Abstract
Objectives
To highlight clinical features and outcome of acute fulminant myocarditis (AFM) in children.
Methods
Diagnostic criteria were (1) the presence of severe and acute heart failure; (2) left ventricular dysfunction on echocardiography; (3) recent history of viral illness; and (4) no history of cardiomyopathy.
Results
Eleven children were included between 1998 and 2003, at a median age of 1 (0 to 9) year. Their mean left ventricular ejection fraction (LVEF) was 22 (SD 9)% at presentation. A virus was identified in five patients: human parvovirus B19 (n = 2), Epstein–Barr (n = 1), varicella zoster (n = 1), and coxsackie (n = 1). The median intensive care unit course was 13 (2–34) days. Intravenous inotropic support was required by nine patients and eight were mechanically ventilated. All patients received corticosteroid, associated with intravenous immunoglobulin in seven. Five patients experienced cardiocirculatory arrest that was successfully resuscitated in four. At a median follow up of 58.7 (33.8–83.1) months, the 10 survivors are asymptomatic with normalised LVEF.
Conclusion
Despite a severe presentation, the outcome of AFM is favourable. Aggressive symptomatic management is warranted and heart transplantation should be considered only when maximal supportive therapy does not lead to improvement.
Acute myocarditis (AM) is defined as inflammation of the myocardium heralded by a non‐specific flu‐like illness.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 It comprises a wide clinical spectrum from subclinical myocardial dysfunction to severe heart failure.1,2,3,5,9,10,13,17 Acute fulminant myocarditis (AFM) is characterised by brutal and distinct onset of severe congestive heart failure or cardiogenic shock.2,3,6,13,20 Despite this initial severe presentation, favourable long‐term prognosis of AFM with complete recovery of left ventricular function is reported in the adult population.2,3,20 Aggressive treatment is warranted in these patients, including mechanical circulatory support and heart transplantation in patients with persistent myocardial dysfunction.20 In the paediatric age group, several studies have examined the diagnosis and treatment of AM.5,6,7,8,9,10,11,12,18,19,21 However, only a few reports have specifically focused on AFM.6,12,19 Such studies have documented successful outcome of AFM with immunomodulative treatment12 or circulatory support6,19 without a detailed description of its clinical picture and global medical management. Because of the lack of data concerning the natural history of AFM in children, the indication and timing for those treatments as well as the need for heart transplantation remain unclear.17 Moreover, information on mid‐term outcome is scarce and the evolution towards complete myocardial recovery or dilated cardiomyopathy has not been determined.11 We reviewed our experience to highlight clinical, biological and echocardiographic features in children with AFM and to report their outcomes.
METHODS
Patients and data collection
We retrospectively studied all the children (age < 15 years) admitted to La Timone Children's Hospital with an AFM from 1998 to 2003. Patients were included if they had (1) severe and acute heart failure; (2) left ventricular dysfunction assessed by echocardiography; (3) a recent history of viral illness3; and (4) absence of personal or familial history of cardiomyopathy.
Acute heart failure was defined by heart failure symptoms with a time interval between onset and hospital admission shorter than seven days. Severe heart failure was defined as severe systemic hypotension requiring intravenous inotropic support or due to life threatening arrhythmias. Recent viral illness was defined as the existence, within the two weeks before admission, of an acute fever and asthenia episode associated with rash or cutaneous eruption, digestive discomfort (diarrhoea, abdominal pain) or upper or lower airways irritation signs. Complete investigations were performed to rule out congenital heart malformations (for example, coronary artery anomalies). Patients with metabolic perturbations, exposure to cardiotoxic agents, chronic primitive arrhythmia and chronic bacterial sepsis were excluded.
