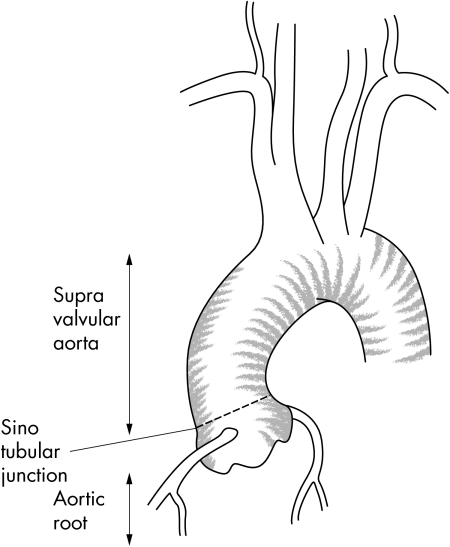
Dilation of the ascending aorta entails a high risk of dissection or aortic rupture in the absence of surgical treatment. Overall, it represents 50% of all thoracic aneurysms, but can be separated into two distinct entities, according to aetiology and surgical management: (1) the aortic root aneurysm, concerning the initial portion, the so called “aortic root”, that includes the sinuses of Valsalva; and (2) the “supravalvular aortic aneurysms” above the sinuses of Valsalva up the brachiocephalic trunk (fig 1). In contrast to the supravalvular aneurysm that can be treated by a simple supracoronary tube graft, the aortic root aneurysm involves the aortic valve which needs to be spared or replaced. Recent surgical advances have been developed for aortic root aneurysms, which are detailed in this report.
Figure 1 Normal ascending aorta: aortic root, sinotubular junction, and supravalvular aorta.
INCIDENCE AND RISK FACTORS
Aortic aneurysms remain the 13th leading cause of mortality in western countries.1,2 The incidence of thoracic aortic aneurysms is estimated to be 4.5 cases per 100 000.1,2,3 Supravalvular aortic aneurysms are less common, and predominantly affect male patients (ratio 3:1); the mean age at the time of diagnosis ranges from 59–69 years.3 In the case of aortic root aneurysms, patients are younger (30–50 years), with a 1:1 sex ratio.
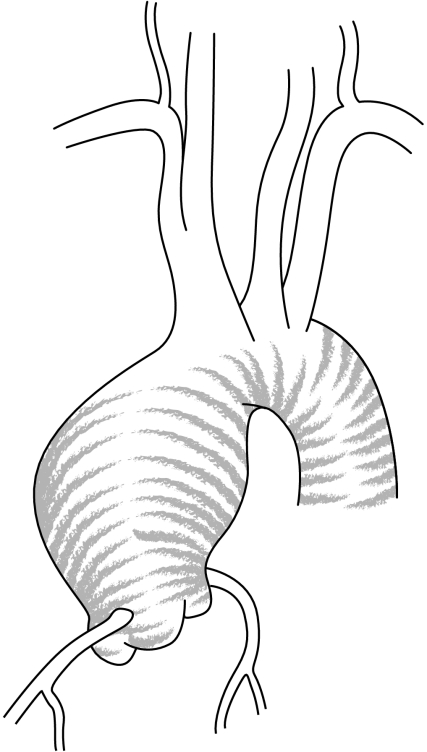
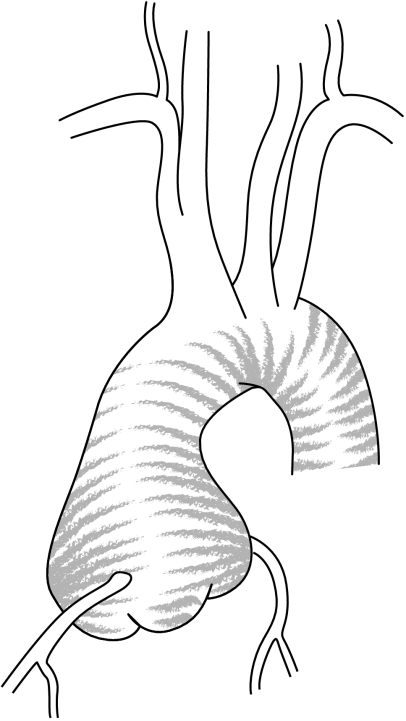
Supravalvular aortic aneurysms are caused by atherosclerosis in relation to hypertension, whereas aneurysm of the aortic root is related to dystrophic degeneration of the aortic wall—so called cystic medial necrosis.1,3 Aneurysm of the aortic root (annulo‐aortic ectasia) can be either idiopathic or associated with well‐defined connective tissue disorders such as Marfan syndrome, Ehler Danlos syndrome, or bicuspid valve1,3 (figs 2 and 3).
Figure 2 Supravalvular aortic aneurysm.
Figure 3 Aortic root aneurysm.
