Abstract
Objective
To examine the effects of intracoronary PhotoPoint photodynamic therapy (PDT) with a new photosensitiser, MV0611, in the overstretch balloon and stent porcine models of restenosis.
Methods
28 pigs were injected with 3 mg/kg of MV0611 systemically 4 h before the procedure. Animals were divided into either the balloon overstretch injury (BI) group (n = 19) or the stented group (n = 9). After BI, a centred delivery catheter was positioned in the artery to cover the injured area, and light (532 nm, 125 J/cm2) was applied to activate the drug (n = 10). Control arteries (n = 9) were not activated by light. In the stented group, the drug was light activated before stent deployment. Serial sections of vessels were processed 14 days after treatment in the BI group and 30 days after treatment in the stented group for histomorphometric or immunohistochemical analysis.
Results
Intracoronary PDT significantly reduced intimal thickness in both BI and stented arteries (about 65%: 0.22 (SEM 0.05) mm v 0.62 (0.05) mm, p < 0.01; and about 26%: 0.40 (0.04) mm v 0.54 (0.04) mm, p < 0.01, respectively). PDT increased luminal area by ⩽ 60% and 50% within BI and stented arteries (3.43 (0.27) mm2v 5.51 (0.52) mm2, p < 0.05; 4.0 (0.02) mm2v 6.0 (0.16) mm2, p < 0.01), respectively. Complete re‐endothelialisation was observed by immunohistochemical and gross histological analyses in all PDT and control arteries. There were no cases of aneurysm formation or thrombosis.
Conclusion
Intracoronary PhotoPoint PDT with MV0611 reduces intimal proliferation without suppressing re‐endothelialisation in a porcine model of restenosis.
Restenosis is a considerable limitation after percutaneous coronary intervention with balloon angioplasty.1 Coronary stenting reduces restenosis rates and improves clinical outcomes.2 Nevertheless, in‐stent restenosis, caused by growth of neointima and extracellular matrix in response to vessel wall injury, remains a major clinical burden with high rates of recurrence and morbidity.3,4 Despite the use of numerous pharmacological agents and interventional devices, restenosis remains a major limitation of percutaneous coronary intervention.
Intracoronary ionising radiation (brachytherapy), with β or γ sources, is an effective treatment shown in randomised clinical trials to reduce the recurrence of in‐stent restenosis.5,6 Radiation therapy, however, has been associated with logistical issues and clinical events such as thrombosis and late reocclusion due to incomplete vessel healing caused by delayed re‐endothelialisation.7,8 Recently, stents coated with sirolimus or paclitaxel have been shown to dramatically prevent in‐stent restenosis in selected patients.9,10 Many other drug–stent combinations, however, have failed to show long‐term benefit. Furthermore, this approach has generated new concerns such as potential vascular complications, chronic inflammation and prohibitive treatment costs for multivessel disease.11,12
Photodynamic therapy (PDT) is clinically approved for the treatment of various proliferative disease states in ophthalmology, oncology and dermatology.13,14,15 More recently, PDT has emerged as a promising treatment of vascular lesions associated with injury and atherosclerotic plaque.16,17 Intravascular PhotoPoint PDT (Miravant Medical Technologies, Santa Barbara, California, USA) is a newly developed proprietary catheter‐based system for photo‐selective delivery of a non‐ionising, non‐thermal energy source of visible light to activate a photosensitiser localised to the artery wall. The novel photosensitiser molecule gallium chloride mesoporphyrin dimethyl ester (MV0611) was discovered through rational drug screening for use in cardiovascular applications.18 The interaction between MV0611 and intravascular light generates reactive oxygen species, which inhibit smooth muscle cell (SMC) proliferation,19 the cell type that is responsible for intimal hyperplasia.20 Unlike other antirestenotic interventions, however, PDT does not appear to delay endothelial regeneration within treated areas.21,22 In the present study, we examined the feasibility and efficacy of a novel catheter‐based intravascular PDT system and MV0611 for the prevention of restenosis in balloon overstretch‐injured or stented porcine coronary arteries.
METHODS
The Institutional Animal Care and Use Committee of the MedStar Research Institute, Washington Hospital Center, approved the protocol. Experiments were conducted according to established guidelines for the humane use of laboratory animals. Male and female domestic juvenile swine (Thomas D Morris, Inc, Reisterstown, Maryland, USA) aged 3 months and weighing 31–38 kg were used in these studies.
