Abstract
Objectives
To evaluate the effect of intensive lipid‐lowering treatment on coronary artery calcification in a substudy of a trial recruiting patients with calcific aortic stenosis.
Methods
In a double blind randomised controlled trial, 102 patients with calcific aortic stenosis and coronary artery calcification were randomly assigned by the minimisation technique to atorvastatin 80 mg daily or matched placebo. Coronary artery calcification was assessed annually by helical computed tomography.
Results
48 patients were randomly assigned to atorvastatin and 54 to placebo with a median follow up of 24 months (interquartile range 24–30). Baseline characteristics and coronary artery calcium scores were similar in both groups. Atorvastatin reduced serum low density lipoprotein cholesterol (−53%, p < 0.001) and C reactive protein (−49%, p < 0.001) concentrations whereas there was no change with placebo (−7% and 17%, p > 0.95 for both). The rate of change in coronary artery calcification was 26%/year (0.234 (SE 0.037) log arbitrary units (AU)/year; n = 39) in the atorvastatin group and 18%/year (0.167 (SE 0.034) log AU/year; n = 49) in the placebo group, with a geometric mean difference of 7%/year (95% confidence interval −3% to 18%, p = 0.18). Serum low density lipoprotein concentrations were not correlated with the rate of progression of coronary calcification (r = 0.05, p = 0.62).
Conclusion
In contrast to previous observational studies, this randomised controlled trial has shown that, despite reducing systemic inflammation and halving serum low density lipoprotein cholesterol concentrations, statin treatment does not have a major effect on the rate of progression of coronary artery calcification.
Coronary artery calcification is an independent risk factor for coronary heart disease, with even low coronary calcium scores doubling the risk of coronary events.1 The relative risk associated with coronary calcification is greater than that associated with established factors such as smoking, hypertension and diabetes mellitus. Progression of coronary artery calcification is associated with a higher incidence of coronary events even among people who are asymptomatic at the time of initial scanning.2 Thus, not only is the presence of coronary artery calcification indicative of atheromatous plaque disease but its progression may correspond with cardiovascular event rates.
Statin treatment has a proven role in the primary3,4 and secondary prevention5,6,7,8 of cardiovascular disease, with incremental benefits seen with more intensive reductions in serum cholesterol concentrations.8 Previous studies9,10 have reported that statins can halt the progression and may even induce regression of coronary artery calcification. Indeed, the rate of progression of coronary artery calcification correlates with the average serum low density lipoprotein (LDL) cholesterol concentration.9 This has led to the use of computed tomography to monitor disease progression and response to treatment, particularly with statins. Two recent trials, however, did not show a benefit of statin on the progression of coronary artery calcification in asymptomatic people.11,12
The SALTIRE (Scottish Aortic Stenosis Lipid lowering Therapy, Impact on Regression) trial was a prospective double blind, randomised controlled study of intensive lipid‐lowering treatment of patients with calcific aortic stenosis.13 As part of this trial, aortic valve and coronary artery calcium scores are measured by helical computed tomography. The objective of this substudy was to assess the effect of atorvastatin 80 mg daily on the rate of progression of coronary artery calcification in patients with calcific aortic stenosis.
METHODS
Patient population
Patients aged > 18 years with calcific aortic stenosis (grade 1–3 calcification on echocardiography14) and a peak post‐valve velocity of ⩾ 2.5 m/s were recruited from eight hospital centres across the southeast of Scotland. Exclusion criteria were women of childbearing potential without contraception, active or chronic liver disease, history of alcohol or drug misuse, severe mitral stenosis (valve area < 1 cm2), severe mitral or aortic regurgitation,15 major left ventricular dysfunction (ejection fraction < 35%), planned aortic valve replacement, intolerance to statins, patients who were taking or would in the opinion of the treating physician benefit from statins, baseline serum total cholesterol of < 4.0 mmol/l, and permanent pacemaker or cardiodefibrillator. For the substudy, we also excluded patients who had no coronary artery calcification on computed tomography. The study was conducted with the approval of all the regional research ethics committees and in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant.