Twenty per cent of patients with Marfan syndrome (autosomal dominant connective tissue disorder, incidence 2/5000) will be operated on for an aortic root aneurysm.4 Similar lesions can also occur in certain families, without phenotypic expression of Marfan syndrome, exhibiting strong histories of ascending aortic aneurysm formation and dissection transmitted in an autosomal dominant fashion.1
The frequent association of bicuspid aortic valve with aortic dissection (10‐fold more than the normal population), aortic dilation, and cystic medial necrosis has been well established.1,5 Patients with bicuspid (incidence 2%) and unicuspid aortic valves display larger aortic sizes at each level, compared with normal tricuspid aortic valves. The degree of dilation seems to be related to the presence of aortic insufficiency, and potentially correlates with intrinsic medial weakness.5
Other aetiologies are uncommon. Patients with chronic dissection of the aortic root usually develop an associated aneurysm, characterised by a high rate of expansion and risk of rupture, as the aortic wall is only limited by the outer third of the media and the adventitia.1,3 Aneurysmal formation from true primary bacterial infection of the ascending aortic wall is rare, and results from either an episode of bacterial endocarditis or from infection of a laminar clot within a previously formed aneurysm. Syphilis, formerly the main cause of ascending aortic aneurysms, is much less common following the development of effective antibiotics. The most common causal organisms include, in order of decreasing frequency, Staphylococcus aureus, Staphylococcus epidermidis, Salmonella species, and Streptococcus species.1,3 Arteritis is even more rare. Takayasu's arteritis usually produces obstructive lesions, but may present with aortic dilation in 15% of cases.1 Giant cell arteritis (temporal arteritis) can also affect the ascending aorta and produce aneurysms. Less commonly, pseudoaneurysms of the ascending aorta occur after trauma or infection, or postoperatively at an aortic suture line or at the site of aortic cannulation.
NATURAL HISTORY
Observational evidence from published series demonstrates a rising incidence of dissection or rupture with expanding aneurysm size.3,6 The law of Laplace predicts that as the size of the aneurysm increases, the wall tension rises. The inherent disease progression usually consists of self‐propagating incremental expansion and possible rupture.
Isolated dilation of the ascending aorta requires particular care in identification of the critical size at which risk of rupture or dissection becomes greater than the risk of elective surgery. The natural history of the ascending aortic aneurysm is often related to its location and primary cause. Ascending aortic aneurysms grow an average of 1–4 mm per year, but patients with bicuspid valves or those with Marfan's syndrome present a more rapid expansion rate, and larger aneurysms grow more rapidly than smaller ones.
Previous studies have suggested other predictors of progressive dilatation such as smoking, diastolic blood pressure, renal failure,1 male sex, fibrocalcific changes in the aortic valve, and left ventricular wall motion abnormalities.
When the aorta reaches 6 cm in size, reported yearly rates of rupture, dissection, and death are 3.6%, 3.7%, and 10.8%, respectively. The cumulative risk of any of these events is 14.1%.6
SYMPTOMS
Acute symptoms suggest that rupture may be imminent, or dissection is indicated by severe “tearing” pain in 75% of patients. However, most ascending aortic aneurysms remain asymptomatic for a long time, being incidentally diagnosed on chest x ray or other imaging studies, such as echocardiographic evaluation performed to assess aortic insufficiency. Chronic anterior pain can result from compression of the overlying sternum and is the first complaint in 25–75% of patients.
Physical examination
In a patient without rupture, the aneurysm can become very large without any physical findings. An aortic root aneurysm is often associated with aortic insufficiency that may lead to widened pulse pressure or diastolic murmur, or symptoms of heart failure. A thorough vascular examination should be carried out to look for any concomitant peripheral vascular disease, carotid disease, abdominal aortic aneurysms (10–20% of cases) or sequelae of distal embolisation.1
Symptoms relate to pressure against or erosion of adjacent structures by the enlarging aorta, such as anterior pain, necrosis of the overlying sternum and ribs (syphilis), cough, wheezing, or haemoptysis from tracheal or bronchial compression or erosion; or hoarseness from compression of the left recurrent laryngeal nerve (involvement of the distal aortic arch). Occasionally signs of superior vena cava or airway compression are present. Less commonly, aneurysms of the ascending aorta can rupture into the right atrium or the superior vena cava, presenting with high output cardiac failure.1
Physical examination findings in the patient with aortic root aneurysm vary, depending on the cause. Patients with Marfan syndrome may be recognised because of their skeletal features including a tall stature with long limbs, arachnodactyly, pectus deformities, and scoliosis.4 It is critical in patients with an unexplained thoracic aortic aneurysm to obtain a careful family history. Marfan syndrome, bicuspid aortic valve, familial aortic aneurysm, and familial aortic dissection are often first diagnosed when screening the “asymptomatic” first‐degree relatives of an affected individual.4,5
DIAGNOSTIC STUDIES
Left ventricular hypertrophy on ECG is a consequence of significant aortic insufficiency. Patients with generalised atherosclerosis may show evidence of concomitant coronary artery disease, or previous myocardial injury.