Pharmacokinetics of MV0611
An initial group of pigs was sedated with a combination of ketamine (25 mg/kg; Fort Dodge Animal Health, Overland Park, Kansas, USA) and xylazine (2 mg/kg; Vedco, St Joseph, Missouri, USA) by intramuscular injection. The photosensitiser drug MV0611 (3 mg/kg) was prepared in a liposomal formulation of soybean phospholipid and was injected into the ear vein of 12 animals. Femoral arterial blood was sampled at baseline, 30 min after drug administration, and at death. The blood samples were centrifuged and the plasma was stored in microtubes at −70°C. Animals were killed at 1 (n = 3), 4 (n = 3) and 8 (n = 3) hours after MV0611 injection. Control animals (n = 3) were injected with soybean phospholipid drug vehicle (same volume/animal weight) and were killed 4 h later. Traverse sections (about 3 mm) of coronary arteries from all animals were rapidly excised and rinsed with 0.9% saline to remove all traces of blood. Arterial sections in tissue blocks were snap frozen with precooled isopentane and stored at −70°C for later fluorescence detection. Other artery sections were cleaned of surrounding tissue and stored at −70°C in microtubes for subsequent analysis by high performance liquid chromatography. Reverse phase high performance liquid chromatography analysis with fluorescence detection (excitation λ = 396 nm, emission λ = 569 nm) was used to determine concentrations of MV0611 extracted from plasma and coronary arteries. Four sections of frozen coronary artery tissue were sliced (about 10 µm) on a cryotome, and fluorescence distribution of MV0611 was captured on a camera linked to the fluorescence microscope. Background autofluorescence was determined in vehicle control artery sections.
Animal preparation
All animals were orally treated daily with 325 mg of aspirin (Bayer, Morristown, New Jersey, USA) and 75 mg of clopidogrel bisulfate (Plavix; Bristol Meyers Squibb/Sanofi, New York, New York, USA) beginning three days before the procedures and continued until death. To prevent coronary vasospasm, pigs also received 30 mg oral administration of nifedipine (Procardia; Pfizer, Jersey City, New Jersey, USA) one day before procedures. On the day of the procedures, the pigs were premedicated with a combination of ketamine (25 mg/kg; Fort Dodge Animal Health), xylazine (2 mg/kg; Vedco) and acepromazine (0.2 mg/kg; Phoenix Pharmaceuticals, St Joseph, Missouri, USA) by intramuscular injection. Endotracheal intubation was performed and the animals were ventilated with oxygen (2 l/min) and room air (1.5 l/min). Anaesthesia was continued with inhaled 0.9% isoflurane (Abbott Laboratories, Chicago, Illinois, USA) and was confirmed by the absence of a limb withdrawal reflex. After placement of an 8 French introducer sheath in the left carotid artery through a midline neck incision, each animal received an intra‐arterial dose of heparin (300 U/kg; American Pharmaceutical Partners, Los Angeles, California, USA). An 8 French J‐tipped guiding catheter (Cordis Corp, Miami, Florida, USA) was then positioned in the coronary ostium under fluoroscopic guidance. Vital signs and cardiovascular parameters such as ECG, heart rate and blood pressure were routinely monitored throughout all procedures.
Animals were divided into two groups. One group of animals received a balloon overstretch injury (BI) and the other group received a stent. Four hours after photosensitiser drug administration, a baseline angiogram was obtained and the mid‐portion of segments of the left anterior descending, right coronary or left circumflex artery were identified. The drug was photoactivated after injury in the BI group (n = 19) and before stent deployment in the stented group (n = 9).
Balloon overstretch injury
BI was obtained with an angioplasty balloon (1.25 balloon to artery ratio) positioned in the mid‐portion of segments of the left anterior descending, right coronary or left circumflex artery and inflated to 10 atm three times for 30 s. After completion of the injury, the angioplasty balloon was withdrawn, angiography was performed to assess the vessel patency and degree of injury, and vessels were randomly assigned to PDT (n = 10) or control (n = 9).
PhotoPoint PDT procedure
The photosensitiser drug MV0611 (3 mg/kg intravenously; Miravant Pharmaceuticals Inc) was prepared in a liposomal formulation of soybean phospholipid. MV0611 was systemically administered as a slow bolus injection into the ear vein. Four hours after MV0611 injection, a light diffuser (25–30 mm length) centred within a proprietary modified percutaneous transluminal coronary angioplasty‐type balloon (3.0–3.5 mm × 25–30 mm; Miravant) was inserted into the coronary artery over a flexible 0.014 inch guidewire (Hi‐Torque Ironman, Guidant, Santa Clara, California, USA). The diffuser was connected to a 2.5 W diode‐pumped solid‐state laser (Melles Griot, Carlsbad, California, USA), which delivered light in the visible spectrum at 532 nm to activate MV0611, localised to the arterial wall. The total light fluence of 125 J/cm2 at an irradiance of 250 mW/cm2 was delivered in 10–12 equal fractionations. Each light fractionation comprised a low‐pressure balloon inflation (1–2 atm) for 45 s to help remove blood and provide a uniform treatment field. The inflations were separated by a 30 s deflation period to restore coronary perfusion. After the light activation of MV0611, the light delivery catheter was removed and an angiogram was obtained to confirm lumen patency after PDT. In control animals, the drug was not activated by light. The animals were kept in subdued filtered lighting for 24 h after the procedure to avoid exposure to ambient light and possible photosensitivity.
