38 (58%) | 0.042 |
| Primary PCI | 394 (26%) | 25 (38%) | 0.056 |
| Time to primary PCI (min) | 300 (194–569) | 255 (201–445) | 0.383 |
| Rescue PCI | 259 (17%) | 2 (3%) | 0.004 |
| Subsequent PCI | 393 (26%) | 11 (16%) | 0.107 |
Data are number (%) or median (interquartile range).
COA, chronic oral anticoagulant drug treatment; PCI, percutaneous coronary intervention.
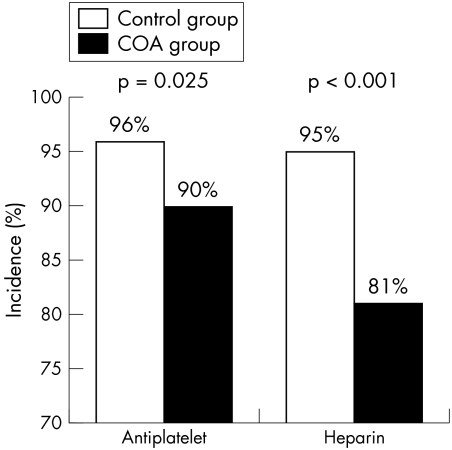
Short term drug treatments were also different for the two groups: fewer in the COA group received antiplatelet (aspirin and thienopyridines) and heparin (fig 1) but the use of glycoprotein IIb/IIIa receptor antagonists did not differ (23% of patients receiving COA v 31%, p = 0.121).
Figure 1 Short term antiplatelet and heparin treatment among patients who received chronic oral anticoagulant drug treatment (COA) and those who did not (control).
Outcome
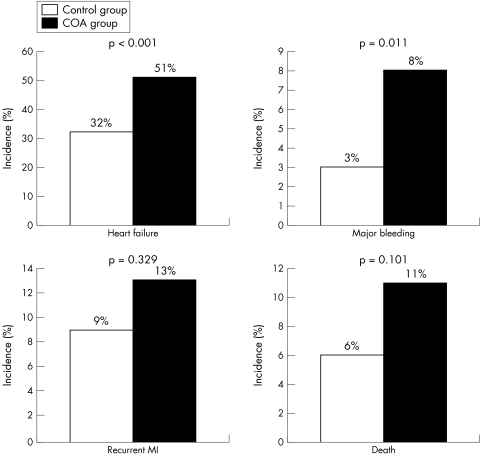
The difference in outcome was highly significant for occurrence of congestive heart failure (defined as Killip class > I) and major bleeding (fig 2). The incidence of ventricular arrhythmia, cardiogenic shock and stroke was similar for the two groups (13% v 12%, p = 0.957; 7% v 11%, p = 0.335; and 1% v 3%, p = 0.170, for no COA v COA, respectively).
Figure 2 Occurrence of in‐hospital major adverse events (MAE). COA, chronic oral anticoagulant drug treatment; MI, myocardial infarction.
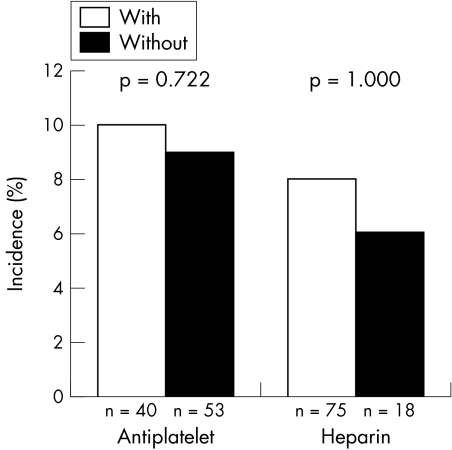
The use of antiplatelets or heparins during the first 48 hours after admission had no impact on the incidence of major adverse events (including recurrent myocardial infarction, major bleeding, in‐hospital heart failure and mortality) among patients with prior COA (fig 3).
Figure 3 Influence of short term antiplatelet and heparin treatments during the first 48 hours after admission on in‐hospital major bleeding in patients with chronic oral anticoagulant drug treatment (COA).
Multivariate analysis
Prior COA was associated with an increased risk of bleeding in univariate analysis. In multivariate analysis, prior COA was not an independent factor of major bleeding. In multivariate analysis, adjustment for known predictors of bleeding showed that age and on‐admission heart failure were the only factors independently associated with major bleeding in this cohort (p = 0.026 and p < 0.001, respectively) (table 4).
