Abstract
Objective
To compare rate control and rhythm control strategies in patients with atrial fibrillation (AF) after percutaneous mitral balloon valvotomy (PMV).
Methods
183 patients with AF after successful PMV, with AF duration ⩽ 12 months and post‐PMV left atrial (LA) size ⩽ 45 mm, were studied in a prospective, randomised trial. The primary end point was improvement in AF‐related symptoms. Secondary study end points were 6 min walk tests, quality of life (QOL), normalisation of LA size, number of hospital admissions and duration of hospital stay.
Results
Over one year, 2% patients in the rate control group had sinus rhythm, as compared with 96% of patients in the rhythm control group (p < 0.001). A greater proportion of patients reported improvement in symptoms in the rhythm control group than in the rate control group (p < 0.0001 at every visit time). Walking distance in a 6 min walk test, QOL and LA size normalisation were better in the rhythm control group than in the rate control group. The strategy of rhythm control was associated with similar numbers of hospital admissions but with longer duration of hospital admissions. Drug‐related side effect did not differ between the rate control and rhythm control groups. During the follow‐up period, no patients in either group had embolic or transitory ischaemic neurological events.
Conclusions
In patients with AF after PMV, AF duration ⩽ 12 months and post‐PMV LA size ⩽ 45 mm, sinus rhythm was easy and safe to achieve and maintain. Moreover, patients benefited from restoration and maintenance of sinus rhythm in terms of improved AF‐related symptoms, 6 min walk tests and QOL, and of LA size normalisation. Rhythm control should therefore be considered as the preferred initial therapy for this group of patients. The optimal strategy to treat AF after PMV should be individualised.
Most patients with rheumatic mitral valvular stenosis and atrial fibrillation (AF) remain in AF rhythm after percutaneous mitral balloon valvotomy (PMV).1,2,3 AF can adversely affect haemodynamic function. The absence of atrial systole (atrial kick) and a rapid ventricular rate with relative shortening of diastole can increase left atrial (LA) pressure, worsen pulmonary venous congestion and compromise cardiac output. Moreover, stasis of blood in the LA appendage predisposes to development of thrombi and embolic complications.4 AF also generates significant healthcare costs.5
Sinus rhythm is difficult to achieve and maintain in patients with rheumatic mitral valvular stenosis and AF but would be more easily achieved with reduction of LA pressure after successful PMV.6 In our previous study, we found that longer AF history, smaller mitral valve area and higher LA pressure after PMV are the key factors of AF recurrence.1 Sinus rhythm can be maintained in a higher proportion of patients with AF duration ⩽ 12 months and post‐PMV LA size ⩽ 45 mm than of those with AF duration > 12 months and post‐PMV LA size > 45 mm.1 This indicates that rhythm control may be before rate control in this selected group of patients.
Prospective data comparing strategies of rate control and rhythm control in patients with AF after PMV are lacking. The optimal strategy to treat the condition has not been established. This prospective randomised trial was designed to examine this important clinical issue.
METHODS
Study population
This study was designed as a prospective, randomised, single‐centre clinical trial to compare strategies of rate control and rhythm control in patients with AF after successful PMV with the Inoue technique.7 A successful procedure is defined as PMV achieved without acute mitral valve replacement and a mitral valve area after PMV of ⩾ 1.5 cm2. Transoesophageal echocardiography was done one day before PMV. AF was identified by ECG. If another ECG recorded during the 12 months before PMV showed the presence of sinus rhythm, AF duration was defined as ⩽ 12 months. Patients who remained in AF rhythm three days after PMV, with AF duration ⩽ 12 months and post‐PMV LA size ⩽ 45 mm, were eligible if they gave written informed consent. Patients were excluded from the study if they had received an antiarrhythmic drug within five half lives of the time of random assignment. Recruitment began on 1 March 2001 and random assignment was concluded on 30 March 2004. Three days after PMV, patients recruited for this pilot study were randomly assigned to one of the two following treatment arms.
In the rate control group, no attempt was made to terminate AF. Rate was controlled with the administration of digitalis, a non‐dihydropyridine calcium channel blocker and a β blocker, alone or in combination. Rate control during AF was assessed both at rest and during activity. The target was a resting heart rate of 60–80 beats/min and 90–115 beats/min during moderate exercise.8 If patients had intolerable symptoms due to AF or unacceptable adverse effects of the atrioventricular node blocking, or if the ventricular response could not be controlled despite treatment, cardioversion was performed.