Stent deployment
After PDT, a bare metal stent (3.0–3.5 × 13 mm, ACS Multilink Tristar; Guidant) premounted on a balloon catheter was implanted with about 30% overstretch with high‐pressure balloon inflation (10–20 atm for 30–60 s) in the PDT (n = 5) or control (n = 4) arteries. Care was taken to deploy the stent about 5 mm within the proximal and distal margins of the PDT treatment zone as verified by fluoroscopy to prevent geographic miss. In the artery, the PDT treatment field was identified by radio‐opaque marker bands located at the proximal and distal ends of the light catheter. A post‐stent angiogram was obtained to confirm lumen patency and the animals were allowed to recover. After the interventions, the animals were given heparin (50 mg subcutaneously), and a 2% glyceryl trinitrate ointment patch was applied to the skin (Nitro‐Bid; E Fougera & Co, Melville, New York, USA).
Follow up and histological evaluation
Fourteen days after PDT in the BI group or 30 days after PDT in the stented group, animals were anesthetised again and an angiogram of the coronary arteries was obtained followed by death. The heart was rapidly excised, pressure perfusion fixed with 10% phosphate buffered formalin for 15 min and stored overnight in the same fixative. In the BI group, the injured segments were excised from the heart. Serial 2–3 mm transverse segments were processed and embedded in paraffin, and a minimum of four serial cross sections (4–5 μm) were made. In the stented group, the stented segments were harvested and embedded in methyl methacrylate. Four serial cross sections (4–5 μm) within the stent body were cut, leaving the stent wires intact. Sections were stained with Verhoeff van Gieson elastin, 4,6‐diamidino‐2‐phenylindole or haematoxylin and eosin. All histological sections were magnified and digitised. An experienced observer blinded to the treatment groups assessed each cross‐sectional specimen for histopathological and histomorphometric analyses.
A mean injury score and morphometric parameters were calculated for each arterial segment. The vessel injury score was determined by the method used by Schwartz et al.23 In brief, the degree of injury at each wire site was assessed as follows: grade 0, internal elastic lamina intact with media compressed; grade 1, internal elastic lamina lacerated with media compressed; grade 2, internal elastic lamina and media lacerated with the external elastic lamina intact; or grade 3, external elastic lamina lacerated. Inflammation was graded as 0, none; 1, scattered inflammatory cells; 2, inflammatory cells encompassing 50% of a strut in at least 25–50% of the circumference of the artery; or 3, inflammatory cells surrounding a strut in at least 25–50% of the circumference of the artery. The vascularisation score is based on the extent of the circumference of the artery involved by new growth of capillaries within a definite lumen. Scores are 1, < 0.25; 2, 0.25–0.5; or 3, > 0.5. The intimal fibrin content was graded as: 1, focal residual fibrin involving any portion of the artery and for moderate fibrin deposition adjacent to the strut involving < 25% of the circumference of the artery; 2, moderate fibrin deposition involving > 25% of the circumference of the artery or heavy deposition of fibrin adjacent to and between stent struts involving < 25% of the circumference of the artery; or 3, heavy deposition of fibrin involving > 25% of the circumference of the artery. The intimal SMC content was scored as: 1, sparse SMC density involving any portion of the artery and for moderate SMC infiltration less than the full thickness of the neointima involving < 25% of the circumference of the artery; 2, moderate SMC infiltration less than the full thickness of the neointima involving > 25% of the circumference of the artery or dense SMC content the full thickness of the neointima involving < 25% of the circumference of the artery; or 3, dense SMC content in the full thickness of the neointima involving > 25% of the circumference of the artery. Adventitial fibrosis was scored as: 1, light dispersed adventitial fibrosis; 2, moderate and thicker adventitial fibrosis; or 3, heavy and dense adventitial fibrosis. The extent refers to the portion of the circumference of the artery involved. The endothelialisation score was defined as the extent of the circumference of the arterial lumen covered by endothelial cells and was scored from 1 to 3 (1, < 25%; 2, 25–75%; 3, > 75%). Positive remodelling is defined as an expansion of the external elastic membrane (vessel) area at the treated site. Values were corrected for degree of injury. Mean values were calculated for each animal from the analysable sections.
Immunohistochemical analysis
Serial cross sections of the BI group arteries were deparaffinised and pretreated with trypsin (0.01% trypsin; Sigma, St Louis, Missouri, USA) at room temperature for 20 min. Endogenous peroxidase was blocked with 1% peroxidase solution. The sections were then incubated with anti‐human factor VIII (Biomeda, Foster City, California, USA) for 1.5 h at 37°C in a humidified chamber. The adherent antibodies were reacted with a universal biotinylated secondary antibody (Shandon–Lippshaw) followed by horseradish peroxidase conjugated to streptavidin (Vector Laboratories, Burlingame, California, USA). Staining was visualised with A DAB Substrate Kit I (Vector Laboratories). Sections were then counterstained with Mayer's haematoxylin. To confirm re‐endothelialisation, some of the sections were also incubated with fluorescein isothiocyanate‐conjugated CD31 (MCA 1746F (platelet endothelial cell adhesion molecule 1); Serotec, Raleigh, North Carolina, USA) or with fluorescein isothiocyanate‐conjugated mouse IgG 1 (MCA 928F; Serotec) as control.
