Abstract
Objective
To study the role of β1 integrins in left ventricular (LV) remodelling after myocardial infarction (MI).
Methods and results
LV structural and functional alterations were determined in wild‐type (WT) and β1 integrin heterozygous knockout (hKO) mice one month after MI. MI increased β1 integrin expression in both groups; however, the increase was lower in hKO. Infarct size was similar in WT and hKO mice, whereas lung wet weight to dry weight ratio was increased in the hKO‐MI mice (5.17 (SE 0.13) v 4.60 (0.15) in WT‐MI, p < 0.01). LV end systolic and end diastolic diameters were significantly higher and percentage fractional shortening was significantly lower in hKO‐MI. The ratio of peak velocity of early LV filling (E wave) to that of the late LV filling (A wave) and the isovolumic relaxation time (IVRT) were increased in both MI groups but the increase in IVRT was significantly higher in hKO‐MI group than in WT‐MI mice. Langendorff perfusion analysis indicated reduced peak LV developed pressure and increased LV end diastolic pressure in both MI groups. The reduction in peak LV developed pressure (36.7 (2.2) v 53.4 (1.9) mm Hg, p < 0.05) and increase in LV end diastolic pressure was higher in hKO‐MI than in WT‐MI. Increase in fibrosis was not different between the two MI groups. The increase in myocyte circumference was higher in the hKO‐MI group (p < 0.001 v WT‐MI). The number of apoptotic myocytes was significantly higher in hKO‐MI than in WT‐MI mice (p < 0.005) three days after MI. The number of necrotic myocytes was not different between the two MI groups.
Conclusion
β1 integrins are crucial in post‐MI remodelling with effects on LV function, hypertrophy and apoptosis.
Myocardial infarction (MI) is one of the major causes of death in patients with heart failure, due either to left ventricular (LV) enlargement or to systolic dysfunction. Cardiac myocyte loss due to apoptosis is proposed to be a major factor in the pathogenesis of cardiac disease.1 Apoptosis occurs in the heart during MI and heart failure in humans and in animal models.1,2
Integrins, a family of αβ heterodimeric cell surface receptors, link the extracellular matrix proteins and the intracellular cytoskeleton.3,4 Integrins have an important role in the regulation of gene expression, cell proliferation, differentiation, migration, apoptosis and cardiac myocyte hypertrophy.4,5 Cardiac myocytes predominantly express β1 integrins.6 β1 integrins serve as mechanotransducers during normal development and in response to physiological and pathophysiological signals.7 Expression of β1 integrins increases in the heart after MI.8 Disruption of β1 integrin function in murine myocardium, by expression of a chimeric molecule encoding the transmembrane and extracellular domains of the Tac subunit of the interleukin 2 receptor fused to the cytoplasmic domain of β(1A) integrin (Tacβ1A), leads to an increased hypertrophic response with reduced basal contractility and relaxation.9 By using Cre‐LoxP technology to inactivate the β1 integrin gene, Shai et al10 observed that β1 integrin knockout mice were intolerant to pressure overload imposed by seven days of transverse aortic constriction. Evan's blue dye staining indicated disruption of cardiac myocyte membrane integrity in the β1 integrin knockout mice. These studies provide evidence that β1 integrins have an important role in the development of cardiomyopathies. The potential role of β1 integrins in post‐MI remodelling has not yet been studied.
Recently, our laboratory has shown that stimulation of β1 integrins inhibits β adrenergic receptor‐stimulated apoptosis in adult rat ventricular myocytes.11 This study was undertaken to investigate the role of β1 integrins in modulating post‐MI remodelling with respect to physiological function, hypertrophy and apoptosis.
METHODS
Vertebrate animals
All experiments conform to the protocols approved by the Institutional Animal Care and Use Committee. Heterozygous knockout (hKO) mice for β1 integrins and wild‐type (WT) mice were from Jackson Research Laboratory (Bar Harbor, Maine, USA) and are of 129xblack Swiss hybrid background. We are using hKO mice because β1 integrin homozygous mice die of inner cell mass failure and peri‐implantation lethality.12
Age‐matched mice (four months) were subjected to MI by coronary artery ligation as described previously.13 The left anterior descending coronary artery was ligated about 3 mm below the tip of the left auricle. Mice in the sham group underwent the same procedure except for ligation of the left anterior descending artery. Pulmonary fluid accumulation was measured as a ratio of lung wet weight to dry weight.