The hospital records were reviewed to collect clinical features, ECG and biological, echocardiographic, haemodynamic and histological data. One case of AFM due to Epstein–Barr virus was previously reported.22
Echocardiography
The following data were collected or calculated: left ventricular end diastolic and end systolic diameters, left ventricular ejection fraction (LVEF) by modified Simpson's rule, left ventricular shortening fraction, presence and degree of mitral regurgitation (graded none, mild, moderate or severe, according to the jet width at its origin and area as seen by colour Doppler flow mapping) and systolic pulmonary artery pressure measurement by the peak velocity of tricuspid incompetence. The end diastolic and end systolic dimensions were recorded and expressed as z values based on body surface area from non‐linear regression equations derived from a normal population of children.23
Endomyocardial biopsy
Right ventricular endomyocardial biopsy was performed in three patients under general anaesthesia by a standard percutaneous venous femoral approach with a 6 French bioptome. Three to five myocardial samples were obtained from each procedure in different areas of the right ventricle. Biopsies were fixed in 10% formalin and stained with haematoxylin and eosin for light microscopy. Histological diagnosis was based on the presence of lymphocytic infiltrate associated with myocyte degeneration or necrosis. Classification of AM was based on the Dallas criteria.24
Viral assessment
Each patient underwent serial serology for viruses on admission and this was repeated 15 days later. Evidence of infection for a specific agent was defined as seroconversion (significant rise in Ig G) or presence of a positive polymerase chain reaction (PCR) in the blood for a specific viral agent. Endomyocardial biopsy samples were subsequently analysed by electronic microscopy and myocardial viral PCR when needed and when possible.
Statistical analysis
Continuous data are expressed as mean (SD) or median value when appropriate. Data were statistically analysed by SPSS V.10.0 for Windows software (SPSS Inc, Chicago, Illinois, USA). Parameters of left ventricular function were analysed at presentation and at the latest follow up by a Wilcoxon test for paired samples. A double unilateral Student's t test was used to determine whether our population's z scores were within ±2 SD from an age and body surface area calculated mean. The level of significance was fixed at 0.05.
RESULTS
Initial presentation and echocardiography
From June 1998 to June 2003, 109 consecutive patients were hospitalised with severe heart failure due to dilated cardiomyopathy (table 1). Eleven (10.1%) of these patients (three boys and eight girls) met the inclusion criteria for AFM. All the patients were admitted to the paediatric intensive care unit (ICU). Of the 11 children, nine were directly admitted to our centre, whereas two were transported to our ICU after being managed elsewhere. One patient (patient 5) was transferred for cardiac catheterisation from another paediatric ICU with a diagnosis of idiopathic dilated cardiomyopathy 15 days after his initial presentation. Despite a typical clinical picture of heart failure, another child (patient 2) was referred from a local hospital for “liver transplantation” after one week of ICU course. In his case, acute liver failure was secondary to congestive heart failure. Age at presentation varied from 6 days (maternofetal contamination) to 9.5 years (median 1.05 year) and weight from 3.3–25 kg (median 9.2 kg).
Table 1 Characteristics of patients on admission.
| Patient | Age on admission (months) | Initial LVEF (%) | Initial sPAP (mm Hg) | Reduced QRS amplitude on ECG | CK/TnI rise | Viral agent | EMB results |
|---|---|---|---|---|---|---|---|
| 1 | 21.7 | 25 | 25 | Yes | No | PV B19 | |
| 2 | 12 | 24 | 44 | No | No | AM | |
| 3 | 17 | 30 | 40 | No | No | ||
| 4 | 12.8 | 10 | 30 | No | No | PV B19 | |
| 5 | 13.6 | 20 | 45 | No | No | EBV | BM |
| 6 | 11 | 30 | 45 | No | No | VZV | |
| 7 | 16.5 | 40 | 55 | No | No | ||
| 8 | 4.1 | 25 | 50 | Yes | No | Coxsackie | |
| 9 | 7.1 | 15 | 50 | No | Yes | AM | |
| 10 | 0.2 | 10 | 60 | Yes | Yes | ||
| 11 | 114 | 15 | 50 | No | No |
AM, active myocarditis; BM, borderline myocarditis; CK, creatine kinase; EMB, endomyocardial biopsy; EBV, Epstein–Barr virus; LVEF, left ventricular ejection fraction; PV B19, parvovirus B19; sPAP, systolic pulmonary artery pressure; TnI, troponin I; VZV, varicella zoster virus.