Statistical analyses
All data values are expressed as the mean (SEM) where appropriate. Two means were compared by Student's non‐paired t tests with statistical software (Graph Pad Prism, San Diego, California, USA). In all cases, a probability level of p < 0.05 was considered to be significant compared with control.
RESULTS
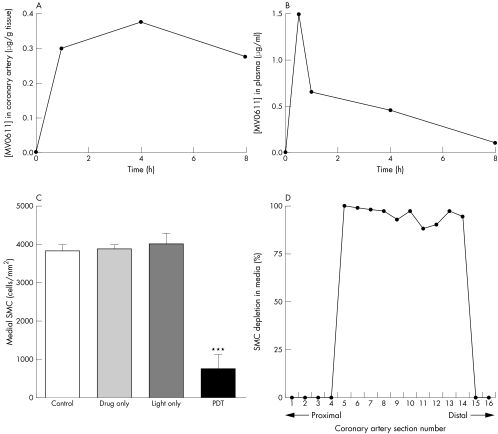
Arterial localisation and distribution of MV0611 peaked in the medial layer 4 h after injection (fig 1A). At 1 h after drug injection, MV0611 fluorescence was localised only to endothelium and adventitia (data not shown), possibly due to relatively higher circulating plasma concentrations (fig 1B). Concentrations of MV0611 also peaked at (0.38 µg/g tissue) 4 h after drug injection (fig 1A). At 8 h after drug injection, MV0611 fluorescence was still present in the media (data not shown) but the arterial drug concentration was lower than that at 4 h. Thus, 4 h was selected for the post‐drug light administration time to maximise PDT in the artery wall. Three days after PDT, medial SMC numbers were reduced to 945 (200) cells/mm2 as compared with 3863 (109) cells/mm2 in control (p < 0.001) (fig 1C). The PDT efficacy was relatively uniform along the length of the treatment field (fig 1D).
Figure 1 MV0611 concentrations and medial smooth muscle cell (SMC) numbers in control and photodynamic therapy (PDT) ‐treated porcine coronary arteries. MV0611 concentrations in (A) coronary artery and (B) plasma at various time points after intravenous injection of 3 mg/kg MV0611. (C) Bar chart showing medial SMC numbers (cells/mm2). ***p < 0.001 compared with any control. (D) Percentage SMC depletion in media along the sectional (∼3 mm apart) length of a coronary artery after PDT.
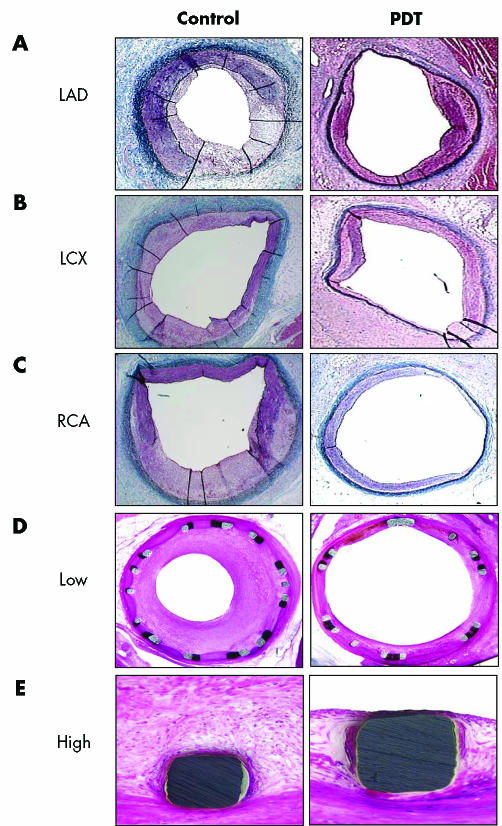
In this study we examined 28 animals in which 28 arteries were treated. Of the 28, 19 arteries had BI (controls, n = 9; PDT, n = 10) and the remaining nine were stented (controls, n = 4; PDT, n = 5). The procedural, BI and stent deployment characteristics were similar in the control and PDT groups. All the arteries were patent at the time of death and there were no cases of aneurysm formation or thrombosis. Figure 2 shows representative photomicrographs of sections of BI and stented arteries.
Figure 2 Representative histological appearances of balloon overstretch‐injured (BI) and stented arteries subjected to photodynamic therapy (PDT) and control. Porcine coronary arteries were subjected to BI followed by PDT. (A) Left anterior descending artery (LAD); (B) left circumflex artery (LCX); (C) right coronary artery (RCA). Animals were killed 14 days later. Porcine coronary arteries were subjected to stenting followed by PDT. Animals were killed 30 days later. (D) Low magnification 20×; (E) high magnification 200×. Sections of perfusion‐fixed arteries were stained with haematoxylin and eosin.