In the rhythm control group, antiarrhythmic drugs with or without electrical cardioversion were used to restore sinus rhythm. The preferred initial treatment was amiodarone at a daily dose of 600 mg for one week. If sinus rhythm had not been restored within one week, patients underwent electrical cardioversion followed by administration of low‐dose amiodarone (100–200 mg daily) aimed at preventing recurrent AF at the lowest effective level. In the case of recurrent AF, sinus rhythm was again restored in two weeks by the same method.
Both randomisation groups received adequate anticoagulation with a target international normalised ratio of 2.0–3.0. If sinus rhythm was present at one month, the oral anticoagulant was changed to aspirin (300 mg daily).
This trial was done at Renmin Hospital of Wuhan University, China. The research and ethics committee of Wuhan University approved this protocol. Written informed consent was obtained from all patients.
Clinical follow up
All randomly assigned patients were followed up for 12 months with regular visits scheduled at three days (baseline), three weeks, and three, six and 12 months after PMV. On hospital discharge, patients were given diaries to list symptoms, follow‐up physician appointments and rehospitalisation. Patients were advised to telephone at any time if they experienced new symptoms.
Study procedure
At baseline and at follow‐up visits, patients underwent one‐dimensional and two‐dimensional echocardiography, a 6 min walk test and assessment of quality of life (QOL). Laboratory studies to assess liver and thyroid function and chest radiographs were performed at least every six months.
End points
As patients undergoing PMV in Mainland China are mostly younger9 and AF is usually not life threatening, death was not an end point in this short‐term study. The primary end point was improvement in AF‐related symptoms in symptomatic AF. Secondary study end points were 6 min walk tests, QOL, normalisation of LA size, number of hospital admissions and duration of hospital stay (measured from the date of admission to discharge). Improvement in AF‐related symptoms in symptomatic AF was assessed during each follow‐up visit by the changes compared with baseline in the three most frequently reported symptoms: palpitations, dyspnoea and dizziness.10 Symptomatic improvement was defined (in hierarchical order) as the elimination of palpitations (if present at baseline), a reduction in the frequency of episodes of dyspnoea (if palpitations were not eliminated) or a reduction in the frequency of dizzy spells (if palpitations were not eliminated and dyspnoea was unchanged). For patients who did not have palpitations at baseline, symptomatic improvement was defined as the continued absence of palpitations and an improvement in dyspnoea or dizziness, as described above. These symptoms were carefully assessed by interviews with every patient. Another common AF‐related symptom is “easy fatigability”, but this symptom was assessed separately by 6 min walk tests.10
Assessment of the health‐related QOL
Patients completed the MOS (Medical Outcomes Study) 36‐Item Short‐Form Health Survey (SF‐36) at baseline and after 12 months. It measured the multidimensional properties of health‐related QOL on a scale ranging from 0 to 100, with lower scores representing a lower QOL.11,12,13 The SF‐36 is a standardised, generic health‐related QOL measurement instrument that has been validated in the Chinese population.14,15,16
Statistical analysis
Data were statistically analysed according to the intention‐to‐treat principle. Continuous variables are expressed as mean (SD). Categorical variables are presented as frequencies and percentages. Significance was assumed with a two‐sided p < 0.05. We tested continuous variables by one‐way analysis of variance for between‐group comparisons (with the baseline value as the covariable) and Student's t test for within‐group comparisons. If variables were not normally distributed, the Mann–Whitney test was used to test treatment‐group differences and the Wilcoxon signed rank test was used to test within‐group differences. Groups were compared for categorical variables by the χ2 test with continuity correction or Fisher's exact test as appropriate. Data were analysed with SPSS for windows V.11.0 (SPSS Inc, Chicago, Illinois, USA).
The sample size was calculated with the use of Power And Precision (release 2.00; Biostat Inc, Englewood, New Jersey, USA). It was calculated on the assumption that rate control could improve symptoms in 50% of patients with symptomatic AF17; in contrast, rhythm control was estimated to be effective in 80% of patients. To detect significant differences between the two treatment strategies, 52 patients with symptomatic AF after PMV would need to be included in each treatment group to achieve a power of 90% at a 95% confidence level (two sided).