The ECGs showed diffuse ST segment depression in six patients that was associated with reduced QRS complex voltage in three. Two of nine patients had a rise in troponin Ic and creatine kinase. In five of the nine patients troponin Ic and creatine kinase were obtained more than five days after their initial presentation. C reactive protein at admission ranged from 0–72 mg/ml (median 20 mg/l) and was > 10 mg/ml in six children. Initial echocardiography showed decreased left ventricular systolic function in all with a mean LVEF of 22 (9)%. The left ventricle was dilated in all the patients: mean end diastolic diameter (EDD) adjusted to body surface area was 88.2 (21) mm/m2 and mean z score for EDD was 4.39 (2.1) SD. Pulmonary hypertension was observed in 10 children, with a mean systolic pulmonary artery pressure of 44 (10) mm Hg. Six patients had moderate to severe mitral regurgitation. One patient had mild pericardial effusion.
Endomyocardial biopsy and viral assessment
The diagnosis of myocarditis was confirmed by endomyocardial biopsy in three patients (table 1). Two patients had a endomyocardial biopsy because they were referred from another centre where AFM was not diagnosed. The third patient had endomyocardial biopsy, as the patient did not improve after 10 days of inotropic support, mechanical ventilation and corticotherapy. Five patients (45%) had seroconversion on blood samples. One of them had a positive PCR to Epstein–Barr virus.
Hospital course
Median hospitalisation time in the ICU was 13 days (range 2–34 days) (table 2). The hospital course was complicated in eight patients by respiratory distress necessitating mechanical ventilation for a mean duration of 11 (10) days. All patients required intravenous loop diuretics. Intravenous inotropic support was indicated for nine patients for severe haemodynamic compromise: dobutamine (n = 9), dopexamine (n = 2), dopamine (n = 2), milrinone (n = 2) and epinephrine (n = 2). Two patients with sustained ventricular arrhythmia were not given intravenous inotropic support but were treated with an antiarrhythmic drug (amiodarone) and high dose continuous furosemide infusion.
Table 2 Treatment and hospital course.
| Patient | Mechanical ventilation | Circulatory arrest | Use of IV Ig | IV inotropic support | Death | ICU LOS (days) |
|---|---|---|---|---|---|---|
| 1 | No | Yes | No | No | No | 5 |
| 2 | Yes | No | Yes | Yes | No | 28 |
| 3 | Yes | No | Yes | Yes | No | 7 |
| 4 | Yes | Yes | Yes | Yes | No | 17 |
| 5 | Yes | No | Yes | No | 34 | |
| 6 | Yes | Yes | No | Yes | No | 13 |
| 7 | Yes | Yes | Yes | Yes | No | 11 |
| 8 | No | No | Yes | Yes | No | 22 |
| 9 | Yes | No | No | Yes | No | 21 |
| 10 | Yes | No | Yes | Yes | No | 5 |
| 11 | No | Yes | Yes | No | Yes | 2 |
ICU LOS, length of stay in intensive care unit; IV, intravenous.
Five patients experienced a circulatory arrest, related to ventricular tachycardia in four patients and to electromechanical dissociation for extreme myocardial decay in one. Four of these five children were successfully resuscitated. All resuscitated patients had an abnormal EEG pattern in the 24 h after circulatory arrest and one of them experienced seizure. Neurological status improved during hospitalisation for all these patients and was normalised at hospital discharge.
Patient 11, who died, was a 9‐year‐old girl who developed gastrointestinal infection two weeks before onset of acute heart and liver failure. Her initial LVEF was 15%. After two days of an ICU course with inotropic support and intravenous gammaglobulins the patient improved and left the ICU, still treated with diuretics, angiotensin‐converting enzyme inhibitors and corticosteroids. Because 24 h Holter monitoring showed monomorphic ventricular extrasystoles or couplets, oral amiodarone was started. Propranolol was added one week later because of persisting ventricular extrasystoles or couplets. She improved clinically and became strong enough to walk. Fifteen days after her admission, an echocardiogram documented improvement with an LVEF of 30%. Three weeks after the onset of symptoms, she suddenly collapsed after vomiting and could not be resuscitated. Ongoing Holter monitoring showed a rapid ventricular tachycardia degenerating into ventricular fibrillation. Autopsy was not permitted.
Six patients had acute renal failure on admission that evolved to severe organic failure requiring peritoneal dialysis in two patients. Four children had acute liver failure at admission. Liver enzymes progressively normalised under inotropic support. One patient had a left ventricular thrombus that was successfully treated with heparin. In the other patients, anticoagulation was not added systematically.