Neointimal proliferation
At 14 days after BI, the mean intimal thickness in the PDT group was reduced by about 65% (0.22 (0.05) mm v 0.62 (0.05), p < 0.01) and the reduction in intimal area was similar (about 57%, 0.46 (0.11) mm2v 1.07 (0.15), p < 0.05). Lumen area in the PDT group was significantly larger than in the control group (3.43 (0.27) mm2v 5.51 (0.52) mm2, p < 0.05) (table 1). At 30 days after stenting, the percentage area occlusion in the PDT group was significantly reduced compared with controls (39 (3)% v 55 (4)%, p < 0.01) (table 1, fig 2D.) Mean intimal thickness was also reduced in stented arteries treated with PDT compared with controls (0.4 (0.04) mm v 0.54 (0.04), p < 0.01) (table 1). In addition, the luminal area was increased with PDT, from 3.6 (0.3) mm2 in control to 5.8 (0.3) mm2 (p < 0.01; fig 2E). Similarly, the lumen diameter and lumen to artery ratio were greater in stented arteries treated with PDT than in controls (p < 0.01; table 1).
Table 1 Histomorphometric measurements in porcine coronary arteries in controls and after photodynamic therapy (PDT).
| Control | PDT | p Value | |
|---|---|---|---|
| 14 days after balloon injury | n = 10 | n = 9 | |
| Intimal area (mm2) | 1.07 (0.15) | 0.46 (0.11)* | <0.05 |
| Fracture length (mm) | 1.74 (0.18) | 2.14 (0.21) | NS |
| Intimal area:fracture length (mm) | 0.62 (0.07) | 0.24 (0.06)** | <0.01 |
| Maximum intimal thickness (mm) | 0.62 (0.05) | 0.22 (0.05)** | <0.01 |
| Lumen area (mm2) | 3.43 (0.27) | 5.51 (0.52)* | <0.05 |
| Vessel area (mm2) | 6.59 (0.47) | 7.74 (0.52) | NS |
| Adventitial area (mm2) | 0.96 (0.15) | 0.71 (0.1) | NS |
| 30 days after stenting | n = 5 | n = 4 | |
| Baseline vessel diameter (mm) | 2.65 (0.25) | 2.92 (0.24) | NS |
| Artery area (mm2) | 9.84 (0.37) | 11.09 (0.24) | NS |
| Stent area (mm2) | 8.13 (0.35) | 9.65 (0.19) | NS |
| Medial area (mm2) | 0.76 (0.21) | 0.63 (0.18) | NS |
| Artery diameter (mm) | 3.53 (0.07) | 3.76 (0.04) | NS |
| Stent diameter (mm) | 3.21 (0.08) | 3.50 (0.04) | NS |
| Lumen diameter (mm) | 2.12 (0.09) | 2.70 (0.08)** | <0.01 |
| Lumen area (mm2) | 4 (0.02) | 6 (0.16)** | <0.01 |
| Lumen:artery ratio | 0.37 (0.03) | 0.53 (0.03)** | <0.01 |
| Area of occlusion (%) | 55 (4) | 39 (3)** | <0.01 |
| Injury score | 2.06 (0.14) | 1.80 (0.17) | NS |
| Intimal thickness (mm) | 0.54 (0.04) | 0.4 (0.04)** | <0.01 |
| Intimal vasculature score | 0.63 (0.24) | 0.75 (0.20) | NS |
| Intimal fibrin score | 0.81 (0.10) | 1.10 (0.10) | NS |
| Intimal smooth muscle score | 1.50 (0.39) | 1.60 (0.31) | NS |
| Endothelial score | 3 | 3 | NS |
| Inflammation score | 0.75 (0.11) | 0.55 (0.11) | NS |
| Adventitial fibrosis score | 0.50 (0.13) | 1.00 (0.22) | NS |
*p<0.05; **p<0.01.
Re‐endothelialisation
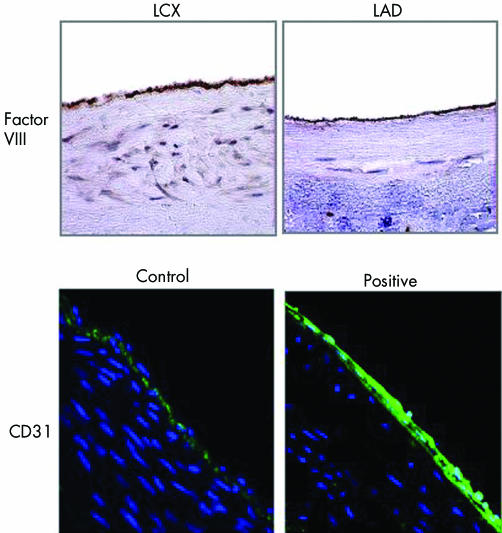
Results from immunohistochemical analysis of the BI group (fig 3) indicated that in the PDT group re‐endothelialisation was complete. A maximum re‐endothelialisation score of 3+ was observed by gross histological analysis in all stented arteries treated with PDT and control, indicating > 75% re‐endothelialisation in treated sections (table 1). Histopathological analysis of stented sections treated with PDT showed that the stent struts were covered with a thin neointima lined with endothelium together with the presence of some intimal fibrin. Control stented sections had more intima and extracellular matrix, whereas fibrinoid deposits were observed adjacent to struts (fig 2D). A detailed summary of the histopathological analysis and histomorphometry indicated that overall scores for stent injury, inflammation and intimal fibrin were similar in the PhotoPoint PDT and the control arteries at 30 days (table 1). There was no excess of thrombosis or inflammation on the PDT‐treated arteries when compared with control. There was mild positive remodelling in the PDT‐treated arteries as compared with the control arteries.
