Abstract
Objective
To look at the presentation, treatment and outcome of patients who developed atrioventricular block after transcatheter closure of a perimembranous ventricular septal defect (PMVSD) with the Amplatzer PMVSD device.
Setting
Three tertiary referral centres for paediatric cardiology in two countries.
Results
All three patients presented within 10 days of the procedure. All three patients were treated with intravenous steroids. A permanent pacemaker was inserted in all patients but no pacemaker required activation after two months.
Conclusion
Complete atrioventricular block occurring in the weeks after device occlusion of a PMVSD appears to resolve quickly. Continued involvement in multicentre device databases is required to monitor safety.
The Amplatzer perimembranous ventricular septal defect (PMVSD) occluder (AGA Medical, Golden Valley, Minnesota, USA) has been shown to be effective in closing haemodynamically significant PMVSDs.1,2,3,4,5 Potential complications are related to the proximity of the PMVSD to the surrounding structures, namely the aortic valve, the tricuspid valve and the atrioventricular conduction bundle.6 The estimated incidence of complete atrioventricular block after transcatheter closure is 1–5%.1,7 We report on three patients who developed complete atrioventricular block after this procedure, from three separate institutions, and their respective outcomes.
CASE REPORTS
Case 1
The first patient was an 11‐year‐old girl weighing 34 kg who had an elective transcatheter closure of a PMVSD. She had no other significant medical history of note. She had good energy levels and did not have any symptoms of left ventricular failure. Her ECG showed sinus rhythm and did not show any conduction abnormalities. Transthoracic echocardiogram showed a 7 mm PMVSD with moderate left ventricular dilatation. No other structural abnormalities were present.
The PMVSD was closed under general anaesthesia. Intravenous cefuroxime 30 mg/kg and heparin 100 IU/kg were given before the procedure was started. The defect measured 6 mm by colour flow Doppler on the transoesophageal echocardiogram. We calculated the ratio of pulmonary to systemic blood flow (Qp:Qs) to be 1.7:1. The device was deployed on the first attempt with a 7 French long sheath (TorqVue delivery system; AGA Medical). No arrhythmias were observed during the procedure. Device placement was confirmed by transoesophageal echocardiography and angiography. The device conformed well and did not appear oversized. There was a small residual shunt. There was no significant aortic or tricuspid regurgitation. The fluoroscopy and procedure times were 20 min and 2 h, respectively. The postprocedural ECG did not show any conduction abnormalities. The day 2 transthoracic echocardiogram showed that the device was in an appropriate position with no residual shunting. She was prescribed aspirin 75 mg/day for six months and was advised to take the recommended endocarditis prophylaxis.8
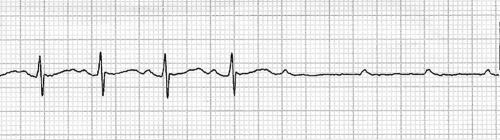
She presented five days later with syncope, which occurred during non‐strenuous exertion. An initial ECG did not show any conduction disturbances. The patient subsequently had a cardiac arrest during transthoracic echocardiography. The ECG showed P waves with absent ventricular response (fig 1). Ventricular standstill was seen on echocardiography for a brief period before cardiorespiratory resuscitation was started. An output was detected after 3 min and she regained consciousness. An ECG showed complete atrioventricular block with a ventricular rate of 40 beats/min. Intravenous isoproterenol was started initially at 0.03 μg/kg/min and titrated to keep the heart rate above 80 beats/min. A temporary pacemaker was inserted the same day and she was paced at a rate of 90 beats/min. Intravenous dexamethasone (1 mg/kg/day) was given for five days followed by prednisone 1 mg/kg for three weeks. She continued to require pacing so a permanent pacemaker was inserted two days later. The pacemaker was periodically activated in the first four weeks after insertion but was not used thereafter. She remains asymptomatic and a follow‐up ECG is entirely normal.
Figure 1 ECG of patient 1, an 11‐year‐old girl in sinus rhythm with subsequent non‐conducted P waves with absent ventricular escape rhythm. The ventricular output recovered after 3 min of resuscitation.