Echocardiography
Transthoracic two‐dimensional M mode echocardiograms and pulsed wave Doppler spectral tracings were obtained with a Toshiba Aplio 80 Imaging System (Tochigi, Japan) equipped with a 12 MHz linear transducer. Echocardiographic studies were performed before and one month after MI on mice anaesthetised with a mixture of isoflurane 1.5% and oxygen 0.5 l/min. The body temperature was maintained at about 37°C with a heating pad. M mode tracings were used to measure LV wall thickness, end systolic diameter (LVESD) and end diastolic diameter (LVEDD). Percentage fractional shortening (%FS) was calculated as described.14 Doppler tracings of mitral and aortic flow were acquired from the apical four‐chamber view. These tracings were used to measure peak velocity of the early ventricular filling (E wave); peak velocity of the late ventricular filling (A wave); peak E:A ratio; and isovolumic relaxation time (IVRT; measured from the aortic valve closure to the mitral valve opening).
Langendorff preparation
After one month of infarction, Langendorff perfusion was carried out by the method described earlier.13 The heart was retroperfused with an oxygenated, normothermic Krebs–Henseleit (KH) buffer ((in mmol/l) NaCl 118, NaHCO3 25, KCl 4.75, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.9 and glucose 11.9) at a constant perfusion pressure of 70 mm Hg. A small fluid‐filled balloon was placed in the LV and was progressively filled in 5 µl increments to establish LV systolic and diastolic pressure–volume relationships.
Analyses
The hearts were arrested in diastole and perfusion fixed with 10% buffered formalin. Hearts were weighed, cut into three slices (apex, mid‐LV and base) and embedded in paraffin. Masson's trichrome‐stained tissue sections were analysed for morphometry including infarct size and myocyte circumference with Bioquant image analysis software (Nashville, Tennessee, USA). Infarct size was determined in a manner similar to that of Pfeffer et al.15
Tissue lysates were prepared from the non‐infarcted LV area with ice‐cold radioimmunoprecipitation assay buffer (158 mM NaCl, 10 mM Tris HCl, pH 7.2, 1 mM EGTA, 1 mM sodium orthovanadate, 0.1% sodium dodecyl sulphate, 1.0% Triton X‐100, 1% sodium deoxycholate and 1 mM phenylmethylsulphonyl fluoride). Proteins (50 µg) were electrophoresed and analysed with monoclonal anti‐β1 integrin antibodies (1:2500, Transduction Lab, San Jose, California, USA) as described.11 Equal protein loading in each lane was verified with anti‐actin antibodies.
The sections (4 µm thick), rehydrated and quenched with 3% hydrogen peroxide, were blocked with 1% goat serum for 1 h and incubated with polyclonal anti‐β1 integrin antibodies (1:100) (Santa Cruz Biotechnology) for 1 h at 37°C in a humidified chamber. The sections were then incubated with secondary antibody (goat anti‐rabbit IgG–horseradish peroxidase conjugate, Santa Cruz Biotechnology) for 45 min at 37°C. The sections were counterstained with haematoxylin and visualised by a microscope (Nikon, Tokyo, Japan).
Terminal deoxynucleotidyl transferase‐mediated dUTP nick end labelling (TUNEL) was carried out on 4 µm thick sections according to the manufacturer's instructions (Cell death detection assay; Roche, Indianapolis, Indiana, USA) and cardiac myocytes were identified with α sarcomeric actin antibodies (1:50; 5C5 clone; Sigma Chemicals, St Louis, Missouri, USA). Hoechst 33258 (10 µmol/l) staining was used to count the total number of nuclei. Sections were visualised by confocal microscopy (Nikon). The index of apoptosis was calculated as the percentage of apoptotic myocyte nuclei in the total number of nuclei.
Apoptosis in the non‐infarct area was confirmed by in situ oligo ligation (ISOL) assay according to the manufacturer's instructions (Intergen, Purchase, New York, USA). Hoechst 33258 (10 µM) staining was used to count the total number of nuclei. Apoptosis is expressed as the percentage of ISOL‐positive myocyte nuclei in the total number of nuclei.
After Langendorff perfusion analysis, the hearts were perfused with 0.1% Evan's blue dye (Sigma Chemicals) in KH buffer for 15 min.16 The hearts were then perfused for 5 min with KH buffer to wash away excess dye. Frozen sections (5 μm) were stained with Hoechst 33258 to count the total number of nuclei. Sections were visualised by fluorescent microscopy. The number of necrotic myocytes in the non‐infarct LV region was calculated as the percentage of Evan's blue dye‐positive myocytes in the total number of nuclei.
Statistical analyses
Data are presented as mean (SE). Data were analysed by Student's t tests or one‐way analysis of variance and a post hoc Tukey's test. Probability values of p < 0.05 were considered to be significant.
