Fulminant myocarditis (FM) is characterised by a rapid onset of heart failure, usually following a flu‐like illness.1 Intensive cardiovascular support including mechanical assist devices can be lifesaving; however, reports of experience with providing mechanical circulatory support to paediatric patients with FM is quite limited.2 In addition, the clinical presentation has rarely been described in detail. We report our experience treating paediatric patients with FM who were rescued by the use of extracorporeal membrane oxygenation (ECMO) during the acute stage of the illness. We describe their clinical courses and treatment strategies in detail.
PATIENTS AND METHODS
From June 1997 to March 2005, nine children (three boys and six girls with a mean age of 9.1 (SD 3.3) years, range 5–15 years) with FM were identified in our institute. Each patient had a clinical picture compatible with FM and had ECMO instituted because of a clinical presentation of imminently lethal cardiogenic shock, pulseless ventricular arrhythmia or cardiac arrest.
The ECMO circuit consisted of a centrifugal pump and a hollow‐fibre, microporous‐membrane oxygenator with an integrated heater (CB2505; Medtronic Inc, Anaheim, California, USA), similar to the rapid deployment ECMO that was described by Jacobs et al.3 The ECMO pump blood flow was started initially at 50–70 ml/kg/min. Catecholamine infusion was adjusted according to haemodynamic status. Daily echocardiography and ECG assessed each patient's cardiac function. Patients received intravenous immunoglobulin (2 g/kg) (Gamimune N; Bayer Corp, Elkhart, Indiana, USA) according to the protocol recommended by McNamara et al.4 Patients did not receive other immunosuppressants.
We used temporary pacemakers to compensate for atrioventricular block and bradycardia in two patients. Two larger patients received intra‐aortic balloon pump therapy before institution of ECMO. The fluid status was controlled by diuretics and haemofiltration on the ECMO circuit, if needed. If the patient had severe lung oedema and distended left atrium and ventricle, left atrial drainage was added.
The medical records were reviewed and descriptive statistics were used. Data are shown as mean (SD), unless otherwise specified.
RESULTS
All nine patients had flu‐like symptoms two days to one week before admission. Symptoms on admission included persistent vomiting in four patients and episodes of consciousness change (seizures, drowsiness or syncope) in five patients. Their clinical conditions deteriorated rapidly after admission. The mean duration from admission to haemodynamic collapse requiring ECMO support was 11.1 (7.1) h (range 3–23 h). Three patients were put on ECMO under the scenario of external cardiac massage. Cardiomegaly or lung oedema on admission chest radiography was present in only three patients.
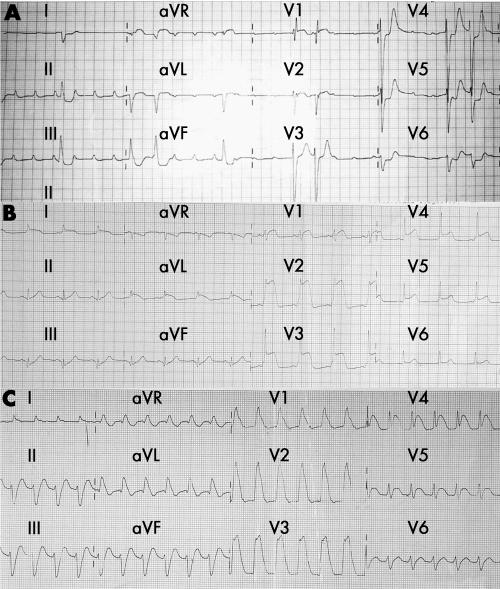
ECG on admission showed high‐degree atrioventricular block in two patients (fig 1A), sinus tachycardia with diffuse ST‐T segment changes in two patients (fig 1B) and wide QRS tachycardia in five patients (fig 1C). All patients had raised cardiac enzymes before institution of ECMO, with creatine kinase 4320 (3995) U/l, creatine kinase MB fraction 327 (189) U/l and troponin I 47.0 (29.1) ng/ml, showing the severity of myocardial damage. Echocardiography on admission showed left ventricular systolic dysfunction (left ventricular ejection fraction 31.4 (15.6)%), with a nearly normal left ventricular diastolic dimension and increased septal thickness.