Case 2
The second patient was a 12‐year‐old girl weighing 45 kg who had an elective transcatheter closure of a PMVSD. She had no pre‐existing medical problems. Transthoracic echocardiogram showed a 5 mm PMVSD, 4 mm from the aorta, with an aneurysm measuring 8 mm at the entrance. An ECG recorded before the procedure showed normal PR intervals and QRS complexes. The defect was closed under general anaesthesia. Intravenous antibiotics and heparin were given before the procedure was started. The PMVSD was measured to be 5 mm by transoesophageal echocardiography with colour flow Doppler. The Qp:Qs was measured to be 1.8:1 with a mean pulmonary pressure of 20 mm Hg. Temporary first‐degree atrioventricular block and left bundle branch block occurred during manipulation of the long sheath (8 French; AGA Medical). After further discussion, a 10 mm PMVSD device was inserted with the intention of closing the entrance to the aneurysm. The right ventricular disk conformed appropriately but the left ventricular disk was mushrooming inside the aneurysm after the device was released (fig 2). There was minimal residual shunting. No significant aortic or tricuspid regurgitation was seen on transoesophageal echocardiography. The fluoroscopy and procedure times were 22 min and 100 min, respectively. The ECG reverted to normal sinus rhythm with no bundle branch block after 3 h.
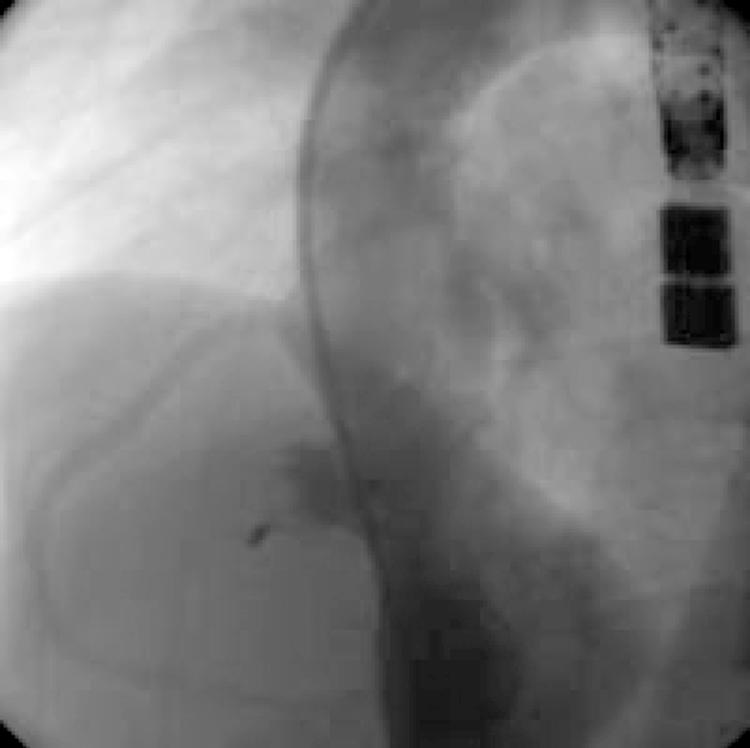
Figure 2 Cine angiogram of patient 2, a 12‐year‐old girl, after device occlusion of a perimembranous ventricular septal defect. The device has a slightly elongated appearance and may have been oversized.
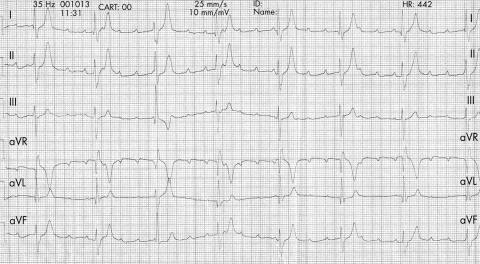
She presented 10 days after the procedure with non‐exertional syncope. The ECG on admission showed first‐degree heart block with a right bundle branch block configuration. This then progressed to third‐degree heart block (fig 3) requiring an intravenous isoproterenol infusion. Treatment with intravenous dexamethasone 1 mg/kg/day was started. A temporary pacemaker was inserted, followed by a permanent one, as she continued to require pacing. She was prescribed naproxen 250 mg orally twice daily for two weeks on discharge form hospital. The pacemaker was active in the first two months after insertion. Follow‐up ECGs showed sinus rhythm with a right bundle branch block configuration.