RESULTS
Characteristics of patients
We recruited 183 consecutive eligible patients who consented to participate in the study. There were 122 women and 61 men, aged 36.9 (5.4) years (range 25–56 years). They had chronic AF for an average of 158.7 (SD 120.3) days (range 28–365 days) and the mean post‐PMV LA size (anteroposterior dimensions) was 39.9 (3.9) mm (range 32–45 mm). Ninety one patients were randomly assigned to the rate control strategy and 92 to the rhythm control strategy. Fifty two patients in the rate control group and 58 in the rhythm control group had symptomatic AF. Baseline characteristics of the two groups with respect to age, sex, AF duration, AF‐related symptoms, post‐PMV echocardiography parameters and pharmacological treatment were similar (table 1).
Table 1 Baseline characteristics of eligible patients.
| Variable | Rate control (n = 91) | Rhythm control (n = 92) | p Value |
|---|---|---|---|
| Age (years) | 37.3 (6.4) | 36.5 (4.2) | 0.34 |
| Women | 59 (64.8%) | 63 (68.5%) | 0.60 |
| Mean AF duration (days) | 160.9 (122.2) | 156.5 (119.0) | 0.81 |
| AF‐related symptoms | 52 (57.1%) | 58 (63.0%) | 0.42 |
| Palpitation | 38 (73.1%) | 37 (63.8%) | 0.30 |
| Dyspnoea | 32 (61.5%) | 30 (51.7%) | 0.30 |
| Dizziness | 5 (9.6%) | 6 (10.3%) | 0.90 |
| Post‐PMV echocardiography parameters | |||
| LA size (mm) | 39.8 (4.1) | 40.0 (3.7) | 0.80 |
| MVA (cm2) | 1.84 (0.15) | 1.81 (0.16) | 0.21 |
| MLAP (mm Hg) | 11.9 (2.8) | 12.5 (2.7) | 0.13 |
| Pharmacological treatment | |||
| Digoxin | 80 (87.9%) | 82 (89.1%) | 0.80 |
| β blockers | 15 (16.5%) | 20 (21.7%) | 0.37 |
| Diltiazem | 9 (9.9%) | 15 (16.3%) | 0.20 |
| Aspirin | 88 (96.7%) | 85 (92.4%) | 0.20 |
| Anticoagulants | 12 (13.2%) | 9 (9.8%) | 0.47 |
| Nitrates | 36 (39.6%) | 28 (30.4%) | 0.20 |
| Diuretics | 45 (49.5%) | 54 (58.7%) | 0.21 |
Values are mean (SD) or number (%).
AF, atrial fibrillation; LA, left atrial; MLAP, mean left atrial pressure; MVA, mitral valve area; PMV, percutaneous mitral balloon valvotomy.
Drug treatment
None of the patients was lost to follow up and none died during the study. In the rate control group, nearly 58% of patients were taking digoxin. To achieve adequate rate control, 73% of patients required two drugs. Cardioversion was performed in five (5.5%) patients: one patient had intolerable symptoms due to AF, three patients had unacceptable adverse effects of atrioventricular node blocking, and in one patient the ventricular response was not controlled despite treatment.
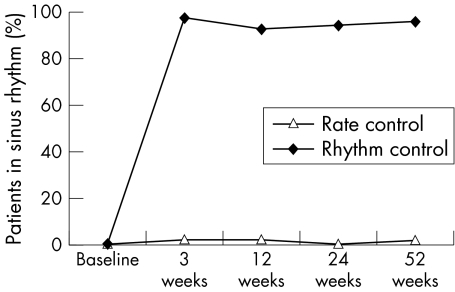
In the rhythm control group, pretreatment with amiodarone restored sinus rhythm in 26% of patients before electrical cardioversion. In the remaining patients, at least one electrical cardioversion was done (twice in 6% of patients and three times in 2%). The cardioversion success rate was 100%. The mean amiodarone maintenance dose was 130 mg/day (87) at 24 weeks and 121 mg/day (56) at 52 weeks. In the rate control group, only 2% of patients were in sinus rhythm at the end of the observation period compared with 96% patients in the rhythm control group (p < 0.001) (fig 1).