Study protocol
Between March 2001 and April 2002, the blinded study coordinator randomly assigned eligible patients by the minimisation technique16 with a dedicated locked computer program (Edinburgh University), which incorporated eight baseline variables: age, sex, smoking habit, hypertension, diabetes mellitus, serum cholesterol concentration, peak aortic jet velocity and aortic calcium score. Patients were assigned either to atorvastatin 80 mg daily or matched placebo (Pfizer, Tadworth, UK) as a single daily dose in numbered containers.
Patients were assessed at baseline, two months, six months and every six months thereafter for a minimum of two years. Clinical evaluation included assessment of functional status, adverse events and biochemical blood analysis. Serum high sensitivity C reactive protein (CRP) concentrations were determined by a highly sensitive immunonephelometric method (Dade Behring, Milton Keynes, UK) as previously described.17 All patients underwent computed tomography within the month before randomisation to study treatment and at each annual visit. Randomly assigned patients who were later treated with an open label statin by their attending physician were immediately scanned and withdrawn from further observation.
Computed tomography
A single blinded operator performed computed tomography with a double helix scanner (Twin II Flash; Philips Medical Systems (UK), Stevenage, UK) calibrated against a standard phantom. Images were acquired in 2.7 mm slices (with a 0.75 s full 360° scan mode) through the region of the coronary arteries with a pitch of 0.7 and an increment of 1.3 mm during held inspiration. Exposure factors were 120 kV at 270 mA and the scan angle was 360°. Images were analysed off line with an automated, computerised software program (Picker cardiac scoring). This uses an Agatston scoring method,18 producing sensitivity and specificity comparable with electron beam computed tomography.17 Scans were scored by both the Agatston (130 HU threshold) and the modified Agatston (90 HU threshold) methods.19 The Agatston method has been shown to reduce interobserver and interscan variation compared with the threshold of 90 HU.20 To assess the reproducibility of the method, repeated baseline computed tomography scans were recorded within four weeks of each other in an unselected random sample of 16 patients.
Data analysis and statistics
Coronary artery calcium scores are expressed in arbitrary units (AU) based on the 130 HU threshold. The calcium scores and high sensitivity CRP concentrations were not normally distributed and data are presented as median (interquartile range) or mean (SD) after logarithmic transformation (log AU). The primary end point, the rate of change of coronary calcium scores, was analysed with random coefficient models13,21 after logarithmic transformation of the scores. In summarising the data, we calculated the change in coronary artery calcium scores by dividing the change between the baseline and final scores by the duration of follow up. Rate of change in coronary calcium score is expressed as percentage change per year or as absolute change in the logarithm of the coronary artery calcium score. Reproducibility was assessed by the method of Bland and Altman.21 As well as tests of significance, 95% confidence intervals are reported as appropriate. Significance was taken as a two‐sided p < 0.05.
RESULTS
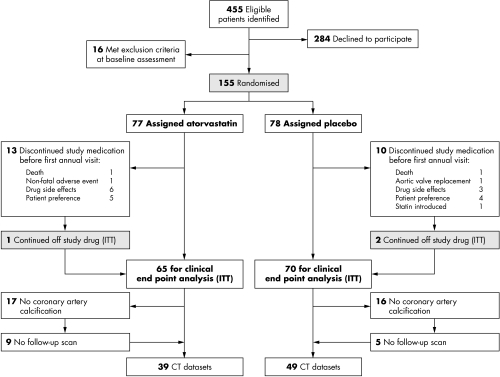
Of 155 patients recruited into the SALTIRE trial, 102 had coronary calcification at baseline (fig 1), of whom 88 had at least two scans. Coronary calcification predominated in the left anterior descending artery (100% of patients) although it was also present in the circumflex (33%) and right (27%) coronary arteries. Baseline characteristics and coronary artery calcium scores were well matched in both treatment groups (table 1) in the 88 evaluable participants.