Table 4 Multivariate analysis of baseline characteristics and short term treatments as predictors of major bleeding.
| Model 1 | p Value | Odds ratio | 5–95% CI |
|---|---|---|---|
| Prior COA | 0.608 | 1.253 | 0.457 to 3.434 |
| Log (age) | 0.026 | 2.277 | 1.679 to 2.649 |
| Heart failure on admission | <0.001 | 3.434 | 1.939 to 6.081 |
Adjusted covariates: female sex, diabetes, heparin, aspirin, glycoprotein IIb/IIIa inhibitors, thrombolysis, primary percutaneous coronary intervention, low creatinine clearance.
COA, long term oral anticoagulant drug treatment.
COA was an independent predictor of in‐hospital heart failure, along with age, low left ventricle ejection fraction and low creatinine clearance after adjustment for baseline characteristics and treatments received within the first 48 hours (p = 0.012) (table 5).
Table 5 Multivariate analysis of baseline characteristics and short term treatments as predictors of heart failure during in‐hospital stay.
| Model 2 | p Value | Odds ratio | 5–95% CI |
|---|---|---|---|
| Prior COA | 0.012 | 2.30 | 1.20 to 4.43 |
| Log (age) | <0.0001 | 2.79 | 2.13 to 3.66 |
| LVEF <40% | <0.001 | 6.61 | 4.72 to 9.25 |
| Altered creatinine clearance | <0.0001 | 4.67 | 2.61 to 8.35 |
Adjusted covariates: heparin, aspirin, glycoprotein IIb/IIIa inhibitors, lysis, primary percutaneous coronary intervention.
COA, chronic oral anticoagulant drug treatment; LVEF, left ventricular ejection fraction.
Among patients eligible for reperfusion therapy, COA was a negative predictor for coronary angiography, as table 6 shows. Moreover, admission heart failure and altered creatinine clearance were also negative predictive factors, whereas use of thrombolytics was positively associated with the use of coronary angiography (table 6). Multivariate analysis also showed that only ejection fraction, current smoking and multiple vessel disease were independent predictive factors for major adverse events (odds ratio 2.44, 95% CI 1.78 to 3.35, p < 0.0001; odds ratio 0.55, 95% CI 0.43 to 0.77 p < 0.001; and odds ratio 1.41, 95% CI 1.20 to 1.65, p < 0.001, respectively), whereas age, sex, diabetes and anterior infarction location were not significant.
Table 6 Multivariate analysis of baseline characteristics of patients eligible for short term reperfusion as predictors of coronary angiography.
| Model 3 | p Value | Odds ratio | 5–95% CI |
|---|---|---|---|
| Prior COA | 0.005 | 0.371 | 0.185 to 0.743 |
| Heart failure | 0.001 | 0.437 | 0.267 to 0.717 |
| Altered creatinine clearance | 0.010 | 0.307 | 0.168 to 0.563 |
| Lysis | 0.0001 | 2.303 | 1.216 to 4.360 |
Adjusted covariates: female sex, diabetes, age.
COA, chronic oral anticoagulant drug treatment.
DISCUSSION
In this large, population based registry of patients with STEMI, the major findings are that a substantial fraction of patients with STEMI were receiving COA (4%). These patients have a higher risk profile than patients without COA and yet receive less reperfusion therapy and less aggressive initial antiplatelet and heparin treatment. These patients also have a lower odds of undergoing coronary angiography during their index hospital stay. After adjustment of major predictors of risk at baseline, COA was a powerful independent predictor of major adverse events, particularly of major bleeding, despite the less aggressive use of antithrombotic drugs. In contrast, short term administration of antiplatelet and heparin to these patients did not significantly increase the risk of bleeding.