Figure 1 Maintenance of sinus rhythm during the study.
Only five (5.5%) patients crossed over from rate control to rhythm control and three (3.3%) patients crossed over from rhythm control to rate control (p = 0.72).
Primary end point
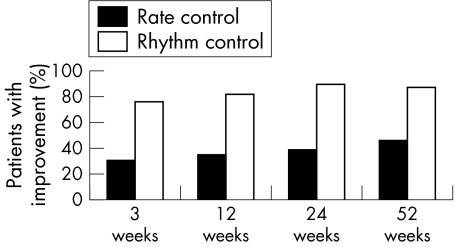
A greater proportion of patients reported improvement in symptoms in the rhythm control group than in the rate control group at every follow up visit. The primary end point differed significantly between the groups at every time point during the trial (fig 2).
Figure 2 Proportion of patients reporting improvement in clinical symptoms during follow up. Rate control versus rhythm control at all time points, p < 0.0001.
Exercise tolerance
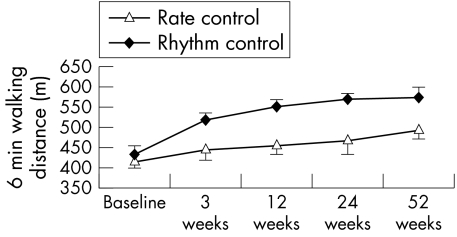
The two treatment strategies differed significantly as measured by the 6 min walk test (baseline: p = 0.57; first visit: p < 0.0001; second visit: p < 0.0001; third visit: p = 0.02; fourth visit: p = 0.01) (fig 3).
Figure 3 Mean data on exercise tolerance as assessed by repeated 6 min walk tests. Error bars show 95% confidence interval.
Quality of life
The scores of the eight SF‐36 subscales were similar in the two groups at baseline. QOL scores, however, were significantly lower than those reported for healthy people.16 At one year, most measures had improved, and QOL scores showed greater improvement in the rhythm control group than in the rate control group (table 2).
Table 2 Assessment of quality of life (SF‐36).
| SF‐36 variable | Rate control | Rhythm control | p Value | China norm† |
|---|---|---|---|---|
| Physical functioning | ||||
| Baseline | 54.8 (17.2) | 52.3 (19.0) | 0.06 | 82.2 |
| Month 12 | 67.8 (36.9)* | 80.9 (27.1)** | 0.01 | |
| Physical role function | ||||
| Baseline | 42.0 (19.2) | 39.2 (20.8) | 0.4 | 81.2 |
| Month 12 | 57.1 (15.6)** | 74.3 (21.0)** | <0.0001 | |
| Bodily pain | ||||
| Baseline | 58.5 (22.4) | 61.0 (25.1) | 0.8 | 81.5 |
| Month 12 | 70.4 (26.4)** | 79.4 (20.3)** | 0.07 | |
| General health | ||||
| Baseline | 36.3 (38.3) | 34.6 (35.1) | 0.9 | 56.7 |
| Month 12 | 38.5 (43.1) | 52 (42.8)* | 0.001 | |
| Vitality | ||||
| Baseline | 35.3 (12.8) | 33.4 (15.5) | 0.7 | 52.0 |
| Month 12 | 41.0 (19.2)* | 49.5 (20.1)* | 0.04 | |
| Social functioning | ||||
| Baseline | 61.2 (43.1) | 63.6 (36.0) | 0.5 | 83.0 |
| Month 12 | 73.2 (32.4)* | 79.1 (28.4)** | 0.7 | |
| Emotional role function | ||||
| Baseline | 64.4 (35.3) | 65.4 (34.2) | 0.9 | 84.4 |
| Month 12 | 66.3 (27.3) | 75.6 (28.1)* | 0.06 | |
| Mental health | ||||
| Baseline | 39.0 (19.1) | 37.7 (21.0) | 0.7 | 59.7 |
| Month 12 | 47.6 (18.3)* | 54.1 (18.0)** | 0.04 | |
36‐Item Short‐Form Health Survey (SF‐36) scores presented as mean (SD).
*p<0.05, **p<0.01 for within‐group difference from baseline.
