Abstract
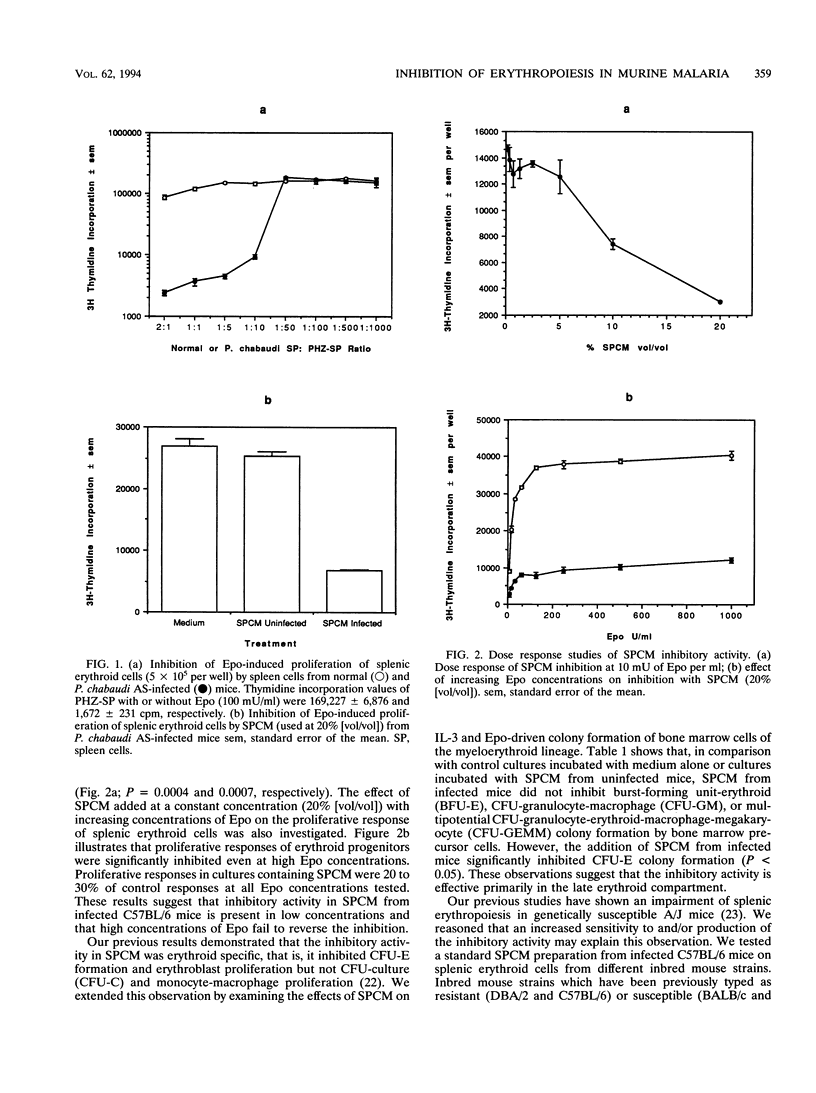
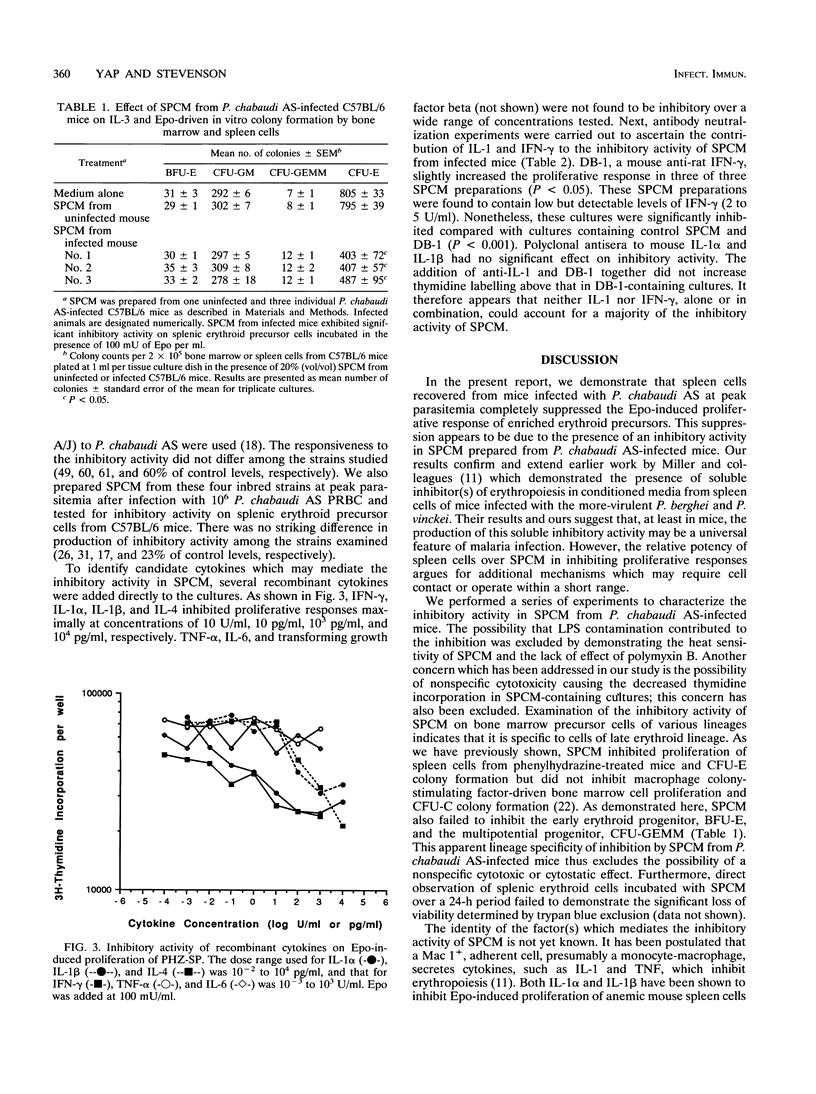
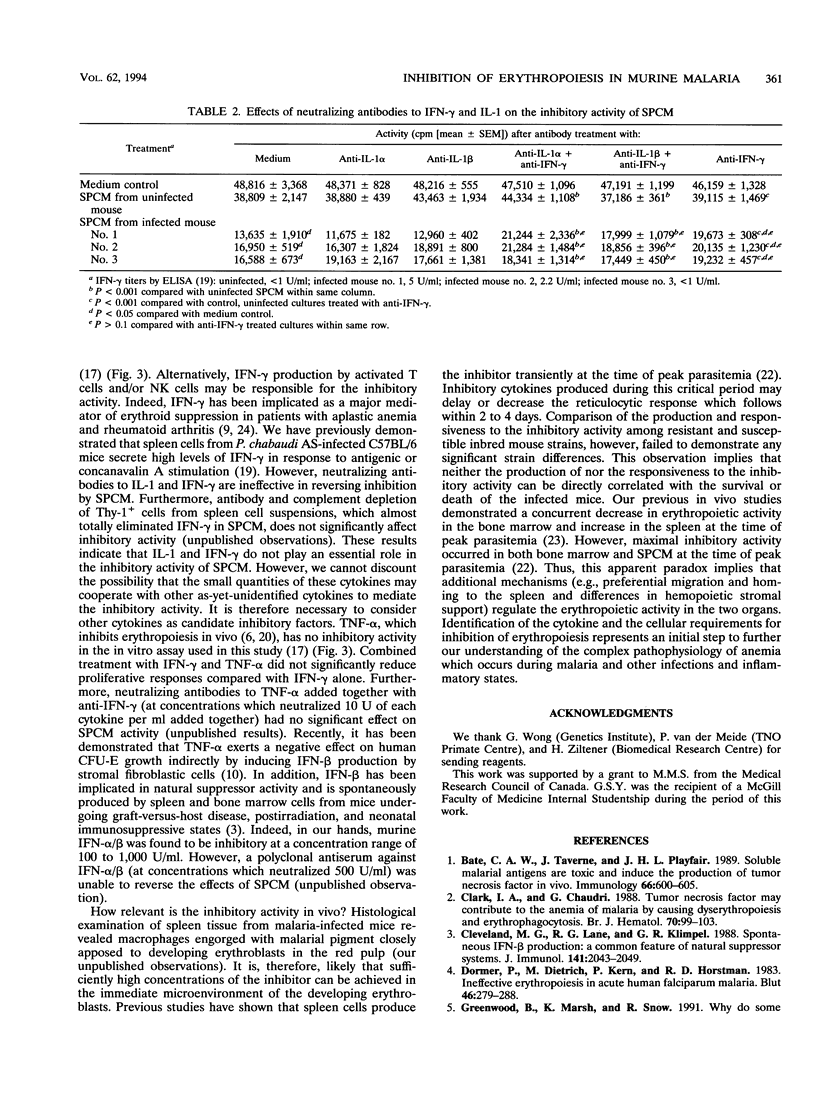
By using erythropoietin-dependent proliferation of splenic erythroid cells as an in vitro erythropoiesis model system, we demonstrate that spleen cells from Plasmodium chabaudi AS-infected C57BL/6 mice potently inhibited erythroid cell proliferation. Inhibitory activity was detected in spleen cell conditioned media (SPCM) prepared from infected mice but not from uninfected mice. The inhibitory activity in SPCM was characterized as being heat sensitive, macromolecular, and host derived. The inhibitory activity was not reversed by increasing the erythropoietin concentration and was found to be specific for the late erythroid lineage. Mouse strains, which differ in their resistance to P. chabaudi AS infection, produced and responded to the inhibitory activity to a similar extent. Putative immune mediators, interleukin 1 alpha, interleukin 1 beta, and gamma interferon, were found to be potent inhibitors of erythroid cell proliferation. However, antibody neutralization experiments failed to demonstrate a major role for these cytokines in the inhibitory activity of SPCM. Our results suggest that the elaboration of inhibitor(s) of erythropoiesis in hemopoietic organs of Plasmodium-infected mice may impair erythroid regeneration. The identity of the inhibitory mediator(s) is presently unknown but is distinct from interleukin 1, tumor necrosis factor alpha, and gamma interferon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988 Sep;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Cleveland M. G., Lane R. G., Klimpel G. R. Spontaneous IFN-beta production. A common feature of natural suppressor systems. J Immunol. 1988 Sep 15;141(6):2043–2049. [PubMed] [Google Scholar]