RESULTS
Expression of β1 integrins in the heart after MI
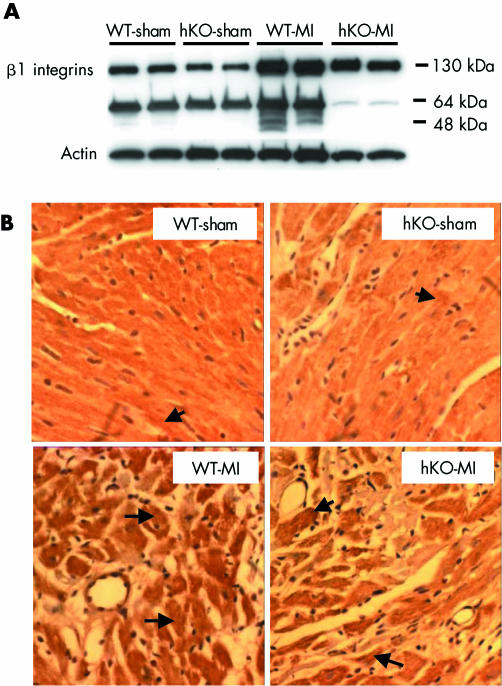
Western blot analysis showed lower levels of intact β1 integrins in the myocardium of hKO‐sham group than in WT‐sham (WT‐sham, 1.0 (0.02); hKO‐sham, 0.62 (0.09); p < 0.05, n = 5). The levels of intact β1 integrins were increased in both MI groups three days after MI (fig 1A) but the increase in β1 integrin was significantly higher in WT‐MI than in hKO‐MI (fold increase v WT‐sham: WT‐MI, 1.51 (0.05); hKO‐MI, 0.84 (0.07); p < 0.05, n = 5). Western blot analysis showed the presence of an about 64 kDa β1 integrin immunoreactive band. The identity and significance of this band is unknown. It may be the extracellular domain of β1 integrin in the extracellular environment. Immunohistochemical analysis showed low basal staining for β1 integrins in both WT‐sham and hKO‐sham hearts (fig 1B). Immunoreactivity for β1 integrins in the heart was increased one month after MI. Most of the increased staining for β1 integrins was detected in cardiac myocytes. The number of cardiac myocytes exhibiting increased β1 integrin expression, however, appeared lower in hKO‐MI hearts than in WT‐MI hearts.
Figure 1 (A) Western blot analysis of β1 integrin in the heart after myocardial infarction (MI). Total left ventricular (LV) lysates, prepared from non‐infarct LV three days after MI, were analysed with anti‐β1 integrin antibodies. The upper band (about 130 kDa) is intact β1 integrin. Equal loading of proteins in each lane is indicated by actin immunostaining. hKO, heterozygous knockout; WT, wild type. (B) Cross sections of hearts, 30 days after MI, immunostained with anti‐β1 integrin antibodies. Arrows indicate positive staining for β1 integrins in cardiac myocytes.
MI and morphometric studies
The MI size was about 20% (percentage of the LV circumference) and was not different between the WT‐MI and hKO‐MI groups (NS) (table 1). The heart weight to body weight ratio was increased in the hKO‐MI group (p < 0.01 v hKO‐sham; p < 0.05 v WT‐MI) (table 1). The lung wet weight to dry weight ratio was greater in the hKO‐MI group, but not in WT‐MI mice, than in their respective shams (p < 0.05 v hKO‐sham; p = 0.01 v WT‐MI) (table 1). There was a trend towards greater fibrosis in the hKO‐MI group than in WT‐MI, but the difference was not significant. Myocyte size was increased in both WT and hKO hearts after MI (p < 0.001 v sham) (table 1). This increase was significantly higher in hKO‐MI hearts than in WT‐MI hearts (p < 0.001 v WT‐MI).
Table 1 Morphometric measurements.
| Parameter | WT‐sham (n = 9) | hKO‐sham (n = 9) | WT‐MI (n = 8)* | hKO‐MI (n = 9)* | p Value |
|---|---|---|---|---|---|
| Infarct size (%LV circumference) | – | – | 19.80 (0.99) | 20.94 (0.95) | NS |
| Lung wet:dry weight (mg/mg) | 4.69 (0.12) | 4.71 (0.11) | 4.60 (0.15) | 5.17 (0.13)†‡ | <0.05†; 0.01‡ |
| HW:BW (mg/g) | 6.50 (0.34) | 6.40 (0.30) | 7.17 (0.72) | 9.78 (0.91)†‡ | <0.01†; <0.05‡ |
| Fibrosis (pixels) | 0.31 (0.08) | 0.30 (0.13) | 21.09 (4.71)† | 26.42 (6.25)† | <0.001† |
| Myocyte size (μm) | 89.86 (2.06) | 89.83 (2.99) | 125.63 (1.91)† | 175.48 (4.48)†‡ | <0.001†‡ |
Values are mean (SE).
*Except for fibrosis, where n = 4 in wild type (WT) myocardial infarction (MI) and n = 5 in heterozygous knockout (hKO) ‐MI; †versus sham; ‡versus WT‐MI.
HW:BW, heart weight to body weight ratio; LV, left ventricular.