†Data from the general population of Hangzhou, Mainland China assessed by SF‐36.16
Normalisation of LA size
In comparison with baseline, significant (both p < 0.0001) reductions of LA dimension were noted at one year in the rate control group (39.8 (4.1) to 36.4 (3.6) mm) and the rhythm control group (40.0 (3.7) to 33.5 (3.8) mm). LA size decreased more in the rhythm control group than in the rate control group (6.4 (3.8) v 3.4 (2.8) mm, respectively; p < 0.0001). LA size (⩽ 30 mm) had normalised at follow up in 14.4% of 90 patients in the rhythm control group but in only 1.1% of 88 patients in the rate control group (p < 0.001).
Thromboembolic prophylaxis and embolic or ischaemic neurological events
In the rate control arm, oral warfarin was used as thromboembolic prophylaxis by 54.9% patients. Aspirin and ticlopidine were used by 34.1% and 5.5% of patients, respectively. In the rhythm control group, 76.9% patients received warfarin for one month before sinus rhythm was present. In 81.5% of study participants, thromboembolic prophylaxis was continued with aspirin, and 3.3% of patients received ticlopidine. During the follow‐up period, no patients in either group experienced embolic or transitory ischaemic neurological events.
Adverse effects
The adverse event rate did not differ between the groups. At least one drug‐related side effect was seen in 15 (16.5%) of 91 rate control patients compared with 10 (10.9%) of 92 rhythm control patients (p = 0.269). The most frequently encountered diltiazem‐associated side effect was peripheral oedema (seven patients). Bronchospasm (n = 1) and hypotension (n = 4) were associated with β blocker. In the rhythm control group, six significant adverse effects were attributed to amiodarone including symptomatic bradycardia (n = 2), asymptomatic hypothyroidism (n = 2) and symptomatic hyperthyroidism (n = 1). All five patients discontinued amiodarone, while two patients remained in normal sinus rhythm and the other three patients crossed over to rate control. Minor bleeding occurred in eight patients (five in the rate control group, three in the rhythm control group).
Hospital admissions
In the rate control group, 10 (11%) of 91 patients were readmitted to hospital at least once compared with 11 (12%) of 92 in the rhythm control group (p = 0.837). In the rate control group, the most frequent cause of hospital readmission was drug‐related side effects (70%). In the rhythm control group, most readmissions were for electrical cardioversion (64% of all hospital readmissions). But patients in the rhythm control group were in hospital longer than their rate control counterparts (13.17 (2.79) v 7.71 (2.50) days, respectively; p = 0.04). The main reason was amiodarone loading and electrical cardioversion in the rhythm control group.
DISCUSSION
To the best of our knowledge, this study is the first randomised trial to compare two different therapeutic strategies, rate or rhythm control, in patients with AF after PMV. Our study showed that during 12 months of follow‐up, patients with AF duration ⩽ 12 months and post‐PMV LA size ⩽ 45 mm benefited from restoration and maintenance of sinus rhythm in terms of improvement in AF‐related symptoms, 6 min walk tests and QOL, and of LA size normalisation. Rhythm control should therefore be considered the preferred initial therapy for this group of patients. This finding also may have important implications for patients with AF who have extensive co‐morbid conditions and require symptom relief not provided by rate control.