Figure 3 Re‐endothelialisation in balloon overstretch‐injured porcine arteries after photodynamic therapy (PDT). Porcine coronary arteries (left anterior descending (LAD) and left circumflex (LCX)) were subjected to balloon overstretch injury followed by PDT. Animals were killed 14 days later. Sections of perfusion‐fixed arteries were stained for factor VIII or CD31.
DISCUSSION
This is the first study to report the inhibition of neointimal formation after BI or stent injury in porcine coronary arteries by means of a novel photosensitiser and centred light delivery catheter system. This reduction of neointima formation was also associated with positive remodelling of the BI arteries and early re‐endothelialisation of the stented arteries, without any evidence of thrombosis or inflammation. Earlier PDT studies in BI coronary arteries and stented femoral arteries of swine have used deeper penetrating red light wavelengths to activate photosensitiser drugs, which were originally indicated for oncological applications.24,25,26 To lower the risk of complications such as perforation and fistula due to excessive propagation of light that have been described with longer wavelengths (red or infrared),25 in our experiments we used less penetrating lower wavelength green light to activate MV0611. The use of green light appeared to restrict the PDT effects of MV0611 to the depth of the arterial wall. In the present study, no major vascular complications were observed in the arteries treated with PDT and all vessels were patent at follow up. In addition, there was no evidence of cellular damage to the underlying media, adventitia or periadventitial area. Arteries treated with intracoronary PDT did not display abnormal healing responses as evidenced by the near complete re‐endothelialisation and an absence of thrombosis or aneurysm formation. Previous studies of porcine stented arteries showed that most of the luminal surface was covered by endothelium after seven days, whereas at four weeks neointima peaked and healing was completed.27 In contrast to intravascular radiation that was associated with lack of healing and delayed re‐endothelialisation contributing to adverse late thrombosis and reocclusion,7,8 arteries treated with PDT have been observed to have medial cell repopulation and enhanced re‐endothelialisation.21,22
The pathophysiological process of restenosis consists of local injury to SMC, removal of endothelium, thrombus formation, inflammation and growth factor release leading to proliferation and migration of SMCs or myofibroblasts.27 We have previously shown that the mechanism of effect of PDT with MV0611 is the depletion of medial SMCs and adventitial myofibroblasts at acute time points when these cells are known to proliferate in response to arterial injury.19 Recently we observed that PDT reduces macrophages by apoptosis. The major cause of cell eradication after vascular PDT has been shown to be apoptosis, without weakening of the vessel wall integrity.28,29 The acute effects of PDT correlated with a chronic reduction of neointimal growth in BI porcine coronary arteries and were similar in magnitude to results described with brachytherapy.7 Chung et al3 have shown that extracellular matrix accumulation, rather than cell proliferation, contributes to the later stages of in‐stent restenosis. The abundant matrix may be another mechanistic target for PDT, as PDT has been shown to inactivate cytokines and growth factors derived from extracellular matrix.21
The possible mechanism by which PDT induces apoptosis is by generation of reactive oxygen species. Evidence is mounting that many agents that induce apoptosis act either as oxidants or as stimulators of oxidative metabolism.30,31 Reactive oxygen species serve as important signal transduction molecules in apoptosis induced by ultraviolet light,32 ionising radiation33 and anthracyclines.34 Chen et al35 have shown that apoptosis of vascular cells induced by PDT with motexafin lutetium is mediated by reactive oxygen species; this apoptotic effect may be enhanced by depletion of intracellular antioxidants and attenuated by addition of antioxidants.
To date, the clinical use of endovascular PDT has been limited to small, non‐randomised, non‐placebo‐controlled trials, which have assessed red light activation of 5‐aminolaevulinic acid at 630 nm or motexafin lutetium (Antrin) at 730 nm.36,37,38,39 The largest cardiovascular clinical trial experience with PDT has been with Antrin. Phototherapy with Antrin was assessed in a dose‐ranging phase I study of 80 patients with lesions in native coronary arteries undergoing stent implantation.39 Although Antrin is well tolerated at lower doses (⩽ 2 mg/kg), a significant number of patients had infusion‐related events such as paraesthesia.39 The use of deep penetrating red light in peripheral vessels may be better tolerated than in the vasculature of the heart, where endovascular PDT with 5‐aminolaevulinic acid at 630 nm has shown evidence of myocardial scarring in porcine coronary arteries.40 Thus, less penetrating green light such as the one tested in our experiments would potentially have a better safety profile.
Catheter‐based intracoronary PDT with MV0611 has some practical advantages. Firstly, MV0611 can be administered intravenously < 4 h before percutaneous interventions. Secondly, the drug is activated at a minimally penetrating wavelength of 532 nm with a centring light delivery balloon catheter. The low inflation balloon removes blood from the green light field allowing a more uniform and photo‐selective PDT only to the depth of the arterial wall.