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patients recruited into the trial and substudy. CT, computed tomography; ITT, intention to treat.
Table 1 Baseline characteristics of participants with calcific aortic stenosis in the treatment groups.
| Characteristic | Atorvastatin (n = 39) | Placebo (n = 49) |
|---|---|---|
| Age (years) | 70 (8) | 70 (9) |
| Men | 74% | 78% |
| Body mass index (kg/m2) | 29 (5) | 28 (5) |
| Cardiovascular risk factors | ||
| Hypertension | 22 | 28 |
| Hyperlipidaemia | 3 | 2 |
| Diabetes mellitus | 0 | 2 |
| Current smoker | 5 | 10 |
| Cardiovascular disease | ||
| Coronary heart disease | 7 | 13 |
| Cerebrovascular disease | 5 | 7 |
| Peripheral vascular disease | 3 | 7 |
| Drug history | ||
| Aspirin | 17 | 26 |
| ACE inhibitor | 7 | 8 |
| β blocker | 11 | 15 |
| Warfarin | 4 | 8 |
| Blood pressure (mm Hg) | ||
| Systolic | 143 (18) | 140 (19) |
| Diastolic | 82 (11) | 78 (11) |
| Lipid profile | ||
| Total cholesterol (mmol/l) | 5.7 (0.9) | 5.5 (0.9) |
| LDL cholesterol (mmol/l) | 3.6 (0.8) | 3.4 (0.7) |
| HDL cholesterol (mmol/l) | 1.5 (0.4) | 1.5 (0.4) |
| Total cholesterol:HDL | 4.2 (1.2) | 4.0 (1.0) |
| Triglycerides (mmol/l) | 1.5 (0.8) | 1.4 (0.7) |
| Coronary calcification score (AU) | ||
| Left anterior descending artery | 112 (40–285) | 207 (76–461) |
| Circumflex artery | 0 (0–9) | 0 (0–4) |
| Right coronary artery | 0 (0–29) | 0 (0–0) |
| Total coronary score | 195 (57–448) | 235 (83–526) |
| Log total coronary score (log AU) | 2.16 (0.68) | 2.30 (0.65) |
Continuous variables stated as mean (SD) or median (interquartile range).
ACE, angiotensin‐converting enzyme; AU, arbitrary unit; HDL, high density lipoprotein; LDL, low density lipoprotein.
Reproducibility
The reproducibility of the left anterior descending coronary score and of the total coronary score was examined with the approach of Bland and Altman.21 Without transformation, the difference between replicate observations tended to increase with the magnitude of the measurement. After logarithmic transformation, higher values showed stable differences, but differences were higher at the lowest scores. Overall, the differences on the log scale correspond to a coefficient of variation of 28% for both variables, but when the analysis was restricted to the 10 pairs with a geometric mean score above 100, the coefficient of variation was 10% for both variables.