The location and shape of the aortic root dilatation may provide clues to the aetiology of the ascending aortic aneurysm. The aorta in Marfan syndrome is typically pear‐shaped, being largest at the sinuses of Valsalva, with the ascending aorta tapering to normal calibre at the origin of the innominate artery (fig 3). Aortic aneurysm in the setting of a bicuspid aortic valve may involve the sinuses but often extends into the sinotubular junction and the ascending aorta. Supravalvular aortic aneurysms gradually increase and diminish in size, and are usually related to the atherosclerotic process (fig 2). In this case dilation spares the aortic annulus and sinuses of Valsalva, and begins at the level of the sino‐tubular junction. Saccular aneurysms are balloon‐shaped, with the dilation being relatively focal, and are related to syphilitic and other infectious causes.
On chest x ray, the enlarged ascending aorta produces a convex contour of the right superior mediastinum. In the lateral view, there is loss of the retrosternal air space. Aneurysms confined to the aortic root can be obscured by the cardiac silhouette and may not be evident on chest radiograph.
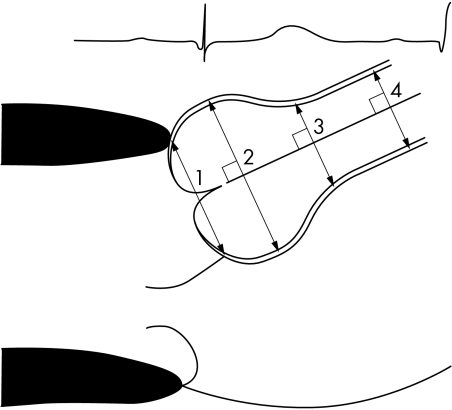
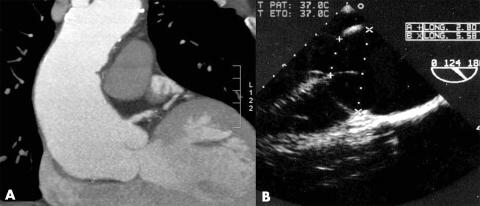
Cross sectional echocardiography is the primary clinical investigation for assessing ascending aorta size and its progression with time.7 Transoesophageal echocardiography may be performed to define the anatomy of the valve and ascending aorta. It differentiates between ascending aortic aneurysms, dissections, and intramural haematoma. However, the distal extent of the aneurysm is not well visualised. Aortic root diameters are measured on echocardiography at end diastole, in the parasternal long‐axis view, at four levels (annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta).7 Measurements are made perpendicular to the long axis of the aorta using the leading edge technique in views showing the largest aortic diameters (figs 4 and 5).
Figure 4 Echocardiographic measurement of aortic root diameters: (1) aortic annulus diameter (internal diameter); (2) sinuses of Valsalva (external diameter, comprising the aortic wall, from leading edge to leading edge); (3) sinotubular junction (external diameter, comprising the aortic wall, from leading edge to leading edge); (4) ascending aorta (external diameter, comprising the aortic wall, from leading edge to leading edge).
Figure 5 (A) Tomodensitometric and (B) echocardiographic views of an aortic root aneurysm.
Most patients are initially evaluated and followed with helical computed tomographic (CT) scans, complete with a three‐dimensional reconstruction of the data to increase the accuracy of aneurysm measurements, determination of its proximal and distal extent, and differentiation between dissection, penetrating ulcer or intramural haematoma (fig 5).
Magnetic resonance imaging (MRI) is useful to avoid contrast and radiation exposure. Cardiac imaging with MRI may provide evaluation of cardiac perfusion, myocardial function, and coronary and valve anatomy. Currently, however, MRI remains expensive, is less readily available and is more time consuming than CT scanning.
Traditional contrast angiography is performed to assess the relationship of the aneurysm to the arch vessels, the degree of aortic regurgitation, and to check the presence of coronary disease or left ventricular dysfunction. Presence of laminar clot can lead to underestimation of the aneurysm size.
As routine, it is recommended to perform both echocardiography and helical CT in order to evaluate and confirm the size of the aneurysm. A coronary angiogram is useful before planning surgery.
MEDICAL MANAGEMENT
In the asymptomatic patient with mild to moderate aortic root enlargement, medical treatment based upon β blocker therapy and serial follow‐up with echocardiography once or twice a year is recommended. β blockers have a negative inotropic and chronotropic effect, lessening the rate and rise of the arterial pulse over time. They are known to retard aortic root dilatation and improve survival in patients with Marfan syndrome.1,4 Whether other causes of ascending aortic aneurysms, including the bicuspid aortic valve, also benefit from prophylactic β blocker therapy is unknown, but this treatment is recommended by most authors in all cases of ascending aortic aneurysms.