Baseline characteristics and management
Our study, like previous studies, showed that 4% of patients with STEMI were under COA, which is far from negligible,8 yet this patient subset is usually excluded from randomised clinical trials and needs to be better described. In the non‐selected group of patients from the RICO survey, such patients had a high risk profile resulting in a higher TIMI (thrombolysis in myocardial infarction) risk score. This high risk profile, associated with the risk of drug interactions between COA and drugs used during the acute phase, create a particularly difficult medical management dilemma. To the best of our knowledge, no data are available on this topic. Our results thus have a direct bearing on decisions regarding the use of anticoagulant and antiaggregant drugs during the acute phase of myocardial infarction. Moreover, our findings highlight the association between COA and heart failure during in‐hospital stay and the low use of coronary angiography. In contrast, in our study population, COA even when associated with antiplatelet or heparin did not significantly increase the risk of major bleeding. The relatively low statistical power due to the small size of the COA group, however, does not allow exclusion of any increased risk of bleeding associated with COA.
Reperfusion procedures
We found that up to a third of all eligible patients did not benefit from reperfusion therapy. The national registry of myocardial infarction study and the GRACE registry reported similar results.9,10 Moreover, reperfusion therapy was used less commonly in the COA group than in the non‐COA group. The low use of lysis complies with recent guidelines but, even though PCI increased correspondingly, this did not fully make up for the lower use of lysis. The specific profile of patients in the COA group partly explains these results. As in the GRACE registry, patients who were denied reperfusion therapy were older, had diabetes and a history of congestive heart failure or myocardial infarction, or had previously undergone coronary bypass surgery.10
Predictors of major bleeding
The incidence of major bleeding reported from clinical studies of patients with myocardial infarction depends on clinical presentation and treatment.11 Previous studies have identified several predictors of major bleeding during AMI, such as female sex, advanced age, renal insufficiency and a history of bleeding.8,9,10,11,12,13,14 Advanced age has been found to be associated with an increased risk of death, vascular complications and a need for transfusion after PCI, with an increased risk of intracranial bleeding after administration of thrombolytic or antithrombotic drugs.14,15,16 The presence of local vascular changes or of more advanced vascular disease has been postulated as a potential explanation for the increased incidence of bleeding complications among elderly patients. The relationship observed between female sex and bleeding risk is generally explained by an increased propensity for the development of vascular complications and an older age, as well as a different threshold for transfusion as a result of lower baseline values.17,18,19
The beneficial effect of warfarin compared with placebo in preventing adverse events after myocardial infarction is well established but was associated with a higher risk of bleeding.4 The beneficial effect of warfarin, either in combination with aspirin or alone, was restricted to non‐fatal reinfarction and thromboembolic stroke but there were no statistical differences in overall mortality between the groups.4 In the present study, 33% (n = 31) of the population had a history of myocardial infarction in the COA group compared with only 11% (n = 226) in the non‐COA group (p < 0.001). Our findings are consistent with a high incidence of major bleeding in the COA group but COA is not an independent predictor of major bleeding. Surprisingly, PCI and pharmacological interventions, particularly the use of thrombolytic agents and glycoprotein IIb/IIIa receptor blockers, were not predictive factors for major bleeding. Patients given PCI, lysis and glycoprotein IIb/IIIa blockers may, however, have been carefully selected to be at lowest risk and were therefore less likely to have major bleeding. Lastly, our work underlines the poor outcome of patients with co‐morbidity and the paradoxical underuse of drugs in this high risk group.
Heart failure
COA was an independent predictor of in‐hospital heart failure, along with age, diabetes and low creatinine clearance, after adjustment for baseline characteristics and treatments received within the first 48 hours. Similar findings were reported in a recent study: patients with heart failure were significantly older and were less likely to be men or smokers. Moreover, a history of co‐morbidity was more common.20 In addition, patients with heart failure were less likely to undergo cardiac catheterisation procedures than were patients without heart failure. In view of the poor prognosis of heart failure, aggressively identifying patients who are suitable for revascularisation appears to be justified to preserve left ventricular function, prevent left ventricular remodelling and improve survival. Our data did not further explain co‐morbidity as a potential cause of heart failure, due to our relatively small population. Further studies are needed to define precisely the relationship between co‐morbidity associated with COA and the development of heart failure in the setting of AMI.
Drug interactions: antithrombin and antiplatelet drugs
Although the combination is generally avoided, antiplatelet drugs and COA were used in the present study. The combination of antiplatelet drugs and oral anticoagulants was reported to increase the risk of bleeding in several ways: their added effects on platelet function and interference with COA metabolism, as well as unique adverse effect profiles that can increase the risk of bleeding.