Symptomatic treatment at ICU discharge was furosemide (n = 6), spironolactone (n = 5), captopril (n = 10), amiodarone (n = 2) and propranolol (n = 1). No patient was listed for heart transplantation during the hospital course.
In addition to symptomatic treatment for cardiopulmonary failure, all patients received anti‐inflammatory corticotherapy (oral prednisone 2 mg/kg/day for one month followed by a tapered dose of 0.5–1 mg/kg/day for 1–2 months) during hospitalisation. After 1999, a gammaglobulin infusion (2 g/kg over 24 h) was systematically added (n = 7 children).
Follow up
Follow up of the 10 survivors (91%) ranges from 33.8–83.1 (median 58.7) months. At last follow up, all the patients were asymptomatic without any drugs. All of them had normal subsequent development with no neurological sequelae, even in children with a history of cardiocirculatory arrest.
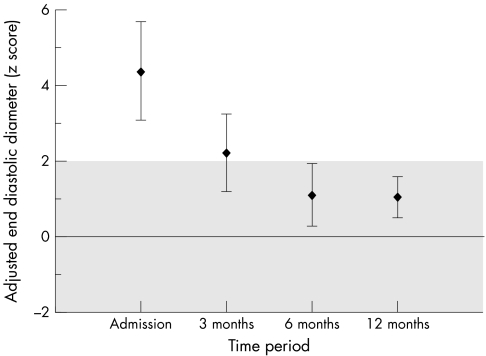
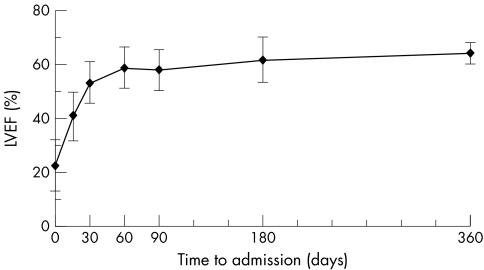
Echocardiographic examination showed normalisation of left ventricular function (fig 1). After one year of follow up, mean LVEF increased significantly from baseline to 64.5 (3.8)% (p = 0.005), whereas mean EDD adjusted to body surface area and z score for EDD decreased significantly to 59.4 (4.95) mm/m2 (p = 0.005) and 1.07 (0.9) SD (p = 0.005). LVEF increased mainly during the first month of evolution (fig 2). ECG and Holter monitoring showed no evidence of residual arrhythmia.
Figure 1 Evolution of left ventricular end diastolic diameter z score adjusted for body surface area, according to time after presentation. Plots represent mean end diastolic diameter in patients and vertical lines represent the 90% confidence intervals. The grey area represents the expected mean value ±2 SD of left ventricular end diastolic diameter derived from a normal population of children.22
Figure 2 Evolution of mean left ventricular ejection fraction (LVEF) in survivors according to time after presentation. Plots represent mean LVEF and vertical lines represent the confidence intervals (mean (SD)). Measures of LVEF are given for admission and days 15, 30, 60, 90, 180 and 360 of evolution.
DISCUSSION
Clinical data are found to be relatively unreliable in diagnosing AM.11 The clinical spectrum varies widely, from asymptomatic patients to patients with signs of moderate heart failure and cardiomegaly on the chest radiography and to patients with severe heart failure.2,3,5,7,9,10,11,12,13,14,17,19,20,21 In AFM, the presentation is typically abrupt: patients present with severe congestive heart failure, cardiogenic shock or severe arrhythmias.1,2,3,6,12,19,20
In our study, diagnosis of AFM was largely based on clinical features with echocardiography. ECG and biological markers served as additional non‐invasive diagnostic modalities. Endomyocardial biopsy has historically been the reference standard in diagnosing myocarditis.1,2,3,5,6,7,8,9,10,11,12,13,14,17,18,19,20,21 Despite the Dallas criteria, created to define the histopathological diagnosis of myocarditis, EMB is far from ideal. Because inflammation of the myocardium may be patchy and easily missed, the sensitivity of EMB remains low. In the study of Duncan et al6 of patients with a typical clinical picture of AFM, only two thirds had positive biopsy results. Moreover, the risk of cardiac perforation with EMB is increased in young children requiring inotropic support.25 Thus, EMB seems to be indicated only when mechanical support is necessary or when there is no myocardial recovery after 15 days of an ICU course. Over recent years promising diagnostic tools have been developed, including assays for autoimmune serum markers or the induction of major histocompatibility and intercellular adhesion molecules on cardiac myocytes, autoimmune scintigraphy, contrast enhanced magnetic resonance imaging, cine magnetic resonance angiography and echocardiographic digital image processing.17 Similarly, advances in molecular biology may further increase the diagnostic sensitivity of EMB.11,17,26 The impact these techniques will have on diagnosis and treatment strategies is unknown.