Associated cardiovascular risk factors should be aggressively controlled. Activities or lifestyle should be modified, because high intensity, competitive and collision sports are potentially dangerous and may precipitate aortic dissection or rupture. Patients with a dilated aortic root can practise isokinetic activities, with low static and dynamic components, such as golf, cricket or other sports, but at a decreased level of intensity (table 1).8
Table 1 Classification of sports, based on peak dynamic and static components during competition8.
| A Low dynamic | B Moderate dynamic | C High dynamic | |
|---|---|---|---|
| I Low static | Billiards, bowling, cricket, curling, golf | Baseball, softball, table tennis, tennis (doubles), volleyball | Badminton, cross‐country skiing, field hockey*, orienteering, race walking, racquetball, running, soccer*, squash, tennis (singles) |
| II Moderate static | Archery, autoracing*, diving*, equestrian*, motorcycling* | Fencing, field events (jumping), figure skating*, football (American)*, rodeoing*, rugby*, running (sprint), surfing*, synchronised swimming* | Basketball*, ice hockey*, cross country skiing, football (Australian)*, lacrosse*, running (middle distances), swimming, team handball |
| III High static | Bobsledding*, field event (throwing), gymnastics*, karate/judo, luge*, sailing, rock climbing*, waterskiing*, weight lifting*, windsurfing* | Body building*, downhill skiing*, wresting* | Boxing*, canoeing/kayaking, cycling*, decathlon, rowing, speed skating |
*Danger of bodily collision.
Pregnancy is discouraged in patients with Marfan syndrome, especially if the aortic root diameter exceeds 40 mm. In the case of pregnancy in a patient with an aortic root diameter < 40 mm, close clinical and echocardiographic follow‐up is necessary, in association with β blocker therapy.4,9,10
In patients with Marfan syndrome, or a young patient with an aortic root aneurysm, investigation of the family is necessary.
SURGICAL STRATEGY
Surgical emergency operation is indicated in the setting of acute ascending aortic dissection or rupture into the pericardium (acute cardiac tamponade). Operative mortality remains significant and death is almost certain in the case of rupture or acute dissection if not surgically corrected.
Based on the natural history of ascending aortic aneurysms, prophylactic surgery seems appropriate when the diameter reaches 5–5.5 cm, depending on the aetiology. Intervention criteria are summarised in table 2 and fig 6.9,10 Elective surgery of the ascending aorta is much safer than emergency intervention (mortality 4.3%).
Table 2 Surgical indications in ascending aortic aneurysms9,10.
| • Surgical emergency | Rupture or aortic dissection |
| Unexplained pain | |
| Compression of adjacent organs | |
| • Elective operation | Aortic insufficiency requiring surgical correction |
| Size ⩾55 mm | |
| Size ⩾50 mm in patients with Marfan syndrome or bicuspid valves | |
| Growth rate ⩾1 cm/year |
Figure 6 Elective medical and surgical management of dilated ascending aorta.
Symptomatic aortic insufficiency or stenosis may be the primary indication for operation. When replacing or repairing a diseased valve a decision must be made regarding the moderately dilated aorta. Approximately 25% of patients operated on for aortic insufficiency with an aortic diameter > 4 cm will require subsequent surgery for aortic replacement.1
For patients with Marfan syndrome and bicuspid valves the size criterion is somewhat lower. In these patients, most authors feel that prophylactic repair is warranted for an intervention criterion of 4.5–5.0 cm diameter.9,10 Dissection or rupture have been reported at sizes < 5.0 cm in several cases, and an increase rate of aneurysm dilatation > 5% per year is known to lead to a 4.1‐fold risk of complications. Other risk factors for aortic dissection in patients with Marfan syndrome include familial history of aortic dissection and a ratio between observed diameter and predicted diameter above 1.3. Moreover, these individuals are often young and otherwise healthy, and thus prophylactic intervention may confer substantial benefits.
SURGICAL TECHNIQUES
Choice of surgical technique depends on the location of the aneurysm, the distal extent of aortic involvement, underlying pathology, life expectancy of the patient, and desired anticoagulation status.
The two types of aneurysm require different surgical management. The supravalvular aneurysm is treated by a simple supracoronary tube graft, whereas the aortic root aneurysm involves the coronary ostia which need to be reimplanted as well as the aortic valve which needs to be spared or replaced.