- Dörmer P., Dietrich M., Kern P., Horstmann R. D. Ineffective erythropoiesis in acute human P. falciparum malaria. Blut. 1983 May;46(5):279–288. doi: 10.1007/BF00319868. [DOI] [PubMed] [Google Scholar]
- Greenwood B., Marsh K., Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991 Oct;7(10):277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Waddelow T. A., Caro J., Oliff A., Roodman G. D. Chronic exposure to tumor necrosis factor in vivo preferentially inhibits erythropoiesis in nude mice. Blood. 1989 Jul;74(1):130–138. [PubMed] [Google Scholar]
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol. 1983 Aug;11(7):649–660. [PubMed] [Google Scholar]
- Looareesuwan S., Merry A. H., Phillips R. E., Pleehachinda R., Wattanagoon Y., Ho M., Charoenlarp P., Warrell D. A., Weatherall D. J. Reduced erythrocyte survival following clearance of malarial parasitaemia in Thai patients. Br J Haematol. 1987 Dec;67(4):473–478. doi: 10.1111/j.1365-2141.1987.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Means R. T., Jr, Krantz S. B. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest. 1993 Feb;91(2):416–419. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means R. T., Jr, Krantz S. B. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992 Oct 1;80(7):1639–1647. [PubMed] [Google Scholar]
- Miller K. L., Schooley J. C., Smith K. L., Kullgren B., Mahlmann L. J., Silverman P. H. Inhibition of erythropoiesis by a soluble factor in murine malaria. Exp Hematol. 1989 May;17(4):379–385. [PubMed] [Google Scholar]
- Monette F. C., Sigounas G. Sensitivity of murine multipotential stem cell colony (CFU-GEMM) growth to interleukin-3, erythropoietin, and hemin. Exp Hematol. 1987 Aug;15(7):729–734. [PubMed] [Google Scholar]
- Pasvol G. The anaemia of malaria. Q J Med. 1986 Mar;58(227):217–219. [PubMed] [Google Scholar]
- Phillips R. E., Looareesuwan S., Warrell D. A., Lee S. H., Karbwang J., Warrell M. J., White N. J., Swasdichai C., Weatherall D. J. The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q J Med. 1986 Mar;58(227):305–323. [PubMed] [Google Scholar]