In recent years, several randomised trials10,18,19,20,21,22 have compared heterogeneous groups of patients, and the data are consistent and strong enough to promote rate control as the initial strategy for the vast majority of patients with persistent AF.23 Firstly, with current antiarrhythmic drugs a rhythm control approach does not lead to improved symptom control or QOL or a reduction in clinical events in the short to medium term. In the longer term, mortality may increase. Secondly, maintenance of sinus rhythm remains poor, even with an aggressive strategy combining electrical cardioversion and current antiarrhythmic drugs. Hence, long‐term therapeutic anticoagulation should be advocated for most patients treated with rhythm control, even if sinus rhythm is achieved in the short term. Thirdly, more hospital admissions and adverse side effects remain problems. But it should be pointed out that the above clinical trial samples consisted of elderly patients (mean age ⩾ 60 years) and mainly patients with non‐valvular AF. Those data suggest that there was no benefit in attempting rhythm control in these patients with a high risk of arrhythmia recurrence. It remains unclear whether the results in the rhythm control group would have been better if sinus rhythm had been maintained in a higher proportion of patients.22
Sinus rhythm is difficult to achieve and maintain in patients with rheumatic mitral valvular stenosis and AF, but would be more easily achieved with reduction of LA pressure after successful PMV.6 Our study confirms previous uncontrolled studies that indicated electrical cardioversion plus low‐dose amiodarone is safe and effective in restoring and maintaining sinus rhythm in patients with AF after PMV.1,6,24 Our results show that 100% of patients were successfully cardioverted and 96% of patients could be maintained in sinus rhythm on continued low‐dose amiodarone treatment over the observation period. This percentage was much higher than that in non‐valvular fibrillation (56% in the PIAF (Pharmacological Intervention in Atrial Fibrillation) study).10 The reasons for the high efficacy observed in patients after PMV are not entirely clear. Perhaps reduction of LA pressure after successful PMV induces a more easily achievable and maintainable sinus rhythm.6 Among patients with shorter AF duration and a smaller LA, post‐PMV LA pressure is lower; thus, sinus rhythm is easier to achieve and maintain.1 In our study, LA dimension was significantly reduced at one year compared with baseline; this indicated that reductions of LA dimension help to maintain sinus rhythm. On the other hand, only 2% patients in the rate control group were in sinus rhythm at one year. This percentage was lower than that in non‐valvular fibrillation (10% in PIAF).10 This result suggests that chronic AF after PMV is not associated with spontaneous reversion to sinus rhythm and measures should be taken to restore sinus rhythm.3 Low‐dose amiodarone was well tolerated in this study. Serious toxicity necessitating discontinuation of treatment is infrequent. The actual crossover rate from rhythm control to rate control was 3.3% at one year, and patients crossed over primarily because of drug intolerance.
Our data suggest that results in the rhythm control group would have been better if sinus rhythm could have been maintained in a higher proportion of patients. One notable advantage is that symptoms are more easily improved by the rhythm control strategy than by the rate control strategy. Sinus rhythm was easily achieved after successful PMV, thus allowing for good management of symptoms. Also, sustained sinus rhythm is associated with an improved QOL and improved exercise performance.25 The study patients randomly assigned to rhythm control had a better exercise tolerance than did patients who underwent rate control. This finding may be due to improvement in haemodynamic function after restoration of sinus rhythm.26,27 The improvement in AF‐related symptoms and exercise tolerance translated to an overall improved QOL when both treatment groups were compared. These findings are in agreement with data from CRRAFT (Control of Rate versus Rhythm in rheumatic Atrial Fibrillation),4 which assessed patients with chronic rheumatic AF, and 72.9% of patients had undergone valvular interventions.
Successful PMV results in significant long‐term reduction in LA size in most patients, but normalisation of LA size is unusual.28 The outcomes of our trial showed that the rhythm control strategy resulted in a partial reversal of LA remodelling, which has been found in other studies.18,28 Results of these studies may apply as powerfully to young patients, who have a longer‐term risk for developing the potential complications of structural remodelling.
During the follow‐up period, neither embolic nor transitory ischaemic neurological events occurred, but we must mention that we cannot exclude the possibility that our sample size was too small or our time period too short to detect significant differences in stroke rate between the two treatment strategies. The patients were also young (aged 36.9 (SD 5.4) years). Permanent anticoagulation may still be needed, although PMV may help prevent systemic embolism in patients with mitral stenosis.29 This issue requires further studies. Most of our patients were taking aspirin at baseline, which reflects common clinical practice in mainland China.
The strategy of rhythm control was associated with similar numbers of hospital admissions for our patients but with longer duration of hospital stay. This meant higher medical costs. The higher cost for rhythm control confirms our intuition that this strategy often requires hospitalisation for antiarrhythmic drug loading, cardioversion or acute rate control for recurrent rapid AF.30 But clinical outcomes are better with rhythm control than with rate control.
Is there still a place for rate control? It should be noted that patients with AF duration > 12 months or post‐PMV LA size > 45 mm were excluded from our study. We came to this decision because of the high risk of AF recurrence in such patients. Rhythm control is therefore not necessary before rate control in those patients. In particular, rate control may be an acceptable alternative to rhythm control in patients who have recurrent AF after initial rhythm control therapy. The treatment for AF should be individualised.