Supravalvular aortic aneurysms
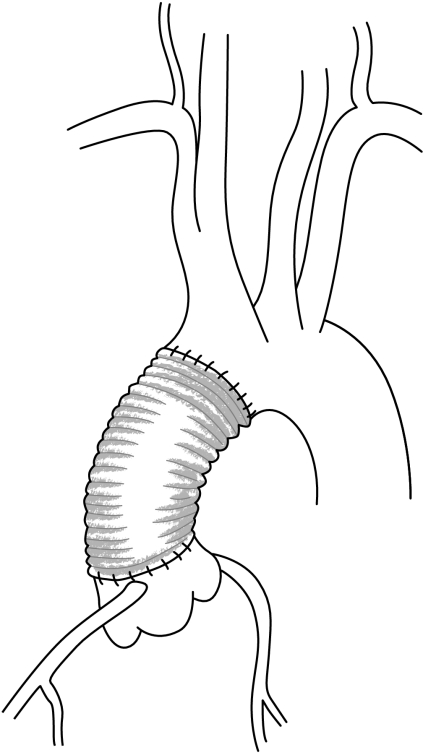
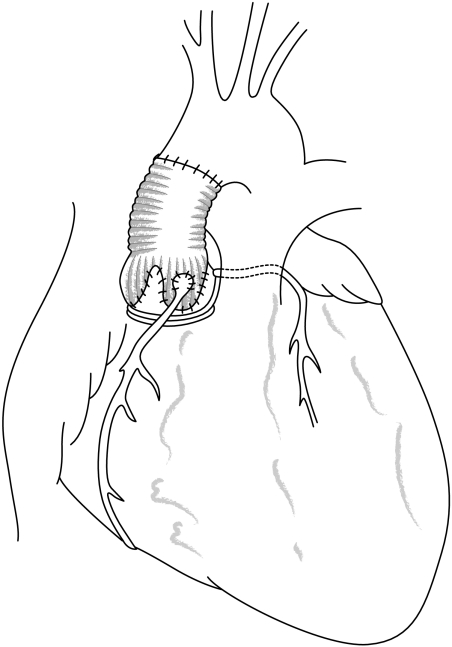
In the case of atherosclerotic aneurysm of the ascending aorta, located at the supravalvular level, a supracoronary tube graft is performed (fig 7). If the aortic valve is diseased, it can be replaced separately. If the sinuses of Valsalva are dilated, a supracoronary tube graft should not be performed because of the frequent need for reoperation by further dilatation of the aortic root.
Figure 7 Supracoronary tube graft.
Aortic root aneurysms
Patients with dilation of the aortic root should undergo a replacement of the root and supravalvular ascending aorta. The dilation of the aortic root frequently leads to secondary aortic regurgitation, despite the presence of morphologically normal valve leaflets in most of the cases.
Two different surgical approaches are available: either radical, replacing the aortic valve and root; or conservative, replacing the aortic root while sparing the aortic valve.
Replacement of the aortic valve and root
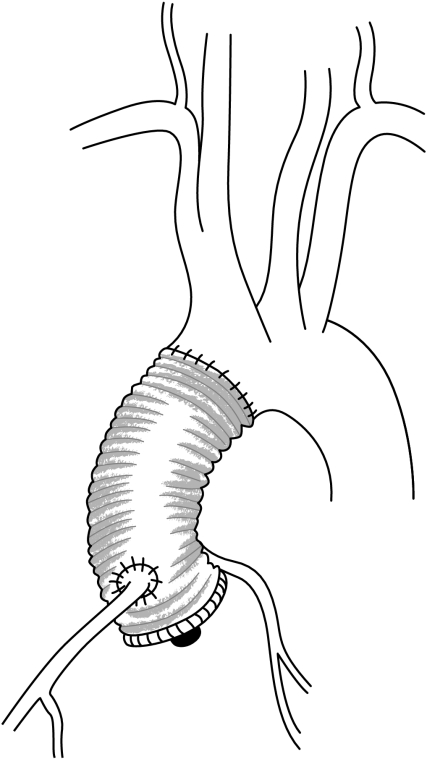
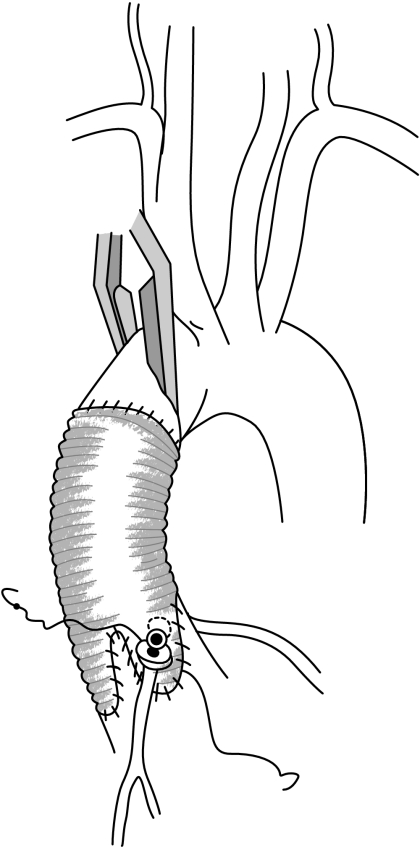
Replacement of the aortic valve and root is performed using a composite graft including a prosthetic valve (most often a mechanical valve). The coronary arteries are reimplanted into the tube graft using the buttons technique (fig 8). This operation was first described by De Bono and Bentall in 1968, and is associated with the need for lifelong oral anticoagulation because of the presence of a mechanical aortic valve.11 Although good results using this technique have been achieved for more than 20 years, with an operative mortality rate of 5% or less, its benefits have to be weighed against the risks of life‐long anticoagulation (thromboembolic and haemorrhagic complications). Indeed, the majority of patients with aortic root aneurysms are young, and although the risks of thromboembolism and bleeding in the presence of mechanical valves are low in these patients, their long life expectancy will increase the cumulative risk of valve‐related morbidity.