Echocardiographic measurements
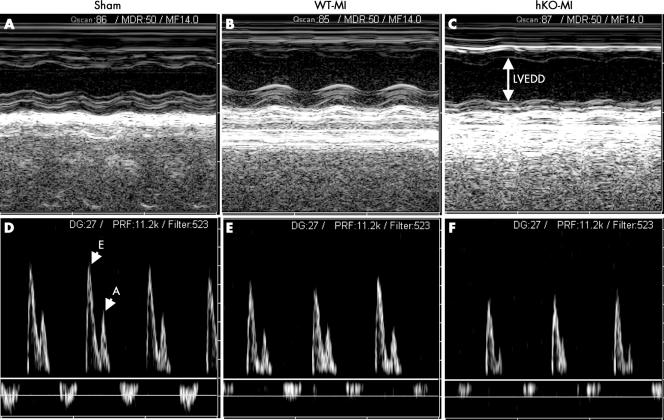
LVESD, LVEDD and %FS did not differ between the WT‐sham and hKO‐sham groups. After one month of MI, LVESD and LVEDD were significantly increased and %FS was significantly decreased in both MI groups (fig 2, table 2). Interestingly, the increase in LVESD and LVEDD, and the decrease in %FS was significantly higher in hKO‐MI group than in WT‐MI (p < 0.05 v WT‐MI group). Heart rates were not significantly different among the sham or MI groups. Doppler measurements (fig 2, table 2) showed increased peak E:A ratio in both MI groups with no significant difference between the two MI groups. The IVRT was increased in both MI groups, but the increase in IVRT was significantly higher in hKO‐MI group than in WT‐MI (p < 0.05 v WT‐MI).
Figure 2 (A, B, C) M mode echocardiographic images obtained from wild type (WT) ‐sham, WT‐myocardial infarction (MI) and heterozygous knockout (hKO) ‐MI hearts one month after MI, respectively. (D, E, F) Doppler tracings obtained from WT‐sham, WT‐MI and hKO‐MI hearts one month after MI, respectively. n = 5 in each group. A, peak velocity of the late ventricular filling (A wave); E, peak velocity of the early ventricular filling (E wave); LVEDD, left ventricular end diastolic diameter.
Table 2 Echocardiographic measurements.
| Parameter | WT‐sham (n = 5) | hKO‐sham (n = 5) | WT‐MI (n = 5) | hKO‐MI (n = 5) | p Value |
|---|---|---|---|---|---|
| M mode | |||||
| LVEDD (mm) | 4.13 (0.23) | 4.05 (0.20) | 5.52 (0.11)* | 6.40 (0.26)*† | <0.001*; <0.05† |
| LVESD (mm) | 3.10 (0.20) | 3.18 (0.23) | 4.58 (0.17)* | 5.69 (0.28)*† | <0.001*; <0.01† |
| %FS | 25.13 (1.99) | 21.73 (2.43) | 17.11 (1.84)* | 11.14 (1.36)*† | <0.05*; <0.05† |
| HR (beats/min) | 376 (18.66) | 369 (8.53) | 375 (15.74) | 346 (6.33) | NS |
| Doppler | |||||
| Peak E (cm/s) | 67.12 (4.00) | 71.45 (3.43) | 68.39 (2.86) | 59.97 (1.54)* | <0.05* |
| Peak A (cm/s) | 49.73 (5.22) | 52.57 (5.21) | 33.00 (1.45)* | 27.79 (2.37)* | <0.05* |
| E:A ratio | 1.38 (0.06) | 1.41 (0.09) | 2.09 (0.11)* | 2.22 (0.18)* | <0.01* |
| IVRT (ms) | 13.45 (2.40) | 13.90 (2.43) | 28.20 (2.65)* | 36.25 (2.27)*† | <0.05*; <0.05† |
Values are mean (SE).
*versus sham; †versus wild type (WT) ‐myocardial infarction (MI).
%FS, percentage fractional shortening; hKO, heterozygous knockout; HR, heart rate; IVRT, isovolumic relaxation time; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter.