- Phillips R. E., Warrell D. A. The pathophysiology of severe falciparum malaria. Parasitol Today. 1986 Oct;2(10):271–282. doi: 10.1016/0169-4758(86)90136-5. [DOI] [PubMed] [Google Scholar]
- Schooley J. C., Kullgren B., Allison A. C. Inhibition by interleukin-1 of the action of erythropoietin on erythroid precursors and its possible role in the pathogenesis of hypoplastic anaemias. Br J Haematol. 1987 Sep;67(1):11–17. doi: 10.1111/j.1365-2141.1987.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Belosevic M., van der Meide P. H., Podoba J. E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990 Oct;58(10):3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Yin S. Tumor necrosis factor exerts dose-dependent effects on erythropoiesis and myelopoiesis in vivo. Exp Hematol. 1990 May;18(4):311–315. [PubMed] [Google Scholar]
- Wognum A. W., Lansdorp P. M., Humphries R. K., Krystal G. Detection and isolation of the erythropoietin receptor using biotinylated erythropoietin. Blood. 1990 Aug 15;76(4):697–705. [PubMed] [Google Scholar]
- Yap G. S., Stevenson M. M. Plasmodium chabaudi AS: erythropoietic responses during infection in resistant and susceptible mice. Exp Parasitol. 1992 Nov;75(3):340–352. doi: 10.1016/0014-4894(92)90219-z. [DOI] [PubMed] [Google Scholar]
- Yap G. S., Stevenson M. M. Production of soluble inhibitor of erythropoiesis during Plasmodium chabaudi AS infection in resistant and susceptible mice. Ann N Y Acad Sci. 1991;628:279–281. doi: 10.1111/j.1749-6632.1991.tb17257.x. [DOI] [PubMed] [Google Scholar]
- Zoumbos N. C., Gascon P., Djeu J. Y., Young N. S. Interferon is a mediator of hematopoietic suppression in aplastic anemia in vitro and possibly in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):188–192. doi: 10.1073/pnas.82.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]