The results of the current study support the strategy of using intravascular PDT with a systemic photosensitiser as an adjunct for percutaneous coronary intervention with balloon angioplasty or stenting for the prevention of restenosis. On the basis of the preclinical results presented here, it is possible that this strategy can be an alternative to other methods that are already proved to prevent restenosis, such as drug‐eluting stents and intravascular brachytherapy. Furthermore, intracoronary PDT may reduce the need for stenting altogether, as it has been shown to prevent negative remodelling while inhibiting restenosis after balloon angioplasty injury. In conclusion, intracoronary PDT inhibited vascular neointima formation without impairing endothelial regeneration in porcine BI and stent injury models of restenosis.
Study limitations
Although we have shown that intracoronary PhotoPoint PDT with MV0611 reduces intimal proliferation in both balloon‐injured and stented porcine coronary arteries, unlike patients whose arteries are diseased, the arteries of the animals we used showed no signs of disease.
Abbreviations
BI - balloon overstretch injury
PDT - photodynamic therapy
SMC - smooth muscle cell
Footnotes
Miravant Medical Technologies supported this study. Advanced Cardiovascular Systems, Inc, a subsidiary of Guidant Corporation, supplied stents for this study. PhotoPoint is a trade name of Miravant Medical Technologies, Santa Barbara, California, USA.
Competing interests: None declared.
References
- 1.Miller J M, Ohman E M, Moliterno D J.et al Restenosis: the clinical issues. In: Topol EJ, ed. Textbook of interventional cardiology. Philadelphia: Saunders, 1999379–414.
- 2.Serruys P W, de Jaegere P, Kiemeneij F.et al A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994331489–495. [DOI] [PubMed] [Google Scholar]
- 3.Chung I M, Gold H K, Schwartz S M.et al Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol 2002402072–2081. [DOI] [PubMed] [Google Scholar]
- 4.Kastrati A, Schomig A, Elezi S.et al Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol 1997301428–1436. [DOI] [PubMed] [Google Scholar]
- 5.Leon M B, Teirstein P S, Moses J W.et al Localized intracoronary gamma‐radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med 2001344250–256. [DOI] [PubMed] [Google Scholar]
- 6.Waksman R, Ajani A E, White R L.et al Two‐year follow‐up after beta and gamma intracoronary radiation therapy for patients with diffuse in‐stent restenosis. Am J Cardiol 200188425–428. [DOI] [PubMed] [Google Scholar]
- 7.Salame M, Verheye S, Mulkey S P.et al Effects of endovascular irradiation on platelet recruitment at site of balloon angioplasty. Circulation 20001011087–1090. [DOI] [PubMed] [Google Scholar]
- 8.Cheneau E, John M C, Fournadjiev J.et al Time course of stent endothelialization after intravascular radiation therapy in rabbit iliac arteries. Circulation 20031072153–2158. [DOI] [PubMed] [Google Scholar]
- 9.Sousa J E, Costa M A, Abizaid A.et al Four‐year angiographic and intravascular ultrasound follow‐up of patients treated with sirolimus‐eluting stents. Circulation 20051112326–2329. [DOI] [PubMed] [Google Scholar]
- 10.Grube E, Silber S, Hauptmann K E.et al Two‐year‐plus follow‐up of a paclitaxel‐eluting stent in de novo coronary narrowings (TAXUS I). Am J Cardiol 20059679–82. [DOI] [PubMed] [Google Scholar]
- 11.Gunn J, Morton A C, Wales C.et al Drug eluting stents: maximising benefit and minimising cost. Heart 200389127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virmani R, Farb A, Kolodgie F D. Histopathologic alterations after endovascular radiation and antiproliferative stents: similarities and differences. Herz 2002271–6. [DOI] [PubMed] [Google Scholar]
- 13.Spaide R F, Sorenson J, Maranan L. Combined photodynamic therapy and intra vitreal triamcinolone for nonsubfoveal choroidal neovascularization. Retina 200525685–690. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi S, Tsuda H, Takemori M.et al Photodynamic therapy for cervical intraepithelial neoplasia. Oncology 200569110–116. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert D J. Treatment of actinic keratoses with sequential combination of 5‐fluorouracil and photodynamic therapy. J Drugs Dermatol 20054161–163. [PubMed] [Google Scholar]
- 16.Mansfield R, Bown S, McEwan J. Photodynamic therapy: shedding light on restenosis. Heart 200186612–618.11711449 [Google Scholar]
- 17.Rockson S G, Lorenz D P, Cheong W F.et al Photoangioplasty: an emerging clinical cardiovascular role for photodynamic therapy. Circulation 2000102591–596. [DOI] [PubMed] [Google Scholar]
- 18.Robinson B C, Leitch I M, Greene S.et al Metallotetrapyrrolic photosensitizing agents for use in photodynamic therapy. US patent 6,827,926 2004; filed May 31, 2002, and issued Dec 7, 2004
- 19.Grove R I, Leitch I, Rychnovsky S.et al Current status of photodynamic therapy for the prevention of restenosis. In: Waksman R, ed. Handbook of vascular brachytherapy, 3rd edn. New York: Futura Publishing, 2002339–345.