LV pressure–volume relationships
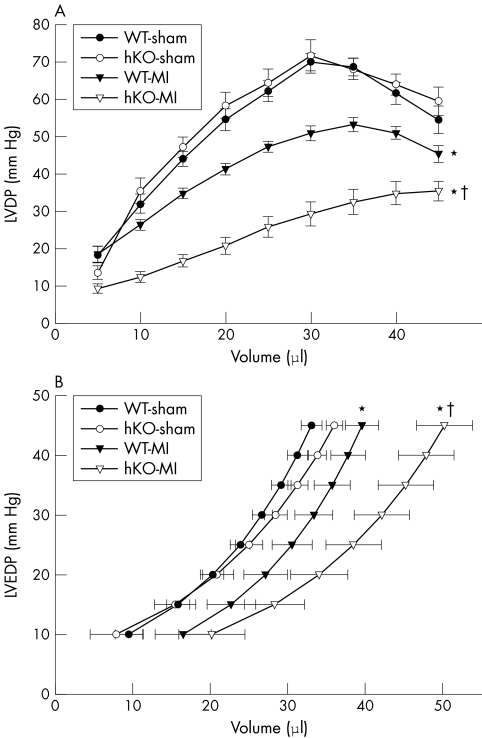
Langendorff perfusion analysis indicated no differences in the LV developed pressure and LV end diastolic pressure–volume relationship between the two sham groups (fig 3). LV developed pressure–volume relationship shifted downward in both groups after MI (p < 0.005, WT‐MI v WT‐sham; p < 0.001, hKO‐MI v hKO‐sham) (fig 3A). Interestingly, the downward shift was significantly higher in the hKO‐MI group than in the WT‐MI group (p < 0.001 v WT‐MI) (fig 3A). The maximum LV developed pressure was depressed to a greater extent in hKO‐MI group than in WT‐MI (hKO‐MI, 36.7 (2.2) mm Hg; WT‐MI, 53.4 (1.9) mm Hg; p < 0.001). The LV end diastolic pressure–volume relationship shifted rightward in both the MI groups (p < 0.05, WT‐MI v WT‐sham; p < 0.01, hKO‐MI v hKO‐sham) (fig 3B), but the shift was significantly higher in the hKO‐MI group than in WT‐MI (p < 0.05 at volumes > 35 µl) (fig 3B).
Figure 3 (A) Analysis of left ventricular developed pressure (LVDP) versus volume. LVDP decreased significantly for a given volume in the heterozygous knockout (hKO) ‐myocardial infarction (MI) group. *p < 0.005 wild type (WT) ‐MI v WT‐sham; *p < 0.001 hKO‐MI v hKO‐sham; †p < 0.001 hKO‐MI v WT‐MI. (B) Analysis of left ventricular end diastolic pressure (LVEDP) –volume relationships. WT‐MI and especially hKO‐MI groups shifted rightward from sham. *p < 0.05 WT‐MI v WT‐sham; *p < 0.01 hKO‐MI v hKO‐sham; †p < 0.05 hKO‐MI v WT‐MI. n = 8 in each group.
Apoptosis and necrosis
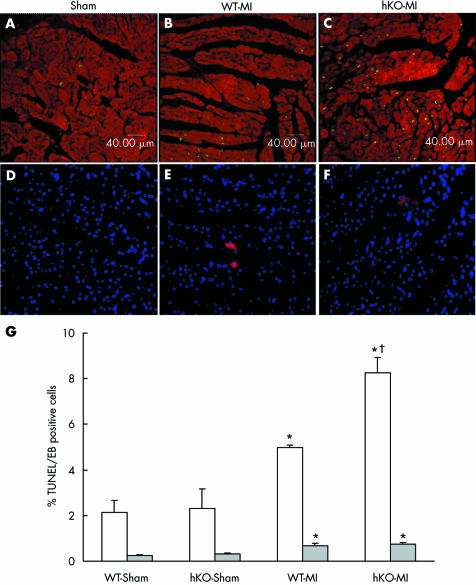
MI increased cardiac myocyte apoptosis in the non‐infarct area of both WT and hKO groups as measured with TUNEL staining (fig 4A–C). The number of apoptotic myocytes (calculated as the percentage of apoptotic myocyte nuclei in the total number of nuclei) was significantly higher in the hKO group three days after MI (p < 0.005 v WT‐MI) (fig 4G). The number of apoptotic myocytes was also higher in the MI groups 30 days after MI than in the sham groups (percentage apoptosis: WT‐sham, 1.2; hKO‐sham, 1.0; WT‐MI, 10.1 (p < 0.05 v sham); hKO‐MI, 10.4 (p < 0.05 v sham)), but myocyte apoptosis was not significantly different between the two MI groups. Similarly, the number of ISOL‐positive cardiac myocytes was significantly higher in the hKO‐MI group three days after MI (percentage apoptosis: WT‐sham, 1.35; hKO‐sham, 1.5; WT‐MI, 3.87 (p < 0.05 v sham); hKO‐MI, 8.32 (p < 0.05 v sham, p < 0.05 v WT‐MI)). ISOL assay showed no significant difference between the two MI groups 30 days after MI (data not shown). The number of apoptotic myocytes did not differ significantly by TUNEL staining versus ISOL assay.