We compared rate control and rhythm control in patients with AF after PMV in a Chinese population composed of mostly young patients (mean age 36.9 (SD 5.4) years, range 25–56 years). Such findings are of interest in countries in which the incidence of rheumatic fever remains relatively high. The problem is different in the United States and Europe, where patients with mitral stenosis and AF are older and more commonly have advanced heart disease.31,32 But our findings may be an important aid to patient selection for rhythm control after PMV.
Patients with AF after PMV often need treatment for decades, even longer. The risk of relapse to AF or side effects with amiodarone rises after 18 months.33 Furthermore, the use of amiodarone in young patients with normal ventricular function cannot be justified.34 Prevention of AF has not yet been proved to prolong survival. Whether rhythm control therapy provides long‐term benefits on prognosis therefore awaits further investigation.
In this study, the actual strategies themselves were not blinded, which created a potential bias. It is logistically and technically difficult, if not impossible, to blind therapies in this setting. Also, our sample was relatively small. This could have affected our results. Despite the limited number of patients, we detected significant differences between the two treatment strategies.
In conclusion, in patients with AF after PMV, AF duration ⩽ 12 months and post‐PMV LA size ⩽ 45 mm, sinus rhythm was easy and safe to achieve and maintain. Moreover, patients benefited from restoration and maintenance of sinus rhythm in terms of improved AF‐related symptoms, 6 min walk tests and QOL, and of LA size normalisation. Rhythm control should therefore be considered as the preferred initial therapy for this group of patients. The optimal strategy to treat AF after PMV should be individualised.
Abbreviations
AF - atrial fibrillation
CRRAFT - Control of Rate versus Rhythm in rheumatic Atrial Fibrillation
LA - left atrial
MOS - Medical Outcomes Study
PIAF - Pharmacological Intervention in Atrial Fibrillation
PMV - percutaneous mitral balloon valvotomy
QOL - quality of life
SF‐36 - 36‐Item Short‐Form Health Survey
Footnotes
Competing interest: None declared.
References
- 1.Jiang H, Huang C X, Li G S.et al Cardioversion treatment in patients with atrial fibrillation and rheumatic mitral stenosis after percutaneous balloon mitral valvuloplasty. Zhonghua Xin Xue Guan Bing Za Zhi 199725284–286. [Google Scholar]
- 2.Qi S H, Jiang H, Wang X H.et al Clinical effect of cardioversion to atrial fibrillation in patients with rheumatic mitral stenosis after percutaneous balloon mitral valvuloplasty. Lin Chuang Xin Dian Xue Za Zhi 20011031 [Google Scholar]
- 3.Langerveld J, van Hemel N M, Kelder J C.et al Long‐term follow‐up of cardiac rhythm after percutaneous mitral balloon valvotomy: does atrial fibrillation persist? Europace 2003547–53. [DOI] [PubMed] [Google Scholar]
- 4.Vora A, Karnad D, Goyal V.et al Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J 200456110–116. [PubMed] [Google Scholar]
- 5.Lumer G B, Roy D, Talajic M.et al Amiodarone reduces procedures and costs related to atrial fibrillation in a controlled clinical trial. Eur Heart J 2002231050–1056. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor A, Kumar S, Singh R K.et al Management of persistent atrial fibrillation following balloon mitral valvotomy: safety and efficacy of low‐dose amiodarone. J Heart Valve Dis 200211802–809. [PubMed] [Google Scholar]
- 7.Inoue K, Owaki T, Nakamura T.et al Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg 198487394–402. [PubMed] [Google Scholar]
- 8.Rawles J M. What is meant by a “controlled” ventricular rate in atrial fibrillation? Br Heart J 199063157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng T O, Chen C R. Late results of percutaneous balloon mitral valvuloplasty: the Chinese experience. Circulation 2000102E18. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser S H, Kuck K H, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet 20003561789–1794. [DOI] [PubMed] [Google Scholar]
- 11.McHorney C A, Ware J E, Jr, Lu J F.et al The MOS 36‐item Short‐Form Health Survey (SF‐36). III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 19943240–66. [DOI] [PubMed] [Google Scholar]