Figure 8 Composite valve and graft replacement.
Replacement of the aortic root while preserving the valve
Because the aortic valve is macroscopically normal in most patients, techniques to replace the diseased aortic root, while preserving the native aortic valve, have been developed since 1980, in order to avoid using prosthetic material and the subsequent complications from anticoagulation therapy.
For aortic root aneurysm, regardless of the aetiology and the degree of aortic regurgitation, the following lesions arise: dilation of the aortic annulus, sinuses of Valsalva and sinotubular junction.12 Therefore, in any case of aortic root aneurysm (Marfan syndrome, bicuspid valve or idiopathic aneurysm), the same surgical principles of treatment can be applied—correction of the annulus and sinotubular junction dilation, and a reconstruction of the aortic root, respecting its dynamic anatomy.
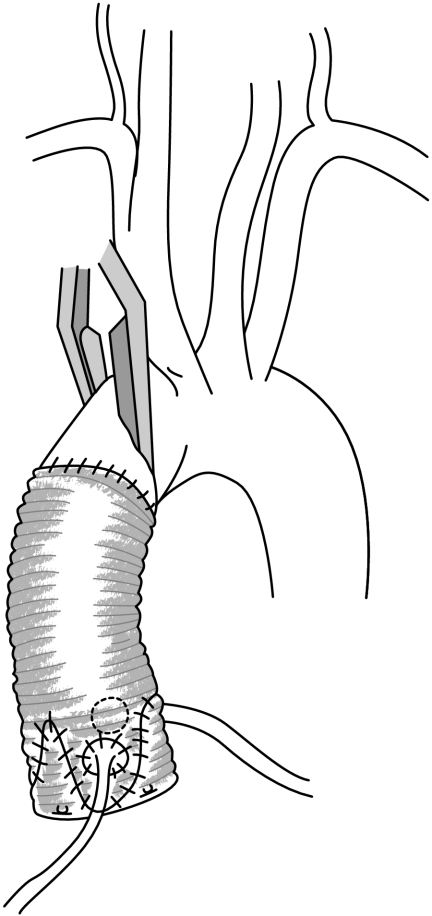
Two types of aortic valve‐sparing operations were originally performed: the remodelling of the aortic root described by Yacoub; and reimplantation of the aortic valve described by David. The remodelling technique reduces the sinotubular junction diameter and creates three neo sinuses of Valsalva by using a scalloped Dacron tube graft sutured in the supravalvular position13 (fig 9). Alternatively, reimplantation of the aortic valve within a cylindrical tube, implanted in a subvalvular position, reduces both the annulus and the sinotubular junction diameters to the detriment of the aortic annulus dynamics, with removal of the sinuses of Valsalva14 (fig 10). Although both techniques spare the aortic valve, indications for the use of either technique remain controversial. Indeed the remodelling technique provides a physiological reconstruction of the aortic root with three neo sinuses of Valsalva enhancing the durability of the repair, but does not address the dilation of the aortic annulus below the valve; the reimplantation technique, on the other hand, treats the dilation of the aortic annulus and sinotubular junction but impairs the dynamics of the valve (inclusion into a rigid tube) and abolishes the sinuses of Valsalva.15 In order to treat the lesions (dilation of the aortic annulus and sinotubular junction) and to preserve the dynamic anatomy of the aortic root to ensure proper valve function, it has been suggested the advantages of both approaches be combined by adding an external subvalvular prosthetic ring annuloplasty to the remodelling procedure to standardise the valve sparing procedure (fig 11).16
Figure 9 Remodelling of the aortic root.
Figure 10 Reimplantation of the aortic valve.
Figure 11 Remodelling associated with a subvalvular aortic annuloplasty.
Original series provide excellent results at 5–10 years follow‐up with a low rate of valve related complications or thromboembolic events.17,18,19,20 Actual results of composite graft replacement and valve sparing procedures are summarised in table 3.