Effect of atorvastatin treatment
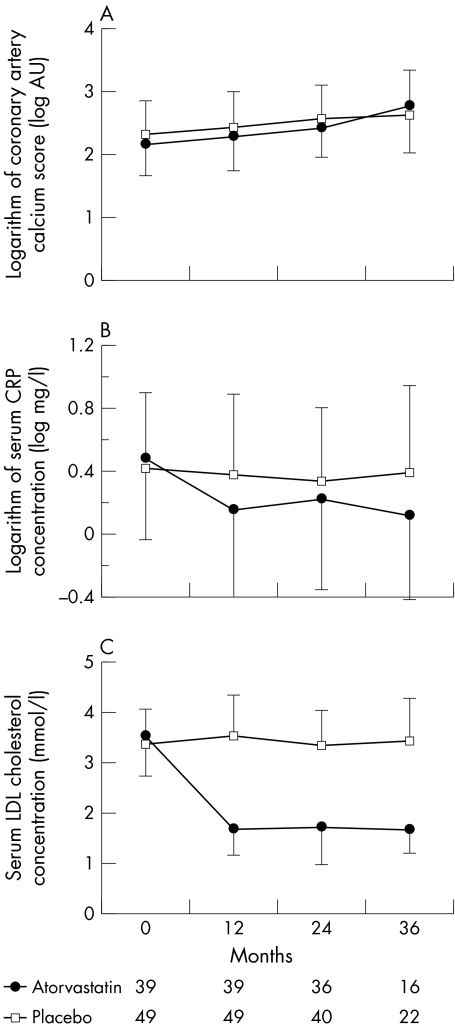
Patients were followed up for a median of 24 months (interquartile range 24–30). Atorvastatin 80 mg daily more than halved serum LDL cholesterol concentrations (53 (SD 19)%, p < 0.001), whereas placebo had no effect (fig 2). This reduction in serum LDL cholesterol concentrations was associated with a major decrease in serum CRP concentrations from 1.95 (interquartile range 1.15–4.86) to 1.00 mg/l (0.49–2.31) (Wilcoxon signed rank test p < 0.001) (fig 2). Atorvastatin was well tolerated: two patients in the placebo group and five patients in the atorvastatin group discontinued the treatment, predominantly as a result of gastrointestinal upset. One patient taking atorvastatin had an increase in creatine kinase of > 5 times the upper limit of normal without symptoms of myositis and was withdrawn at the request of the Data Monitoring Committee. There were no cases of rhabdomyolysis.
Figure 2 Progression of (A) coronary artery calcification, (B) serum C reactive protein (CRP) concentrations (p < 0.001, atorvastatin v placebo) and (C) serum low density lipoprotein (LDL) cholesterol concentrations (p < 0.001, atorvastatin v placebo) in patients treated with atorvastatin 80 mg daily or matched placebo. AU, arbitrary units.
Coronary artery calcium score
Atorvastatin did not affect the rate of progression of the coronary artery calcium score (fig 2). Similar results were obtained when the 90 HU threshold was used (42 (SD 73)%/year in the atorvastatin group and 29 (SD 37)%/year in the placebo group, p = 0.24). Serum LDL cholesterol concentrations did not correlate with the rate of progression of coronary artery calcification (r = 0.05, p = 0.62).
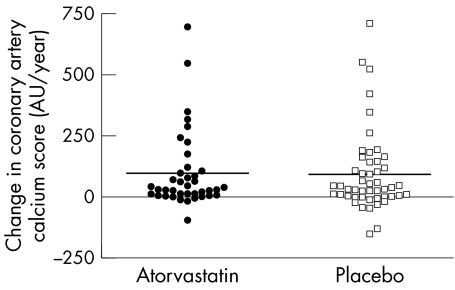
The rates of change of coronary artery calcium scores were primarily analysed on the logarithms of the scores by random coefficients models.22 This showed no difference between the average rates of change in the two treatment arms (p = 0.18). The mean coronary calcium score increased by 0.234 (SE 0.037) log AU/year in the atorvastatin group and 0.167 (SE 0.034) log AU/year in the placebo group. These figures correspond to a 26%/year increase in the atorvastatin group and 18%/year in the placebo group. The geometric mean (adjusted for baseline) is 7% higher at one year with atorvastatin than with placebo, with 95% confidence limits ranging from 3% lower to 18% higher. Figure 3 summarises the observed annual changes in coronary calcium scores, calculated from the first to the last visit.
Figure 3 Absolute rate of change in coronary calcium score expressed in arbitrary units (AU) per year for patients treated with atorvastatin 80 mg or matched placebo.
As anticipated in such a modest clinical trial, all cause mortality, cardiovascular mortality or cardiovascular hospitalisation did not differ significantly between the two groups.
DISCUSSION
We have confirmed that, despite major reductions in serum LDL cholesterol and CRP concentrations, atorvastatin 80 mg daily did not halt the progression, or induce regression, of coronary artery calcification in patients with calcific aortic stenosis. Consistent with recent trials of asymptomatic people,11,12 our findings contrast notably with previous observational studies and suggest that the potential beneficial effects on coronary artery calcification have been overestimated.