- 12.McHorney C A, Ware J E, Jr, Raczek A E. The MOS 36‐Item Short‐Form Health Survey (SF‐36). II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 199331247–263. [DOI] [PubMed] [Google Scholar]
- 13.Ware J E, Jr, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 14.Ren X S, Amick B, 3rd, Zhou L.et al ranslation and psychometric evaluation of a Chinese version of the SF‐36 Health Survey in the United States. J Clin Epidemiol 1998511129–1138. [DOI] [PubMed] [Google Scholar]
- 15.Lam C L, Gandek B, Ren X S.et al Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF‐36 Health Survey. J Clin Epidemiol 1998511139–1147. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Wang H M, Shen Y. Chinese SF‐36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health 200357259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundstrom T, Ryden L. Ventricular rate control and exercise performance in chronic atrial fibrillation: effects of diltiazem and verapamil. J Am Coll Cardiol 19901686–90. [DOI] [PubMed] [Google Scholar]
- 18.Opolski G, Torbicki A, Kosior D A.et al Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest 2004126476–486. [DOI] [PubMed] [Google Scholar]
- 19.Hagens V E, Crijns H J, Van Veldhuisen D J.et al Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 20051491106–1111. [DOI] [PubMed] [Google Scholar]
- 20.Wyse D G, Waldo A L, DiMarco J P.et al A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 20023471825–1833. [DOI] [PubMed] [Google Scholar]
- 21.Van Gelder I C, Hagens V E, Bosker H A.et al A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 20023471834–1840. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson J, Miketic S, Windeler J.et al Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003411690–1696. [DOI] [PubMed] [Google Scholar]
- 23.Boos C J, More R S, Carlsson J. Persistent atrial fibrillation: rate control or rhythm control. Rate control is not inferior to rhythm control. BMJ 20033261411–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavthale S S, Fulwani M C, Vajifdar B U.et al Atrial fibrillation: how effectively can sinus rhythm be restored and maintained after balloon mitral valvotomy? Indian Heart J 200052568–573. [PubMed] [Google Scholar]
- 25.Singh B N, Singh S N, Reda D J.et al Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 20053521861–1872. [DOI] [PubMed] [Google Scholar]
- 26.Gosselink A T, Crijns H J, van den Berg M P.et al Functional capacity before and after cardioversion of atrial fibrillation: a controlled study. Br Heart J 199472161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueshima K, Myers J, Morris C K.et al The effect of cardioversion on exercise capacity in patients with atrial fibrillation. Am Heart J 19931261021–1024. [DOI] [PubMed] [Google Scholar]
- 28.Stefadouros M A, Fawzy M E, Malik S.et al The long‐term effect of successful mitral balloon valvotomy on left atrial size. J Heart Valve Dis 19998543–550. [PubMed] [Google Scholar]
- 29.Chiang C W, Lo S K, Ko Y S.et al Predictors of systemic embolism in patients with mitral stenosis: a prospective study. Ann Intern Med 1998128885–889. [DOI] [PubMed] [Google Scholar]
- 30.Bahnson T D, Grant A O. To be or not to be in normal sinus rhythm: what do we really know? Ann Intern Med 2004141727–729. [DOI] [PubMed] [Google Scholar]
- 31.Shaw T R, Sutaria N, Prendergast B. Clinical and haemodynamic profiles of young, middle aged, and elderly patients with mitral stenosis undergoing mitral balloon valvotomy. Heart 2003891430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leon M N, Harrell L C, Simosa H F.et al Mitral balloon valvotomy for patients with mitral stenosis in atrial fibrillation: immediate and long‐term results. J Am Coll Cardiol 1999341145–1152. [DOI] [PubMed] [Google Scholar]
- 33.Kochiadakis G E, Igoumenidis N E, Hamilos M I.et al Long‐term maintenance of normal sinus rhythm in patients with current symptomatic atrial fibrillation: amiodarone vs propafenone, both in low doses. Chest 2004125377–383. [DOI] [PubMed] [Google Scholar]
- 34.Juneja R, Gulati G. Control of rate versus rhythm in rheumatic atrial fibrillation. Indian Heart J. 2004;56: 356–7; author reply 357, [PubMed]