Table 3 Results of recent series of valve sparing operations, compared with composite graft replacement.
| Total “remodelling”w1–4 | Total “reimplantation”w5–14 | Total of series combining the two techniquesw15–17 | Composite graft replacementw18–28 | |
|---|---|---|---|---|
| Patients (n) | 475 | 700 | 736 | 2453 |
| Mean age | 35.4 (2–76) | 49.7 (8–84) | 50.05 (1.5–81) | 49.1 (38.5–54) |
| Marfan syndrome | 159 (33.5%) | 214 (30.6%) | 155 (21%) | 651 (26.6%) |
| Bicuspid valve | 64 (13.5%) | 17 (2.4%) | 133 (18%) | 139 (18.4%)* |
| Emergency indications | 37 (8%) | 34 (4.8%) | 47 (6.5%) | 262 (14.1%)† |
| Operative mortality | 3.9% (2–4.9) | 1.67% (0–3.8) | 1.9% (0–4) | 5.3% (0–7.8) |
| Mean follow‐up | 86 mo (1–19 yrs) | 30 mo (1–12 yrs) | 29.82 mo (1–87 mo) | 5.7 yrs (3–7.87 yrs) |
| 5 or 10 years survival | 89.8% | 94.15% | 91.5% | 74.9% at 10 yrs |
| Thromboembolic complications | 0% | 0% | 0% | 4.2–20% at 10 yrs |
| Haemorrhagic complications | 0% | 0% | 0% | |
| Reoperation | 7.1% | 4.1% (3,8–9) | 7.2% | 7.2% |
| Mean (SD) postoperative transvalvular gradient (mm Hg) | 4.3 (2.2) | 4.3 | 4.3 | 14 (9) |
| Mean postoperative AR grade | 0.53 | 0.45 | 0.5 | – |
To view study referencesw1–28 visit the Heart website—http://www.heartjnl.com/supplemental
*Results available for 3 series; †results available for 9 series.
AR, aortic regurgitation; mo, months; yrs, years.
Alternative procedures
When a valve sparing operation cannot be performed and anticoagulation therapy is contraindicated, then the aortic root and valve can be replaced by an aortic homograft or a biological stented or not stented substitute.
The pulmonary autograft (Ross procedure) in adults with aneurysmal disease is a contraindication in Marfan syndrome because dystrophic degeneration of the aortic wall is also present on the pulmonary trunk wall. For the same reason, indications are very controversial in bicuspid valve in adults and should not be recommended.
Reoperation
Reoperation may be required because of pseudoaneurysm formation, valve thrombosis or endocarditis, progression of disease in the native valve, or remaining aortic segments. Reported freedom from reoperation and mortality of reoperative ascending aortic surgery depends of the type and indication of the first operation and are summarised in table 3.
Follow‐up
Serial follow‐up evaluation of the operated ascending aorta and remaining thoraco‐abdominal aorta should be performed after the initial operation, based on echocardiography associated with CT or MRI. β blocker therapy must be continued, to prevent or slow down dilation of the remaining aorta.
CONCLUSION
A dilated ascending aorta over the critical diameter of 50 mm is a risk factor for dissection or aortic rupture. Supravalvular aneurysm is treated by a simple supracoronary tube graft. Aortic root aneurysm involves the coronary ostia, which need to be reimplanted, as well as the aortic valve, which needs to be spared or replaced. Although aortic valve sparing represents a very attractive approach, most of the patients are still treated with a composite graft and a mechanical valve. Moreover it remains unknown if a valve sparing procedure will provide better long term results than a composite graft replacement. Further clinical investigation is needed to answer this question. A prospective multicentred randomised trial comparing 120 patients undergoing a valve sparing procedure versus 120 patients receiving mechanical valves is about to be undertaken.