The outcome of AM is poorly characterised. Myocardial recovery may be complete in a significant percentage of patients, whereas others will develop dilated cardiomyopathy with the ultimate need for cardiac transplantation.1,2,3,5,7,8,9,10,11,13,14,15,17,18,19,20,21 AFM seems to behave differently, however, with critical illness at presentation but excellent long‐term survival and complete recovery of left ventricular function.2,3,6,12,19,20 In a prospective study, McCarthy et al3 compared the outcome between 15 patients with AFM and 132 with acute non‐fulminant myocarditis. Ninety three per cent of the patients with AFM survived 11 years after the diagnosis without the need for heart transplantation, whereas only 45% of the patients with AM were still living without having received a heart transplant. Patients younger than 15 years were excluded from their study because of “concern about the heterogeneity of myocarditis in children”.3 In the paediatric age group, a favourable outcome was reported in AFM after circulatory support6,19 and after immunosuppression with monoclonal OKT3.12 No previous study in children has focused on the outcome of AFM, however, and the extrapolation of outcomes from the adult to the paediatric population cannot be assumed.
The present study confirmed the initial severity of AFM in this paediatric population with a high incidence of cardiovascular complications but limited hospital mortality (9%) under aggressive medical support. Myocardial function normalised for all survivors within the first year. In our patients, myocardial recovery started early after hospital admission (fig 2), confirming previous data describing AFM,6,12,19 and allowing withdrawal of mechanical ventilation and ICU management within 15 days to three weeks. Lastly, in our study, myocardial recovery occurred in AFM independently of any circulatory support or new specific immunosuppression. Thus, we agree with McCarthy et al3 that ventricular function may ultimately be expected to normalise in most patients with AFM.
Table 3 summarises the published series including more than three cases of AM and AFM in children.5,6,7,9,10,11,12,19,21 Data concerning the outcome and specific treatment are reported. Two reports were excluded because of lack of data about demographics and patient outcomes.8,18 This overview comprises only cohort studies, as no randomised controlled trials have been performed in the paediatric age group. Anti‐inflammatory and immunomodulative treatments were used in the majority of these studies but treatment strategies differ between centres.5,7,8,10,21 Without a comparable control group, however, it is not possible to conclude that treatment per se influenced the favourable outcome. In our study corticotherapy and intravenous gammaglobulin were administrated according to institutional policy, although their effectiveness remains unproved in the adult and paediatric populations.27 Also, the majority of our patients presented within the first two years of life, including one case of neonatal AFM (table 1). This young age at presentation seems to be associated with better survival.5,9
Table 3 Published series about outcome of myocarditis in the paediatric age group.
| Study | Type of myocarditis | No of patients | Clinical data | Viral doc | EMB | Age (years) | IA drugs | Death or Tx | LVF rec/surv |
|---|---|---|---|---|---|---|---|---|---|
| Matitiau9 | AM + AFM | 9 | Yes | Yes | 100% | 0.6* | Yes (n = 4) | 2 | 5/7 |
| Lee5 | AM + AFM | 35 | Yes | Yes | 100% | 3.3* | Yes | 6 | 27/29 |
| Kleinert10 | AM | 9 | No | No | 100% | 2.1† | Yes | 0 | 9/9 |
| Gagliardi7 | AM | 20 | No | No | 100% | 1.9† | Yes | 0 | 10/20 |
| Duncan6 | AFM | 15 | Yes | Yes | 50% | 4.6* | Yes | 8 | 7/7 |
| Stiller19 | AFM | 4 | Yes | Yes | 100% | 4† | No | 1 | 3/4 |
| Calabrese11 | AM | 28 | Yes | Yes | 100% | 4* | NA | 9 | 2/19 |
| Ahdoot12 | AFM | 5 | Yes | No | 80% | 10* | Yes | 1 | 3/4 |
| English21 | AFM + AM | 41 | Yes | Yes | 85% | 2.2* | Yes | 10 | 27/31 |
*Median age; †mean (SD) age.