- 20.Davis M G, Hagen P O. Pathobiology of intimal hyperplasia. Br J Surg 1994811254–1269. [DOI] [PubMed] [Google Scholar]
- 21.Statius van Eps R G, Adili F, Watkins M T.et al Photodynamic therapy of extracellular matrix stimulates endothelial cell growth by inactivation of matrix‐associated transforming growth factor‐beta. Lab Invest 199776257–266. [PubMed] [Google Scholar]
- 22.Adili F, Scholz T, Hille M.et al Photodynamic therapy mediated induction of accelerated re‐endothelialisation following injury to the arterial wall: implications for the prevention of postinterventional restenosis. Eur J Vasc Endovasc Surg 200224166–175. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz R S, Huber K C, Murphy J G.et al Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol . 1992;19267–274. [DOI] [PubMed]
- 24.Grosjean P, Wagnieres G, Fontolliet C.et al Clinical photodynamic therapy for superficial cancer in the oesophagus and the bronchi: 514 nm compared with 630 nm light irradiation after sensitization with Photofrin II. Br J Cancer 1998771989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins M P, Buonaccorsi G A, Mansfield R.et al Reduction in the response to coronary and iliac artery injury with photodynamic therapy using 5‐aminolaevulinic acid. Cardiovasc Res 200045478–485. [DOI] [PubMed] [Google Scholar]
- 26.Valassis G, Pragst I, Adolfs C.et al Local photodynamic therapy reduces tissue hyperplasia after stenting in an experimental restenosis model. Basic Res Cardiol 200297132–136. [DOI] [PubMed] [Google Scholar]
- 27.Edelman E R, Rogers C. Pathobiologic responses to stenting. Am J Cardiol 1998814E–6E. [DOI] [PubMed] [Google Scholar]
- 28.LaMuraglia G M, Schiereck J, Heckenkamp J.et al Photodynamic therapy induces apoptosis in intimal hyperplastic arteries. Am J Pathol 2000157867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant W E, Buonaccorsi G, Speight P M.et al The effect of photodynamic therapy on the mechanical integrity of normal rabbit carotid arteries. Laryngoscope 1995105867–871. [DOI] [PubMed] [Google Scholar]
- 30.Johnson T M, Yu Z X, Ferrans V J.et al Reactive oxygen species are downstream mediators of p53‐dependent apoptosis. Proc Natl Acad Sci U S A 19969311848–11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irani K. Oxidant signaling in vascular cell growth, death, and survival. Circ Res 200087179–183. [DOI] [PubMed] [Google Scholar]
- 32.Devary Y, Rosette C, DiDonato J A.et al NF‐kappa B activation by ultraviolet light not dependent on a nuclear signal. Science 19932611442–1445. [DOI] [PubMed] [Google Scholar]
- 33.Manome Y, Datta R, Taneja N.et al Coinduction of c‐jun gene expression and internucleosomal DNA fragmentation by ionizing radiation. Biochemistry 19933210607–10613. [DOI] [PubMed] [Google Scholar]
- 34.Quillet‐Mary A, Mansat V, Duchayne E.et al Daunorubicin‐induced internucleosomal DNA fragmentation in acute myeloid cell lines. Leukemia 199610417–425. [PubMed] [Google Scholar]
- 35.Chen Z, Woodburn K W, Shi C.et al Photodynamic therapy with motexafin lutetium induces redox‐sensitive apoptosis of vascular cells. Arterioscler Thromb Vasc Biol 200121759–764. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins M P, Buonaccorsi G A, Raphael M.et al Clinical study of adjuvant photodynamic therapy to reduce restenosis following femoral angioplasty. Br J Surg 1999861258–1263. [DOI] [PubMed] [Google Scholar]
- 37.Mansfield R J, Jenkins M P, Pai M L.et al Long‐term safety and efficacy of superficial femoral artery angioplasty with adjuvant photodynamic therapy to prevent restenosis. Br J Surg 2002891538–1539. [DOI] [PubMed] [Google Scholar]
- 38.Rockson S G, Kramer P, Razavi M.et al Photoangioplasty for human peripheral atherosclerosis: results of a phase I trial of photodynamic therapy with motexafin lutetium (Antrin). Circulation 20001022322–2324. [DOI] [PubMed] [Google Scholar]
- 39.Kereiakes D, Syzniszewski A M, Wahr D.et al Phase I drug and light dose‐escalation trial of motexafin lutetium and far red light activation (phototherapy) in subjects with coronary artery disease undergoing percutaneous coronary intervention and stent deployment: procedural and long‐term results. Circulation 20031081310–1315. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins M P, Buonaccorsi G, MacRobert A.et al Intra‐arterial photodynamic therapy using 5‐ALA in a swine model. Eur J Vasc Endovasc Surg 199816284–291. [DOI] [PubMed] [Google Scholar]