In clinical studies of patients with prosthetic valves, the rate of bleeding when oral anticoagulation is combined with antiplatelet drugs depends on the intensity of treatment and the type of antiplatelet drug. High intensity aspirin (100 mg daily) results in higher rates of major (12.9% v 10.3%) and total (38.7% v 26.1%) bleeding.21
Clinical trials have not provided safety data, as patients taking warfarin have been excluded from studies of glycoprotein IIb/IIIa receptor antagonists. The question of chronic combination treatment with anticoagulant and antiplatelet drugs in patients with indications for both is controversial. In a recent paper Orford and colleagues22 presented data on 66 consecutive patients who received a combination of dual antiplatelet drugs (aspirin and clopidogrel) and systemic anticoagulation (warfarin) after PCI. In this study the authors observed a significant increase in major bleeding, without increased death, myocardial infarction or stent thrombosis.22 Moreover, Lidell and colleagues23 recently reported that the stable anticoagulation status of patients receiving chronic warfarin is unaffected by concomitant administration of clopidogrel 75 mg daily. The COA group remains relatively small but with a proportion similar to such groups in previous studies. Lastly, our work underlines the poor outcome of patients with co‐morbidity and the paradoxical underuse of drugs in this high risk group.
Study limitations
Potential limitations of the study should be acknowledged. Our study is a population based registry on AMI management by routine clinical practice.
At admission 43% of patients were not given adequate anticoagulant drugs (INR < 2), which could have created a bias in our analysis. No difference was observed, however, between patients with an admission INR < 2 and patients with an INR ⩾ 2 in main outcomes (bleeding, heart failure, recurrent myocardial infarction or cardiovascular death) (table 7). Further studies are needed to define precisely the relationship between co‐morbidity associated with COA and development of heart failure in the setting of AMI.
Table 7 Outcome of patients with an international normalised ratio (INR) at admission of ⩾2 or <2.
| INR | ||
|---|---|---|
| ⩾2 (57%) | <2 (43%) | |
| Bleeding | 9% | 9% |
| Congestive heart failure | 44% | 65% |
| Cardiovascular deaths | 14% | 12% |
| Recurrent MI | 12% | 15% |
| CV death/recurrent MI | 26% | 26% |
| CV death/bleeding | 21% | 18% |
| CV death/bleeding/recurrent MI | 33% | 29% |
All differences are non‐significant.
CV, cardiovascular; MI, myocardial infarction.
Conclusion
In this population based registry, patients with STEMI with prior use of COA constituted a fairly large group (4% of all patients with STEMI) who had an overall higher baseline risk profile than that of patients without COA. Yet this group received less reperfusion therapy and less aggressive antithrombotic treatment and experienced a higher incidence of adverse outcomes in hospital than did patients without COA. Thus, our findings strongly suggest the need for large scale randomised controlled trial to examine adequately the effects of COA in the setting of AMI.
ACKNOWLEDGEMENTS
This work was supported by the Association de Cardiologie de Bourgogne and by grants from University Hospital of Dijon, Union Régionale des Caisses d'Assurance Maladie de Bourgogne, Agence Régionale de l'Hospitalisation de Bourgogne and Fédération Française de Cardiologie.
Abbreviations
AMI - acute myocardial infarction
COA - chronic oral anticoagulant drug treatment
INR - international normalised ratio
PCI - percutaneous coronary intervention
STEMI - ST elevation myocardial infarction
TIMI - thrombolysis in myocardial infarction
References
- 1.Hirsh J, Dalen J, Anderson D R.et al Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001119(suppl 1)8S–21S. [DOI] [PubMed] [Google Scholar]
- 2.Ryan T J, Antman E M, Brooks N H.et al 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on management of acute myocardial infarction). Circulation 19991001016–1030. [DOI] [PubMed] [Google Scholar]
- 3.Van de Werf F, Ardissino D, Betriu A.et al Management of acute myocardial infarction in patients presenting with ST‐segment elevation. The task force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J 20032428–66. [DOI] [PubMed] [Google Scholar]
- 4.Hurlen M, Abdelnoor M, Smith P.et al Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002347969–974. [DOI] [PubMed] [Google Scholar]