Previous observational and non‐randomised prospective studies9,10 have suggested that reductions in serum LDL cholesterol concentrations decrease the progression of coronary calcification. Not all observational studies, however, have had consistent findings. In the largest observational study of 182 patients, Hecht and colleagues23 recently found no difference in the progression of coronary calcium scores in patients who were maintained on lipid‐lowering treatment and achieved significant reductions in serum LDL cholesterol concentrations. Observational data may be misleading and prospective randomised controlled trials are necessary to confirm or to refute these interesting preliminary observations. The recent BELLES (Beyond Endorsed Lipid Lowering with EBT Scanning) trial12 found no differential effect between pravastatin (40 mg daily) and atorvastatin (80 mg daily) on the progression of coronary artery calcification in 615 hyperlipidaemic postmenopausal women. Study follow up was brief (one year), however, and there was no placebo control group. The St Francis Heart Study11 randomly assigned 1005 asymptomatic middle‐aged men and women with high coronary artery calcium scores to combination atorvastatin 20 mg, vitamin C 1 g, and vitamin E (α tocopherol) 1000 U daily or to matching placebos. After 4.3 years of follow up, the rate of progression of coronary artery calcification did not differ.
We have conducted a double blind randomised controlled trial with helical computed tomography in patients with aortic stenosis. Minimisation technique ensured good matching of the baseline characteristics of the patient population and reproducibility studies confirmed the validity of our repeated assessments. Although documenting very similar rates of progression of coronary calcification to previous studies,9,10,23 we have not observed a reduction in coronary calcification with intensive lipid‐lowering treatment despite more than halving serum LDL cholesterol concentrations.
Statins have been extremely successful in the primary and secondary prevention of cardiovascular disease. Why then have we and others not observed a beneficial effect of statin on coronary artery calcification? Unstable atherosclerotic plaques have a large lipid‐rich core, a preponderance of macrophages and foam cells, and a thin fibrous cap containing few smooth muscle cells.24 It has been suggested that calcified lesions may be relatively more stable,25 indicating a possible protective role of calcification in coronary plaques. Statins produce many of their beneficial effects through plaque stabilisation. In both primate26 and swine27 models, antiatherosclerotic interventions are associated with an increase in vascular fibrous tissue and calcification. This calcium deposition continues during the initial phase of plaque regression due to the death of foam cells and an increase in necrotic tissue. Thus, vascular calcification may have a role in the initial stabilisation of atherosclerotic plaques. This is consistent with our findings and would account for the lack of effect on the progression of coronary artery calcification despite a reduction in serum CRP concentrations.
After the initial stabilisation of the atherosclerotic plaque, subsequent progression of coronary calcification would be anticipated to be inhibited. The present study was brief, and follow up was only continued for a median of two years. It would be important to extend our observations to five or more years to assess properly the impact of statin on the long‐term progression of coronary artery calcification. It should be acknowledged, however, that the clinical benefits of statin are apparent within the first few years,6,7,8 and in some cases the first few months,28 of treatment. Moreover, the St Francis Heart Study showed no beneficial effects despite 4.3 years of follow up.9
On the basis of previous non‐randomised studies,10 the practice of performing serial computed tomography to monitor disease progression and the response to treatment has become widespread, especially in North America. Our data, and those of the St Francis Heart Study11 and the BELLES study,12 indicate that repeated scanning to assess response to statin is not justified. Indeed, the radiation dose incurred for such serial scans poses potential health risks, particularly when multidetector computed tomography scanners are used.