Figure 3 ECG of patient 2, an 11‐year‐old girl, 10 days after insertion of a perimembranous ventricular septal defect occluder. ECG initially showed first‐degree heart block with a right bundle branch block configuration. This then progressed to third‐degree heart block.
Case 3
The third patient was a 4‐year‐old girl weighing 17 kg. She was asymptomatic and did not have any significant medical history. Her preprocedural ECG did not show any conduction abnormalities and showed a normal QRS pattern. The defect was closed under general anaesthesia. Intravenous antibiotics and heparin were given before the procedure was started. The Qp:Qs at cardiac catheterisation was 1.7:1 and the mean pulmonary artery pressure measured 21 mm Hg. There was an aneurysm measuring 7 mm, with two PMVSDs measuring 1 mm and 5 mm. The arteriovenous loop was recreated once, because of failure of the sheath to pass through the 5 mm PMVSD. An 8 mm Amplatzer PMVSD occluder was successfully deployed. Fluoroscopy showed the left ventricular disk of the device to be slightly elongated and mushrooming into the aneurysm. The right ventricular disk conformed normally. There was a small residual shunt through the device. No new aortic or tricuspid regurgitation was seen on transoesophageal echocardiography. The fluoroscopy and procedure times were 39 min and 60 min, respectively. The postprocedural ECG showed a right bundle branch block with a left anterior hemiblock configuration.
The patient presented five days later with an episode of non‐exertional syncope. The heart rate on admission was 28 beats/min. Initial treatment consisted of temporary pacing, intravenous hydrocortisone for five days, and subsequently oral naproxen. She continued to require pacing so a permanent pacemaker was inserted. Two weeks after insertion, however, it was no longer being activated.
DISCUSSION
Atrioventricular block after device occlusion of a PMVSD is due to the proximity of the conduction bundle to the inferior margin of the defect. By definition, a part of the margin of the PMVSD is the area of fibrous continuity between the atrioventricular valves that forms the posteroinferior border. It is in this area that the atrioventricular conduction bundle emerges from the central fibrous body to become subendocardial and may be prone to damage from a PMVSD occlusion device.6 With inlet extension, PMVSD devices may cause tenting of the mitral and tricuspid valves, further compressing the atrioventricular conduction bundle.6,9
Patients may have atrioventricular block immediately or after a delay of quite some time after device occlusion of a PMVSD. This has led us to believe that there may be different mechanisms of injury to the atrioventricular conduction bundle. Atrioventricular block occurring immediately after device occlusion may result directly from mechanical compression. Atrioventricular block occurring in the weeks and months after a device occlusion may be due to inflammation and fibrosis. The device has been shown to cause a localised inflammatory reaction that can result in extensive scar tissue and cartilaginous metaplasia of the surrounding myocardium.10 This histological change in the tissue that potentially surrounds the conduction bundle may contribute to late onset atrioventricular block. Device flattening is another mechanism that may be responsible for some of the late instances of atrioventricular block. We looked at the shape and size of the devices after insertion and at the time of atrioventricular block in all three cases. The shape and size of any of the devices did not change significantly.
We recommend abandoning the procedure if complete atrioventricular block occurs on crossing the defect with the delivery sheath. From the little evidence that we have to date, oversizing apparently should be avoided. The size of a PMVSD may be overestimated on echocardiography by as much as 21%.11 Hence, we should select a device that is between 0.5–1 mm larger than the PMVSD, as measured on a transoesophageal echocardiogram. The current Amplatzer PMVSD occlusion device, however, is available only in 2 mm increments, which makes device selection more difficult. We have also noticed that some of the devices are quite stiff and consequently may exert excessive pressure on the conducting system. The introduction of softer devices with 1 mm increments in size would help to deal with some of the issues relating to complete atrioventricular block after device occlusion of a PMVSD.