Figure 4 Apoptosis and necrosis after myocardial infarction (MI). (A–C) Confocal microscopic images of the heart to detect cardiac myocyte apoptosis three days after MI with terminal deoxynucleotidyl transferase‐mediated dUTP nick end labelling (TUNEL) staining assay. Green fluorescence shows apoptotic cells and red stain indicates staining for α sarcomeric actin, specific for cardiac myocytes. hKO, heterozygous knockout; WT, wild type. (D–F) Fluorescent images obtained from Evan's blue (EB) dye‐perfused hearts three days after MI. Red fluorescence indicates necrotic cells and blue fluorescence indicates Hoechst‐stained nuclei in the same field (total cell nuclei). (G) Quantitative analysis of cardiac myocyte apoptosis and necrosis three days after MI. *p < 0.001 hKO‐MI v hKO‐sham; *p < 0.005 WT‐MI v WT‐sham; †p < 0.005 hKO‐MI v WT‐MI; n = 6 in each group for TUNEL (white bars). *p < 0.05 MI v sham; n = 3 in each group for EB staining (grey bars).
The two sham groups exhibited only a few necrotic myocytes. At three days after MI, the number of necrotic myocytes was increased in both the MI groups (p < 0.05 v shams) (fig 4D–F) with no significant difference between the MI groups. Overall, the number of necrotic myocytes remained < 0.8% in both the MI groups, which is much lower than apoptosis (fig 4G). The number of necrotic myocytes was not different among the groups 30 days after MI (not shown).
DISCUSSION
The important finding of this study is that the deficiency of β1 integrins increases myocardial dysfunction with increased LV dilatation, reduced %FS and increased IVRT 30 days after MI. MI increased hypertrophy, fibrosis and apoptosis in both WT and β1 integrin‐deficient mice; however, the increase in hypertrophy and apoptosis was significantly higher in β1 integrin‐deficient mice.
Cardiac myocytes predominantly express β1 integrins. Integrins are suggested to have an important role as mechanotransducers in cardiac cells.7 By using western blot analysis, we observed about a 38% decrease in β1 integrin expression in hKO mice as compared with WT at the basal level. Levels of β1 integrin protein were increased in the non‐infarcted LV regions of both WT and hKO mice three days after MI. The levels of β1 integrin protein after MI, however, continued to be higher in the WT than in hKO mice. Immunohistochemical analysis showed increased β1 integrin expression in cardiac myocytes 30 days after MI. It is interesting to note that not all the cardiac myocytes exhibit increased expression of β1 integrins. The number of cardiac myocytes exhibiting increased expression of β1 integrins appears lower in the hKO‐MI mice than in WT‐MI. Previously, expression of β1 integrin was shown to be increased in rat hearts three and seven days after MI.8 The increase in β1 integrin expression was mainly observed in the areas of peri‐infarct, and infarct with β1D integrin expression associated with cardiac myocytes.8 Our findings of increased β1 integrin in the cardiac myocytes are consistent with previous findings in the rat heart.8 However, we observed increased β1 integrin expression in the non‐infarcted portion of the heart. The difference may be due to the difference in the size of the infarct or the age of the animals.
Perinatal lethality and fibrosis has been reported in transgenic mice in which normal β1 integrin function was disrupted in cardiac myocytes by expression of a chimeric molecule, encoding the transmembrane and extracellular domains of the Tacβ1A .9 The surviving lines with the highest transgene expression developed compensatory hypertrophy in the absence of any provoked haemodynamic stimulus. Basal contractility and relaxation was reduced in other surviving lines with less transgene expression. In the present study, measurement of LV systolic and diastolic functions by echocardiography or Langendorff perfusion analysis showed no change in LV structure or function between the two sham groups. Possible reasons for these contrasting findings are the method of preparation of transgenic mice and levels of transgene and β1 integrin expression. Furthermore, Tacβ1A is suggested to function as a dominant negative inhibitor of β1 integrin function in some, but not all, aspects of cell attachment and spreading.17 Cardiac‐specific excision of the β1 integrin gene with Cre‐LoxP technology induced spontaneous heart failure in 6‐month‐old mice.9 These animals had a > 80% decrease in β1D integrin protein in the heart compared with WT mice. The decrease in β1 integrin expression in hKO mice used in our studies is about 38%. The hKO mice did not exhibit signs of cardiac dysfunction during the observation period (about 5 months old). Chimeric mice as well as embryoid bodies constructed from β1 integrin‐null cells showed that β1 integrin is necessary for differentiation and maintenance of a specialised phenotype of cardiac muscle cells.18 Taken together, these observations suggest that varied levels of β1 integrin have different effects on normal heart development and function.
Heart failure is characterised by systolic and diastolic dysfunction caused by reduced LV contractile function and dilatation. Echocardiography is shown to be a valid approach to evaluate LV structure and function in vivo in mice.14,19,20 MI is suggested to be associated with depressed systolic function.13,14,20 LV systolic dysfunction is associated with chamber dilatation.13 Echocardiographic evaluation of the heart one month after MI indicated greater LV diameters and lower %FS in hKO mice than in WT. Impaired LV relaxation has been associated with reduced early to late diastolic transmitral Doppler flow velocity ratios (that is, decreased E:A ratio), prolonged IVRT and prolonged E wave deceleration times.21 Doppler tracings of mitral and aortic flow from the apical four‐chamber view showed an increased E:A wave ratio and IVRT one month after MI, indicating that MI impairs LV relaxation. The increase in IVRT was significantly higher in hKO mice than in WT after MI. Functional analysis of the heart by the Langendorff perfusion technique confirmed the above observations. LV systolic function, as reflected by maximum LV developed pressure, was depressed to a greater extent in the hKO mice. The rightward shift in LV end diastolic pressure–volume relationship was significantly greater in β1 integrin‐deficient mice. Taken together, the structural and functional analyses of the heart suggest that deficiency of β1 integrin impairs cardiac function after MI.