References for table 3 appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References for table 3 appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Isselbacher E M. Thoracic and abdominal aortic aneurysms. Circulation 2005111816–828. [DOI] [PubMed] [Google Scholar]
- 2.Bickerstaff L K, Pairolero P C, Hollier L H.et al Thoracic aortic aneurysms: a population‐based study. Surgery 1982921103–1108. [PubMed] [Google Scholar]
- 3.Coady M A, Rizzo J A, Goldstein L J.et al Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 199917615–635. [DOI] [PubMed] [Google Scholar]
- 4.Jondeau G, Boileau C, Chevallier B.et al Marfan syndrome. Arch Mal Coeur Vaiss 2003961081–1088. [PubMed] [Google Scholar]
- 5.Nistri S, Basso C, Marzari C.et al Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 200596718–721. [DOI] [PubMed] [Google Scholar]
- 6.Davies R R, Goldstein L J, Coady M A.et al Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 20027317–27.Thoracic aneurysm is a lethal condition; its size has a profound impact on rupture, dissection, and death. Expected yearly rate of rupture or dissection is at least 6.9% and a death rate of 11.8% when diameter exceeds 6 cm. [DOI] [PubMed] [Google Scholar]
- 7.Roman M J, Devereux R B, Niles N W.et al Aortic root dilatation as a cause of isolated, severe aortic regurgitation. Prevalence, clinical and echocardiographic patterns, and relation to left ventricular hypertrophy and function. Ann Intern Med 1987106800–807. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell J H, Haskell W, Snell P.et al Task Force 8: classification of sports. J Am Coll Cardiol 2005451364–1367.Exercise is divided into two broad types: dynamic (isotonic) and static (isometric), categorised by the level of intensity (low, medium, high) generally required to perform that sport. Recommendations are provided for competitive athletes with cardiovascular abnormalities. [DOI] [PubMed] [Google Scholar]
- 9.Iung B, Gohlke‐Barwolf C, Tornos P.et al for the Working Group on Valvular Heart Disease. Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J 2002231252–1266.In patients with aortic root dilatation > 55 mm, surgery should be undertaken irrespective of the degree of aortic regurgitation or left ventricular function. In patients with bicuspid aortic valves or with Marfan syndrome an even lower degree of root dilatation (50 mm) can be used as a threshold for surgery, particularly if a valve‐sparing operation is possible or if there is a rapid increase of aortic diameter. [DOI] [PubMed] [Google Scholar]
- 10.Bonow R O, Carabello B, de Leon A C.et al ACC/AHA guidelines for the management of patients with valvular heart disease. Executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on management of patients with valvular heart disease). J Am Coll Cardiol 1998321486–1488.9809971Aortic valve surgery and aortic root reconstruction are indicated in patients with disease of the proximal aorta and aortic regurgitation of any severity when the degree of aortic root dilation is ⩾ 50 mm by echocardiography. [Google Scholar]
- 11.Bentall H H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 196823338–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood M J, El Khoury G, Glineur D.et al The aortic root: structure, function, and surgical reconstruction. Heart 200083376–380.The aortic root complex acts as an individual haemodynamic system. The sinuses of Valsalva and the interleaflet triangles are crucial to proper valve function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yacoub M, Fagan A, Stassano P.et al Result of valve conserving operations for aortic regurgitation [abstract]. Circulation 198368(suppl)III321 [Google Scholar]
- 14.David T E, Feindel C M. An aortic valve‐sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992103617–622. [PubMed] [Google Scholar]
- 15.Leyh R G, Schmidtke C, Sievers H H.et al Opening and closing characteristics of the aortic valve after different types of valve‐preserving surgery. Circulation 19991002153–2160.Echocardiographic study of instantaneous opening and closing characteristics of the aortic valve after using remodelling and reimplantation aortic valve‐sparing surgery, compared to normal aortic valve dynamics. Near‐normal opening and closing characteristics can be achieved by a technique that preserves the shape and independent mobility of the sinuses of Valsalva. [DOI] [PubMed] [Google Scholar]
- 16.Lansac E, Di Centa I, Varnous S.et al External aortic annuloplasty: a useful adjunct to valve sparing procedure. Ann Thorac Surg 200579356–368. [DOI] [PubMed] [Google Scholar]
- 17.Langer F, Aicher D, Kissinger A.et al Aortic valve repair using a differentiated surgical strategy. Circulation 2004110(11 suppl 1)II67–II73.Report of experience with 282 patients undergoing surgery for aortic regurgitation and concomitant aortic root aneurysm. Aortic valve repair is feasible, even for complex mechanisms of aortic regurgitation, with a systematic and individually tailored approach. Operative mortality is low and mid‐term durability is encouraging. The incidence of valve‐related morbidity is low compared with valve replacement. [DOI] [PubMed] [Google Scholar]
- 18.Kallenbach K, Karck M, Pak D.et al Decade of aortic valve sparing reimplantation: are we pushing the limits too far? Circulation 2005112(9 suppl)I253–I259.This single centre study assesses the favourable clinical outcome of aortic valve sparing reimplantation in 284 patients. Lack of anticoagulation and favourable durability should encourage the extension of indications for this technique. [DOI] [PubMed] [Google Scholar]
- 19.Zehr K J, Orszulak T A, Mullany C J.et al Surgery for aneurysms of the aortic root: a 30‐year experience. Circulation 20041101364–1371.Composite valve conduit reconstruction was performed in 149 patients, with low morbidity and mortality. Complications with anticoagulation occurred in 29 patients (valve thrombosis in two cases, haemorrhage in 27 cases). Freedom from thromboembolism and endocarditis at 20 years were 91% and 99%, respectively. [DOI] [PubMed] [Google Scholar]
- 20.Jault F, Nataf P, Rama A.et al Chronic disease of the ascending aorta. Surgical treatment and long‐term results. J Thorac Cardiovasc Surg 1994108747–754.Results of 339 elective composite graft replacement procedures. The 30‐day mortality rate was 7.6% (n = 26). Long‐term survival was 59.6% (±3.7%) at nine years. Reoperation was needed in 14 patients (actuarial freedom from reoperation at nine years: 90% ±0.2%). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.