The effectiveness of pretreatment with local steroids has yet to be determined. As with previous reports, we recommend the use of initial high‐dose intravenous steroids, followed by oral steroids for a period of three weeks.9 Whether or not to remove the device is a difficult decision, with patient symptoms, parental preference and institutional preference all entering the equation. If the atrioventricular block resolves with steroids, we would recommend leaving the device in situ. Insertion of a pacemaker will also depend on institutional preference. Patients who are symptomatic should have a temporary pacemaker inserted and be considered for a permanent one. It is not yet clear whether patients with symptomatic atrioventricular block who revert to sinus rhythm after steroid treatment would benefit from a permanent pacemaker.
There is much uncertainty surrounding the incidence, natural history and treatment of complete atrioventricular block that occurs after device occlusion of a PMVSD. This complication may not be preventable. In addition, it may not be possible to identify patients who have a higher risk of developing atrioventricular block. In view of this, adequate consent, outlining our lack of long‐term follow up of devices implanted and the consequences of complete atrioventricular block, is paramount.
Footnotes
No sponsors were involved in, or contributing financially, to the writing of this paper.
Competing interests: None declared.
Ethical approval to review the patient case notes was obtained from the hospital ethics board in all cases.
References
- 1.Arora R, Trehan V, Kumar A.et al Transcatheter closure of congenital ventricular septal defects: experience with various devices. J Interv Cardiol 20031683–91. [DOI] [PubMed] [Google Scholar]
- 2.Bass J L, Kalra G S, Arora R.et al Initial human experience with the Amplatzer perimembranous ventricular septal occluder device. Catheter Cardiovasc Interv 200358238–245. [DOI] [PubMed] [Google Scholar]
- 3.Pedra C A, Pedra S R, Esteves C A.et al Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv 200461403–410. [DOI] [PubMed] [Google Scholar]
- 4.Thanopoulos B D. Catheter closure of perimembranous/membranous ventricular septal defects using the Amplatzer occluder device. Pediatr Cardiol 200426311–314. [DOI] [PubMed] [Google Scholar]
- 5.Miro J, Dahdah N, Lee S.et al Closure of perimembranous ventricular septal defects with the Amplatzer device: multicentric Canadian experience. Presented at the Fourth World Congress of Pediatric Cardiology and Cardiac Surgery, Buenos Aires 2005
- 6.Ho S Y, McCarthy K P, Rigby M L. Morphology of perimembranous ventricular septal defects: implications for transcatheter device closure. J Interv Cardiol 20041799–108. [DOI] [PubMed] [Google Scholar]
- 7.Masura J, Gao W, Gavora P.et al Percutaneous closure of perimembranous ventricular septal defects with the eccentric Amplatzer device: multicenter follow‐up study. Pediatr Cardiol 200526216–219. [DOI] [PubMed] [Google Scholar]
- 8.Dajani A S, Taubert K A, Wilson W.et al Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin Infect Dis 1997251448–1458. [DOI] [PubMed] [Google Scholar]
- 9.Yip W C, Zimmerman F, Hijazi Z M. Heart block and empirical therapy after transcatheter closure of perimembranous ventricular septal defect. Catheter Cardiovasc Interv 200566436–441. [DOI] [PubMed] [Google Scholar]
- 10.Amin Z, Danford D A, Lof J.et al Intraoperative device closure of perimembranous ventricular septal defects without cardiopulmonary bypass: preliminary results with the perventricular technique. J Thorac Cardiovasc Surg 2004127234–241. [DOI] [PubMed] [Google Scholar]
- 11.Johnson T B, Fyfe D A, Thompson R P.et al Echocardiographic and anatomic correlation of ventricular septal defect morphology in newborn Yucatan pigs. Am Heart J 19931251067–1072. [DOI] [PubMed] [Google Scholar]