Hypertrophy can result from mechanical stress on the heart from pressure or volume loading, as well as cardiac myocyte death. Ventricular hypertrophy is an important adaptive mechanism that allows the heart to maintain its output.10 MI is suggested to be associated with compensatory hypertrophy of the non‐infarcted myocardium.22 β1 integrins participate in the hypertrophic response of cardiac myocytes.5,23 We observed a trend towards greater heart weight to body weight ratio in the WT‐MI group than in WT‐sham, but this increase was significantly higher in the hKO mice. Likewise, cardiac myocyte cross‐sectional area increased in both MI groups. The increase in myocyte cross‐sectional area was significantly higher in hKO mice. The reasons for increased cardiac hypertrophy in hKO mice are not clear, considering that integrins are suggested to function as mechanotransducers, translating mechanical signals to biochemical signals.24,25 One likely possibility is that deficiency of β1 integrin may alter the expression of other integrin subunits. Although β1 isoform is the dominant β integrin subunit, cardiac myocytes also express α1, α3, α5, α7, α9, α10, β3 and β5 subunits. Chronic pressure overload increases expression of α1, α5, α7, β1 and β3 integrin subunits.5 The expression of these subunits may also be altered in hKO mice after MI. Alterations in the expression of one or more integrin subunits can alter heterodimer formation leading to altered signalling and hypertrophy.
Cardiac myocyte apoptosis increases in the infarct and peri‐infarct areas and to a smaller extent in the non‐infarcted areas of the heart after MI.26,27 We observed increased cardiac myocyte apoptosis in the non‐infarcted LV region, but the increase in the number of apoptotic cardiac myocytes was significantly higher in hKO mice, specifically three days after MI. This is consistent with the in vitro findings that the β1 integrin signalling pathway has an anti‐apoptotic role.11 The data presented in fig 1 suggest increased expression of β1 integrins in both MI groups, with higher levels of β1 integrins in WT‐MI than in hKO‐MI mice, therefore possibly exerting more protective effects in WT‐MI hearts. In the present study, apoptosis was the major form of myocardial cell death, rather than necrosis.28 Myocyte membrane integrity (an indication of necrosis) as determined by Evan's blue dye staining was disrupted to a similar extent in both MI groups. Evan's blue dye staining was evident in about 0.8% of total nuclei, which was far less than the number of apoptotic cells. The myocardium of WT mice contained more apoptotic myocytes one month after MI than three days after MI. Sun et al8 reported that after MI, the expression of β1 integrin was significantly increased at day 3, reached a peak at day 7, and gradually declined thereafter at 14 and 28 days. We observed increased β1 integrin levels in some, but not all, cardiac myocytes one month after MI, suggesting that an appropriate increase in β1 integrin may be necessary to maintain cell survival. Also, apoptosis is suggested to be ongoing during MI, and removal of apoptotic debris by phagocytosis may be rate limiting in the heart, resulting in the accumulation of apoptotic markers.27 The available apoptosis detection assays therefore possibly detect one population of myocytes at multiple time points. β1 integrins are involved in the anchorage of cells to the extracellular matrix and loss of attachment to extracellular matrix causes apoptosis (called anoikis) in many cell types, including myocytes.29,30 The possibility of anoikis as a mechanism of cell death subsequently leading to remodelling during heart failure in the present model cannot be ruled out.29
Our data suggest that β1 integrins are crucial in post‐MI remodelling with effects on LV function, hypertrophy and apoptosis. We emphasise, however, that we obtained our data on cardiac dysfunction, apoptosis and hypertrophy in a smaller infarct model. Further work is under way to study the role of β1 integrins in post‐MI cardiac remodelling in a bigger infarct (about 40%).
Abbreviations
%FS - percentage fractional shortening
hKO - heterozygous knockout
ISOL - in situ oligo ligation
IVRT - isovolumic relaxation time
KH - Krebs–Henseleit
LV - left ventricular
LVEDD - left ventricular end diastolic diameter
LVESD - left ventricular end systolic diameter
MI - myocardial infarction
Tacβ1A - Tac subunit of the interleukin 2 receptor fused to the cytoplasmic domain of β1A integrin
TUNEL - terminal deoxynucleotidyl transferase‐mediated dUTP nick end labelling
WT - wild type
Footnotes
This work is supported by National Institutes of Health, Grant number HL‐071519 (KS), a Merit Review Grant from the Department of Veterans Affairs (KS) and a postdoctoral fellowship from the American Heart Association, Southeast Affiliate (PK).