Study limitations
Several factors should be taken into account when considering the results of our study. This was a substudy of the SALTIRE trial13 that recruited only patients with calcific aortic stenosis. Our findings are consistent, however, with two recent randomised controlled trials in asymptomatic younger people without valvular heart disease.11,12 Our study therefore suggests that failure of statins to restrict the progression of coronary artery calcification can be extended to include patients with valvular heart disease as well as older populations. Moreover, our findings suggest that the lack of benefit seen in the St Francis Heart Study is not attributable to the modifying effects of antioxidant vitamins.
When compared with electron beam computed tomography, the accuracy of helical computed tomography in detecting coronary artery calcification has been questioned.18,29 Technological advances have also meant that double helical scanners have now been overtaken by 64‐slice scanners. At trial inception, the double helix scanner was the latest technology, and it would have been inappropriate to replace the scanner during the conduct of the trial. Moreover, our approach has been previously validated21 and we have shown good reproducibility of coronary artery calcification scores in patients with scores of > 100 AU. We do not believe the absence of a major beneficial effect on coronary artery calcification is attributable to our methods. We acknowledge that our population size is modest; however, the 95% confidence intervals can exclude a relative reduction in progression of coronary artery calcification of > 3%/year. We therefore suggest that if lipid‐lowering treatment does reduce the progression of coronary artery calcification then the effect is rather small.
The method of quantification of coronary artery calcification is controversial. The Agatston method is traditionally used but this may overestimate the coronary calcium score in newer generation scanners with reduced slice thickness due to partial voluming. More recent methods include the volume30 and the coronary calcium mass31 scores, although neither is superior to the Agatston score in terms of reproducibility from consecutive scans in an individual patient.32
Conclusion
We conclude that intensive lipid‐lowering treatment does not halt the progression, or induce regression, of coronary artery calcification. Although coronary artery calcium scores correlate well with the presence of atherosclerosis and predict future coronary risk, our findings confirm that monitoring progression of coronary artery calcification to assess the response to lipid‐lowering treatment has no role.
Abbreviations
AU - arbitrary units
BELLES - Beyond Endorsed Lipid Lowering with EBT Scanning
CRP - C reactive protein
LDL - low density lipoprotein
SALTIRE - Scottish Aortic Stenosis Lipid Lowering Therapy, Impact on Regression
Footnotes
The SALTIRE trial was supported by a project grant from the British Heart Foundation (PG/2000/044) and an unrestricted educational grant from Pfizer (UK). Additional support was provided by the Wellcome Trust Clinical Research Facility, Edinburgh.
Competing interests: DEN and NAB hold unrestricted educational grant awards from Pfizer (UK) Ltd. DEN, DBN and NAB have undertaken paid consultancy and served on advisory boards for Pfizer (UK) Ltd.
References
- 1.Pletcher M J, Tice J A, Pignone M.et al Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta‐analysis. Arch Intern Med 20041641285–1292. [DOI] [PubMed] [Google Scholar]
- 2.Raggi P, Cooil B, Shaw L J.et al Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol 200392827–829. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd J, Cobbe S M, Ford I.et al Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 19953331301–1307. [DOI] [PubMed] [Google Scholar]
- 4.Downs J R, Clearfield M, Weis S.et al Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 19982791615–1622. [DOI] [PubMed] [Google Scholar]
- 5. Scandinavian Simvastatin Survival Study Investigators, Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 19943441383–1389. [PubMed] [Google Scholar]
- 6.Lewis S J, Moye L A, Sacks F M.et al Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med 1998129681–689. [DOI] [PubMed] [Google Scholar]
- 7.LIPID Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 19983391349–1357. [DOI] [PubMed] [Google Scholar]
