Abstract
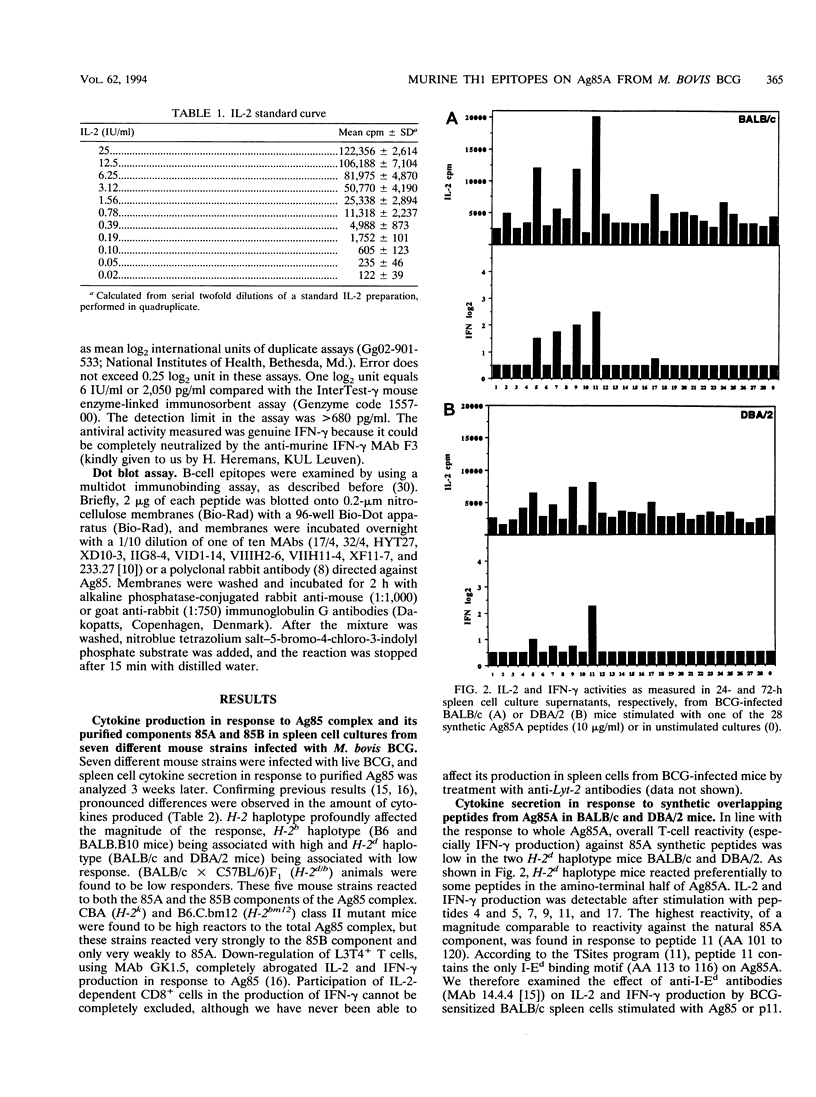
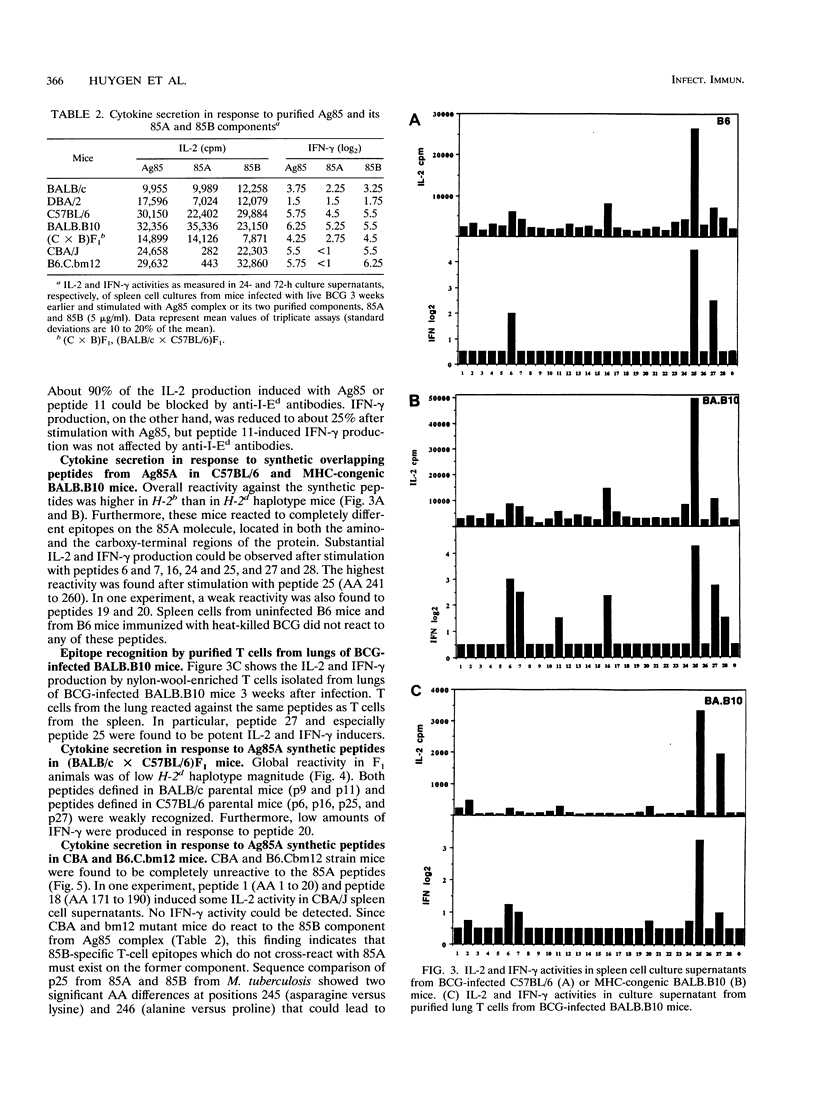
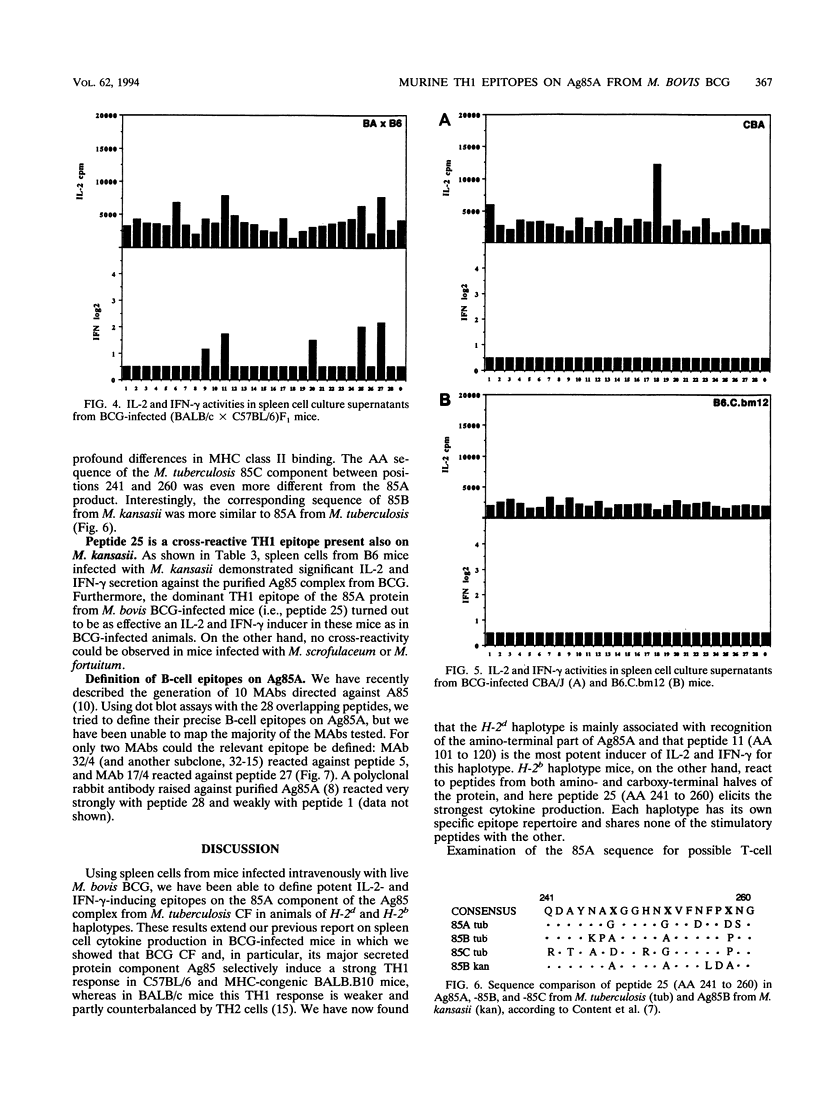
TH1 cytokine secretion was examined in response to synthetic peptides of the 85A component of the major secreted, fibronectin-binding antigen 85 complex from Mycobacterium tuberculosis in seven different mouse strains infected with live M. bovis BCG. Twenty-eight overlapping 20-mer peptides covering the complete mature 295-amino-acid (AA) protein were synthesized. Significant interleukin-2 (IL-2) and gamma interferon (IFN-gamma) secretion could be measured following in vitro stimulation of spleen cells with these peptides. H-2d haplotype mice reacted preferentially against the amino-terminal half of the protein, i.e., against peptide 5 (AA 41 to 60) and especially against peptide 11 (AA 101 to 120), which contained an I-Ed binding motif. H-2b haplotype mice, on the other hand, reacted against peptides from both amino- and carboxy-terminal halves of the protein, peptide 25 (AA 241 to 260) being the most potent stimulator of IL-2 and IFN-gamma production. (BALB/c x C57BL/6)F1 animals with the H-2d/b haplotype weakly recognized peptides specific for both parental lines. Finally, CBA/J (H-2k) and major histocompatibility complex class II mutant B6.C.bm12 mice, carrying a mutant I-A beta bm12 allele on an H-2b background, reacted only very weakly to the 85A peptides. Reactive T cells isolated from lungs of BCG-infected H-2b haplotype mice recognized the same epitopes as spleen cells, especially peptide 25. These data confirm previous findings regarding the powerful IL-2 and IFN-gamma-inducing properties of antigen 85 during infection with live M. bovis BCG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Gottschau A., Bennedsen J., Nagai S., Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992 Dec;36(6):823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Dieli F., Gautam Y., Siew L. K., Zembala M. Major histocompatibility complex regulation of the class of the immune response: the H-2d haplotype determines poor interferon-gamma response to several antigens. Eur J Immunol. 1990 Jun;20(6):1305–1310. doi: 10.1002/eji.1830200616. [DOI] [PubMed] [Google Scholar]