Competing interests: None declared
References
- 1.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease. A novel therapeutic target? FASEB J 200216135–146. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson A B, Gottlieb R A. Mechanisms of apoptosis in the heart. J Clin Immunol 200323447–459. [DOI] [PubMed] [Google Scholar]
- 3.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 19926911–25. [DOI] [PubMed] [Google Scholar]
- 4.Stupack D G, Cheresh D A. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 20021153729–3738. [DOI] [PubMed] [Google Scholar]
- 5.Ross R S. Molecular and mechanical synergy: cross‐talk between integrins and growth factor receptors. Cardiovasc Res 200463381–390. [DOI] [PubMed] [Google Scholar]
- 6.Zhidkova N I, Belkin A M, Mayne R. Novel isoform of beta 1 integrin expressed in skeletal and cardiac muscle. Biochem Biophys Res Commun 1995214279–285. [DOI] [PubMed] [Google Scholar]
- 7.Simpson D G, Majeski M, Borg T K.et al Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res 199985e59–e69. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Opavsky M A, Stewart D J.et al Temporal response and localization of integrins beta1 and beta3 in the heart after myocardial infarction: regulation by cytokines. Circulation 20031071046–1052. [DOI] [PubMed] [Google Scholar]
- 9.Keller R S, Shai S Y, Babbitt C J.et al Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol 20011581079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shai S Y, Harpf A E, Babbitt C J.et al Cardiac myocyte‐specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 200290458–464. [DOI] [PubMed] [Google Scholar]
- 11.Communal C, Singh M, Menon B.et al Beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta‐adrenergic receptor‐stimulated apoptosis. J Cell Biochem 200389381–388. [DOI] [PubMed] [Google Scholar]
- 12.Stephens L E, Sutherland A E, Klimanskaya I V.et al Deletion of beta 1 integrins in mice results in inner cell mass failure and peri‐implantation lethality. Genes Dev 199591883–1895. [DOI] [PubMed] [Google Scholar]
- 13.Trueblood N A, Xie Z, Communal C.et al Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 2001881080–1087. [DOI] [PubMed] [Google Scholar]
- 14.Finsen A V, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol 200598680–689. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer M A, Pfeffer J M, Fishbein M C.et al Myocardial infarct size and ventricular function in rats. Circ Res 197944503–512. [DOI] [PubMed] [Google Scholar]
- 16.Sumida T, Otani H, Kyoi S.et al Temporary blockade of contractility during reperfusion elicits a cardioprotective effect of the p38 MAP kinase inhibitor SB‐203580. Am J Physiol Heart Circ Physiol 2005288H2726–H2734. [DOI] [PubMed] [Google Scholar]
- 17.Bodeau A L, Berrier A L, Mastrangelo A M.et al A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J Cell Sci 20011142795–2807. [DOI] [PubMed] [Google Scholar]
- 18.Fassler R, Rohwedel J, Maltsev V.et al Differentiation and integrity of cardiac muscle cells are impaired in the absence of β1 integrin. J Cell Sci 19961092989–2999. [DOI] [PubMed] [Google Scholar]
- 19.Gardin J M, Siri F M, Kitsis RN et a l. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res 199576907–914. [DOI] [PubMed] [Google Scholar]
- 20.Yang X P, Liu Y H, Rhaleb N E.et al Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 1999277H1967–H1974. [DOI] [PubMed] [Google Scholar]
- 21.Collins K A, Korcarz C E, Lang R M. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics 200313227–239. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Li P, Zhang X. Ischemic myocardial injury and ventricular remodeling. Cardiovasc Res 199327145–157. [DOI] [PubMed] [Google Scholar]
- 23.Brancaccio M, Fratta L, Notte A.et al Melusin, a muscle‐specific integrin beta1‐interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med 2003968–75. [DOI] [PubMed] [Google Scholar]
- 24.Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol 19913841–848. [DOI] [PubMed] [Google Scholar]
- 25.Nebe B, Rychly J, Knopp A.et al Mechanical induction of beta 1‐integrin‐mediated calcium signaling in a hepatocyte cell line. Exp Cell Res 1995218479–484. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Li B, Wang X.et al Overexpression of insulin‐like growth factor‐1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest 19971001991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bialik S, Geenen D L, Sasson I E.et al Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest 19971001363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajstura J, Cheng W, Reiss K.et al Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 19967486–107. [PubMed] [Google Scholar]
- 29.Michel J B. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol 2003232146–2154. [DOI] [PubMed] [Google Scholar]
- 30.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 19979701–706. [DOI] [PubMed] [Google Scholar]