AFM, acute fulminant myocarditis; AM, acute myocarditis; doc, documentation (titres or cultures); EMB, endomyocardial biopsy evidence of myocarditis; IA, immunoactive (corticosteroids, azathioprine, ciclosporin and intravenous polyclonal immunoglobulin); LVF, left ventricular function; NA, not available; rec, recovery; surv, survivors without heart transplantation; Tx, heart transplantation.
Because we have been routinely using circulatory support only since 2002, no patient in our study was placed under mechanical support, although several of them could have been candidates for this therapy. Although mechanical circulatory support may favour the reversibility of ventricular dysfunction in AFM by unloading the left ventricle and by a positive influence on the neurohormonal activation resulting from heart failure,6 such therapy should not be initiated only for the purpose of cardiac functional recovery. Conversely, circulatory support is fully justified when conventional medical treatment is insufficient.6,19,20 Lastly, the favourable outcome of patients with AFM associated with the possibility of viral recurrence in the cardiac graft11 implies a more expectant course of management regarding heart transplantation. Heart transplantation is a final treatment option and patients should only be listed in the case of failed cardiac recovery.
Our study has inherent limitations. Firstly, it was a retrospective and purely descriptive study. Inclusion criteria were mainly based on clinical features. However, the clinical picture in metabolic cardiomyopathies may mimic AFM. In our institution, all the children presenting with Icardiomyopathy undergo systematic metabolic screening, as Bonnet et al28 recommend that most metabolic cardiomyopathies be diagnosed. In patients with metabolic disorders, the diagnosis of AFM was excluded, regardless of their clinical profile. Complete viral assessment was not available for all of our patients with AFM, and gammaglobulin infusions could have altered the antibody profiles. The small number of patients and data do not allow any analysis of risk factors or treatment effectiveness. We cannot comment on the efficacy of the more recent immunosuppressive and antiviral treatments, or of mechanical support, as those therapies were not available to us during the study period.
In conclusion, to our knowledge this is the first study in the paediatric age group to detail clinical presentation and outcome of AFM. Considering the favourable outcome with reversibility of left ventricular dysfunction, beginning aggressive treatment is mandatory if a child has a clinical diagnosis of AFM, without waiting for serological or histopathological confirmation. Heart transplantation should be considered only when maximal supportive therapy does not lead to improvement
ACKNOWLEDGEMENTS
We thank Dr Joffrey Zoll for his valued help in editing of this manuscript.
Abbreviations
AFM - acute fulminant myocarditis
AM - acute myocarditis
EDD - end diastolic diameter
ICU - intensive care unit
LVEF - left ventricular ejection fraction
PCR - polymerase chain reaction
Footnotes
Competing interests: None declared.