- BLOCH H., SEGAL W. Viability and multiplication of vaccines in immunization against tuberculosis. Am Rev Tuberc. 1955 Feb;71(2):228–248. doi: 10.1164/artpd.1955.71.2.228. [DOI] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., de la Cuvellerie A., De Wit L., Vincent-Levy-Frébault V., Ooms J., De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991 Sep;59(9):3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Drowart A., De Bruyn J., Huygen K., Damiani G., Godfrey H. P., Stelandre M., Yernault J. C., Van Vooren J. P. Isoelectrophoretic characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand J Immunol. 1992 Nov;36(5):697–702. doi: 10.1111/j.1365-3083.1992.tb03130.x. [DOI] [PubMed] [Google Scholar]
- Feller D. C., de la Cruz V. F. Identifying antigenic T-cell sites. Nature. 1991 Feb 21;349(6311):720–721. doi: 10.1038/349720a0. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Tse H. Y. Insights into immune-response gene function using an Ia mutant mouse strain. Crit Rev Immunol. 1987;7(3):169–191. [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Joliff G., Mathieu L., Hahn V., Bayan N., Duchiron F., Renaud M., Schechter E., Leblon G. Cloning and nucleotide sequence of the csp1 gene encoding PS1, one of the two major secreted proteins of Corynebacterium glutamicum: the deduced N-terminal region of PS1 is similar to the Mycobacterium antigen 85 complex. Mol Microbiol. 1992 Aug;6(16):2349–2362. doi: 10.1111/j.1365-2958.