- 5.Alpert J S, Thygesen K, Antman E.et al Myocardial infarction redefined: a consensus document of The Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol 200036959–969. [DOI] [PubMed] [Google Scholar]
- 6.Hasdai D, Topol E J, Califf R M.et al Cardiogenic shock complicating acute coronary syndromes. Lancet 2000356749–756. [DOI] [PubMed] [Google Scholar]
- 7.De Queiroz Fernandes Araujo J O, Veloso H H, Braga De Paiva J M.et al Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta‐analysis of randomized, controlled trials. Am Heart J 2004148937–943. [DOI] [PubMed] [Google Scholar]
- 8.Rao A K, Pratt C, Berke A.et al Thrombolysis in myocardial infarction (TIMI) trial. Phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase, J Am Coll Cardiol 1988111–11. [DOI] [PubMed] [Google Scholar]
- 9.Rogers W J, Canto J G, Lambrew C T.et al Temporal trends in the treatment of over 1. 5 million patients with myocardial infarction in the US from 1990 through 1999: the national registry of myocardial infarction 1, 2 and 3, J Am Coll Cardiol 2000362056–2063. [DOI] [PubMed] [Google Scholar]
- 10.Eagle K A, Goodman S G, Avezum A.et al GRACE Investigators. Practice variation and missed opportunities for reperfusion in ST‐segment‐elevation myocardial infarction: findings from the global registry of acute coronary events (GRACE), Lancet 2002359373–377. [DOI] [PubMed] [Google Scholar]
- 11.Moscucci M, Fox K A, Cannon C P.et al Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE). Eur Heart J 2003241815–1823. [DOI] [PubMed] [Google Scholar]
- 12.Moscucci M, O'Connor G T, Ellis S G.et al Validation of risk adjustment models for in‐hospital percutaneous transluminal coronary angioplasty mortality on an independent data set. J Am Coll Cardiol 199934692–697. [DOI] [PubMed] [Google Scholar]
- 13.Califf R M, Topol E J, George B S.et al Hemorrhagic complications associated with the use of intravenous tissue plasminogen activator in treatment of acute myocardial infarction. Am J Med 198885353–359. [DOI] [PubMed] [Google Scholar]
- 14.Madan M, Blankenship J C, Berkowitz S D. Bleeding complications with platelet glycoprotein IIb/IIIa receptor antagonists. Curr Opin Hematol 19996334–341. [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz S D, Granger C B, Pieper K S.et al Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries (GUSTO) I investigators. Circulation 1997952508–2516. [DOI] [PubMed] [Google Scholar]
- 16.Gore J M, Sloan M, Price T R.et al Intracerebral hemorrhage, cerebral infarction, and subdural hematoma after acute myocardial infarction and thrombolytic therapy in the thrombolysis in myocardial infarction study. Thrombolysis in myocardial infarction, phase II, pilot and clinical trial. Circulation 199183448–459. [DOI] [PubMed] [Google Scholar]
- 17.Lansky A J, Mehran R, Dangas G.et al Comparison of differences in outcome after percutaneous coronary intervention in men versus women <40 years of age. Am J Cardiol 200493916–919. [DOI] [PubMed] [Google Scholar]
- 18.Moscucci M, Mansour K A, Kent K C.et al Peripheral vascular complications of directional coronary atherectomy and stenting: predictors, management, and outcome. Am J Cardiol 199474448–453. [DOI] [PubMed] [Google Scholar]
- 19.Robertson T, Kennard E D, Mehta S.et al Influence of gender on in‐hospital clinical and angiographic outcomes and on one‐year follow‐up in the new approaches to coronary intervention (NACI) registry. Am J Cardiol 19978026K–39K. [DOI] [PubMed] [Google Scholar]
- 20.Steg P G, Dabbous O H, Feldman L J.et al Global Registry of Acute Coronary Events Investigators. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the global registry of acute coronary events, Circulation 2004109494–499. [DOI] [PubMed] [Google Scholar]
- 21.Levine M N, Hirsh J, Salzman E W. Side effects of antithrombotic therapy. In: Colman RW, Hirsh J, Marder VJ, et al, eds. Hemostasis and thrombosis: basic principles and clinical practice. 3rd ed. Philadelphia: JB Lippincott, 1994
- 22.Orford J L, Fasseas P, Melby S.et al Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004147463–467. [DOI] [PubMed] [Google Scholar]
- 23.Lidell C, Svedberg L E, Lindell P.et al Clopidogrel and warfarin: absence of interaction in patients receiving long‐term anticoagulant therapy for non‐valvular atrial fibrillation. Thromb Haemost 200389842–846. [PubMed] [Google Scholar]