References
- 1.Feldman A M, McNamara D. Myocarditis. N Engl J Med 20003431388–1398. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman E B, Hutchins G M, Herskowitz A.et al Clinicopathologic description of myocarditis. J Am Coll Cardiol 1991181617–1626. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy R E, 3rd, Boehmer J P, Hruban R H.et al ong‐term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 2000342690–695. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell J B, Dec G W, Goldenberg I F.et al Results of heart transplantation for active lymphocytic myocarditis. J Heart Transplant 19909351–355. [PubMed] [Google Scholar]
- 5.Lee K J, McCrindle B W, Bohn D J.et al Clinical outcomes of acute myocarditis in childhood. Heart 199982226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan B W, Bohn D J, Atz A M.et al Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg 2001122440–448. [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi M G, Bevilacqua M, Squitieri C.et al Dilated cardiomyopathy caused by acute myocarditis in pediatric patients: evolution of myocardial damage in a group of potential heart transplant candidates. J Heart Lung Transplant 199312S224–S229. [PubMed] [Google Scholar]
- 8.Drucker N A, Colan S D, Lewis A B.et al Gamma‐globulin treatment of acute myocarditis in the pediatric population. Circulation 199489252–257. [DOI] [PubMed] [Google Scholar]
- 9.Matitiau A, Perez‐Atayde A, Sanders S P.et al Infantile dilated cardiomyopathy: relation of outcome to left ventricular mechanics, hemodynamics, and histology at the time of presentation. Circulation 1994901310–1318. [DOI] [PubMed] [Google Scholar]
- 10.Kleinert S, Weintraub R G, Wilkinson J L.et al Myocarditis in children with dilated cardiomyopathy: incidence and outcome after dual therapy immunosuppression. J Heart Lung Transplant 1997161248–1254. [PubMed] [Google Scholar]
- 11.Calabrese F, Rigo E, Milanesi O.et al Molecular diagnosis of myocarditis and dilated cardiomyopathy in children: clinicopathologic features and prognostic implications. Diagn Mol Pathol 200211212–221. [DOI] [PubMed] [Google Scholar]
- 12.Ahdoot J, Galindo A, Alejos J C.et al Use of OKT3 for acute myocarditis in infants and children. J Heart Lung Transplant 2000191118–1121. [DOI] [PubMed] [Google Scholar]
- 13.Bowles N E, Vallejo J. Viral causes of cardiac inflammation. Curr Opin Cardiol 200318182–188. [DOI] [PubMed] [Google Scholar]
- 14.Grogan M, Redfield M M, Bailey K R.et al Long‐term outcome of patients with biopsy‐proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 19952680–84. [DOI] [PubMed] [Google Scholar]
- 15.Liu P P, Mason J W. Advances in the understanding of myocarditis. Circulation 20011041076–1082. [DOI] [PubMed] [Google Scholar]
- 16.Garg A, Shiau J, Guyatt G. The ineffectiveness of immunosuppressive therapy in lymphocytic myocarditis: an overview. Ann Intern Med 1998129317–322. [DOI] [PubMed] [Google Scholar]
- 17.Levi D, Alejos J. Diagnosis and treatment of pediatric viral myocarditis. Curr Opin Cardiol 20011677–83. [DOI] [PubMed] [Google Scholar]
- 18.Camargo P R, Snitcowsky R, da Luz P L.et al Favourable effects of immunosuppressive therapy in children with dilated cardiomyopathy and active myocarditis. Pediatr Cardiol 19951661–68. [DOI] [PubMed] [Google Scholar]
- 19.Stiller B, Dahnert I, Weng Y G.et al Children may survive severe myocarditis with prolonged use of biventricular assist devices. Heart 199982237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acker M A. Mechanical circulatory support for patients with acute‐fulminant myocarditis. Ann Thorac Surg 200171(Suppl 3)S73–S76. [DOI] [PubMed] [Google Scholar]
- 21.English R F, Janosky J E, Ettedgui J A.et al Outcomes for children with acute myocarditis. Cardiol Young 200414488–493. [DOI] [PubMed] [Google Scholar]
- 22.Fraisse A, Paut O, Zandotti C.et al Epstein‐Barr virus: an unusual cause of acute myocarditis in children. Arch Pediatr 20007752–755. [DOI] [PubMed] [Google Scholar]
- 23.Sluysmans T, Colan S D. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 200401144–2004. [DOI] [PubMed] [Google Scholar]
- 24.Aretz H T, Billingham M E, Edwards W D.et al Myocarditis: a histopathologic definition and classification. Am J Cardiovasc Pathol 198713–14. [PubMed] [Google Scholar]
- 25.Pophal S G, Sigfusson G, Booth K L.et al Complications of endomyocardial biopsy in children. J Am Coll Cardiol 1999342105–2110. [DOI] [PubMed] [Google Scholar]
- 26.Bowles N E, Ni J, Kearney D L.et al Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 200342466–472. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo J, Mann D L. Antiinflammatory therapy in myocarditis. Curr Opin Cardiol 200318189–193. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet D, de Lonlay P, Gautier I.et al Efficiency of metabolic screening in childhood cardiomyopathies. Eur Heart J 199819790–793. [DOI] [PubMed] [Google Scholar]