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Launois P., Huygen K., De Bruyn J., N'Diaye M., Diouf B., Sarthouj L., Grimaud J., Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991 Nov;86(2):286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launois P., Niang M. N., De Bruyn J., Sarthou J. L., Rivier F., Drowart A., Van Vooren J. P., Millan J., Huygen K. The major secreted antigen complex (Ag 85) from Mycobacterium bovis bacille Calmette-Guérin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J Infect Dis. 1993 May;167(5):1160–1167. doi: 10.1093/infdis/167.5.1160. [DOI] [PubMed] [Google Scholar]
- Lin C. C., Rosenthal A. S., Passmore H. C., Hansen T. H. Selective loss of antigen-specific Ir gene function in IA mutant B6.C-H-2bm12 is an antigen presenting cell defect. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6406–6410. doi: 10.1073/pnas.78.10.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Manca F., Rossi G., Valle M. T., Lantero S., Li Pira G., Fenoglio D., De Bruin J., Costantini M., Damiani G., Balbi B. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun. 1991 Feb;59(2):503–513. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C., Williams V. E., 2nd, Kanter M., McDevitt H. O. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara N., Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and sequencing of the gene for alpha antigen from Mycobacterium avium and mapping of B-cell epitopes. Infect Immun. 1993 Apr;61(4):1173–1179. doi: 10.1128/iai.61.4.1173-1179.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D. B., Mitchison N. A. Immune suppression genes. Clin Exp Immunol. 1989 Feb;75(2):167–177. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Van Vooren J. P., Turneer M., Yernault J. C., De Bruyn J., Burton E., Legros F., Farber C. M. A multidot immunobinding assay for the serodiagnosis of tuberculosis. Comparison with an enzyme-linked immunosorbent assay. J Immunol Methods. 1988 Oct 4;113(1):45–49. doi: 10.1016/0022-1759(88)90380-8. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992 Dec;56(4):648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]