- 8.Medical Research Council, British Heart Foundation MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 20023607–22. [DOI] [PubMed] [Google Scholar]
- 9.Callister T Q, Raggi P, Cooil B.et al Effect of HMG‐CoA reductase inhibitors on coronary artery disease as assessed by electron‐beam computed tomography. N Engl J Med 19983391972–1978. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach S, Ropers D, Pohle K.et al Influence of lipid‐lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation 20021061077–1082. [DOI] [PubMed] [Google Scholar]
- 11.Arad Y, Spadaro L A, Roth M.et al Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 200546166–172. [DOI] [PubMed] [Google Scholar]
- 12.Raggi P, Davidson M, Callister T Q.et al Aggressive versus moderate lipid‐lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005112563–571. [DOI] [PubMed] [Google Scholar]
- 13.Cowell S J, Newby D E, Prescott R J.et al A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. N Engl J Med 20053522389–2397. [DOI] [PubMed] [Google Scholar]
- 14.Rosenhek R, Binder T, Porenta G.et al Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000343611–617. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi W A, Enriquez‐Sarano M, Foster E.et al Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 200316777–802. [DOI] [PubMed] [Google Scholar]
- 16.Treasure T, MacRae K D. Minimisation: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups; minimisation does. BMJ 1998317362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr J J, Crouse J R, 3rd, Goff D C., Jret al E aluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol 2000174915–921. [DOI] [PubMed] [Google Scholar]
- 18.Agatston A S, Janowitz W R, Hildner F J.et al Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 199015827–832. [DOI] [PubMed] [Google Scholar]
- 19.Shemesh J, Apter S, Rozenman J.et al Calcification of coronary arteries: detection and quantification with double‐helix CT. Radiology 1995197779–783. [DOI] [PubMed] [Google Scholar]
- 20.Goldin J G, Yoon H C, Greaser L E., 3rdet al piral versus electron‐beam CT for coronary artery calcium scoring. Radiology 2001221213–221. [DOI] [PubMed] [Google Scholar]
- 21.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986i307–310. [PubMed]
- 22.Brown H, Prescott R.Applied mixed models in medicine. Chichester: John Wiley and Sons, 1999
- 23.Hecht H S, Harman S M. Relation of aggressiveness of lipid‐lowering treatment to changes in calcified plaque burden by electron beam tomography. Am J Cardiol 200392334–336. [DOI] [PubMed] [Google Scholar]
- 24.Davies M J. The composition of coronary‐artery plaques. N Engl J Med 19973361312–1314. [DOI] [PubMed] [Google Scholar]
- 25.Mintz G S, Popma J J, Pichard A D.et al Patterns of calcification in coronary artery disease: a statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995911959–1965. [DOI] [PubMed] [Google Scholar]
- 26.Stary H C. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol 200188(2A)16E–9E. [DOI] [PubMed] [Google Scholar]
- 27.Daoud A S, Jarmolych J, Augustyn J M.et al Sequential morphologic studies of regression of advanced atherosclerosis. Arch Pathol Lab Med 1981105233–239. [PubMed] [Google Scholar]
- 28.Schwartz G G, Oliver M F, Ezekowitz M D.et al Rationale and design of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study that evaluates atorvastatin in unstable angina pectoris and in non‐Q‐wave acute myocardial infarction. Am J Cardiol 199881578–581. [DOI] [PubMed] [Google Scholar]
- 29.Qanadli S D, Mesurolle B, Aegerter P.et al Volumetric quantification of coronary artery calcifications using dual‐slice spiral CT scanner: improved reproducibility of measurements with 180 degrees linear interpolation algorithm. J Comput Assist Tomogr 200125278–286. [DOI] [PubMed] [Google Scholar]
- 30.Callister T Q, Cooil B, Raya S P.et al Coronary artery disease: improved reproducibility of calcium scoring with an electron‐beam CT volumetric method. Radiology 1998208807–814. [DOI] [PubMed] [Google Scholar]
- 31.Hong C, Becker C R, Schoepf U J.et al Coronary artery calcium: absolute quantification in nonenhanced and contrast‐enhanced multi‐detector row CT studies. Radiology 2002223474–480. [DOI] [PubMed] [Google Scholar]
- 32.Rumberger J A, Kaufman L. A Rosetta stone for coronary calcium risk stratification: Agatston, volume, and mass scores in 11,490 individuals. AJR Am J Roentgenol 2003181743–748. [DOI] [PubMed] [Google Scholar]