Figure 1 Example ECGs of patients with fulminant myocarditis showing (A) high‐degree atrioventricular block, (B) diffuse ST‐T change and (C) wide QRS tachycardia.
Echocardiography showed that left ventricular ejection fraction continued to decline even after initiation of ECMO but that it gradually improved around the fourth day after ECMO support in survivors. All patients had wide polymorphic QRS tachycardia during ECMO support, and the QRS interval gradually narrowed as ventricular function improved. The improvement of cardiac contractility shown by echocardiography was relatively parallel to the narrowing of the QRS complex. The patients with atrioventricular block resumed normal sinus rhythm after three days of ECMO. The ST‐T change usually took about two weeks to normalise.
In the cohort, six patients (66.7%) survived to hospital discharge. The duration of ECMO in survivors was 115 (34) (61–216) h. Renal failure was noted in four patients, and three of them died (75%). The mean intubation period of survivors was 6.5 (1.1) days. Three patients died; the first, due to sepsis; the second, due to ECMO circuit occlusion by a thrombus; and the third, due to severe hypoxic encephalopathy after prolonged cardiac massage. All survivors stayed in New York Heart Association functional class I during the follow‐up period of 52.5 (24.8) months.
DISCUSSION
FM carries a high mortality, ranging from 50–75%, without immediate mechanical circulatory support.5 Prompt diagnosis, as well as proper mechanical circulatory support, improves survival. This single‐institute study, which may be the largest series focused on paediatric patients with FM rescued by ECMO, indicates the effectiveness of ECMO support.
All nine patients in this study had non‐specific flu‐like symptoms with mild fever lasting for 2–7 days and then developed haemodynamic compromise. It is noteworthy that their chief complaints on admission such as vomiting, seizure and drowsiness seemed unassociated with the cardiovascular system and were, thus, misleading. Thus, by high index of suspicion, clinicians should consider electrocardiography and determine cardiac enzyme concentrations to judge the critical nature of the condition. We suggest that when the arrhythmias develop, the patient should be referred immediately to a medical centre with mechanical circulatory support facilities because the clinical condition can deteriorate very quickly into multifocal ventricular arrhythmia and cardiogenic shock refractory to medical treatment.
The choice of mechanical circulatory support devices for paediatric patients is limited. The arrhythmia and small femoral artery would preclude proper use of an intra‐aortic balloon pump for paediatric patients. ECMO and ventricular assist device were used for paediatric patients with FM as described by Duncan et al.2 Because FM tends to recover within two weeks, ECMO may be a more appropriate option for this relatively short duration. Furthermore, ECMO can be set up even for patients who are being resuscitated. We therefore suggest that ECMO be regarded as the first‐line mechanical circulatory support for paediatric patients with FM.
Paediatric FM requiring ECMO support was characterised by preceding non‐specific extracardiac symptoms and rapid onset of heart failure associated with ECG changes. Early recognition of the clinical picture and prompt ECMO support may provide better chances of recovery for these patients.
Footnotes
Competing interests: None declared.
References
- 1.Batra A S, Lewis A B. Acute myocarditis. Curr Opin Pediatr 200113234–239. [DOI] [PubMed] [Google Scholar]
- 2.Duncan B W, Bohn D J, Atz A M.et al Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg 2001122440–448. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs J P, Ojito J W, McConaghey T W.et al Rapid cardiopulmonary support for children with complex congenital heart disease. Ann Thorac Surg 200070742–749. [DOI] [PubMed] [Google Scholar]
- 4.McNamara D M, Rosenblum W D, Janosko K M.et al Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation 1997952476–2478. [DOI] [PubMed] [Google Scholar]
- 5.Fenoglio J J, Jr, Ursell P C, Kellogg C F.et al Diagnosis and classification of myocarditis by endomyocardial biopsy. N Engl J Med 198330812–18. [DOI] [PubMed] [Google Scholar]