Abstract
We have reported that P388D1 cell line murine macrophages stimulated with lipopolysaccharide (LPS) from Actinobacillus actinomycetemcomitans release interleukin-1 (IL-1) inhibitor. The IL-1 inhibitor was purified from conditioned media of P388D1 cells stimulated with A. actinomycetemcomitans LPS for 72 h to homogeneity by a four-step procedure: acetic acid extraction from conditioned media; Bio-Gel P-60 gel filtration chromatography; DEAE-Sepharose CL-6B column chromatography; and reverse-phase high-performance liquid chromatography on a C18 hydrophobic support. The purified IL-1 inhibitor gave a single band of protein with a molecular mass of 26 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The purified IL-1 inhibitor was a heat- and acid-stable protein that was inactivated by digestion with trypsin and reduction with dithiothreitol. This inhibitory factor suppressed the proliferation of C3H/HeJ mouse thymocytes and the proliferation of IL-1-dependent cell lines, D10.G4.1 and RPMI 1788, induced by IL-1. However, this inhibitor did not affect the proliferation of IL-2-dependent CTLL-2 cells induced by IL-2, the proliferation of C3H/HeJ mouse thymocytes stimulated with a mitogenic dose of concanavalin A, and the proliferation of IL-6-dependent B9 cells induced by IL-6. Furthermore, the IL-1 inhibitor significantly blocked stimulation of bone resorption in organ cultures of newborn mouse calvaria and inhibited the osteoclast-like cell formation in mouse marrow cultures. A monoclonal antibody prepared against the purified IL-1 inhibitor reacted with mouse recombinant IL-1 receptor antagonist (rIL-1ra), and a polyclonal antibody to mouse rIL-1ra reacted with the IL-1 inhibitor by Western blot (immunoblot) analysis. These results indicate that the IL-1 inhibitor is an identical molecule to rIL-1ra, suggesting that the IL-1 inhibitor (IL-1ra) released by macrophages stimulated with LPS from A. actinomycetemcomitans may play an important mediative role in the development of periodontal disease.
Full text
PDF


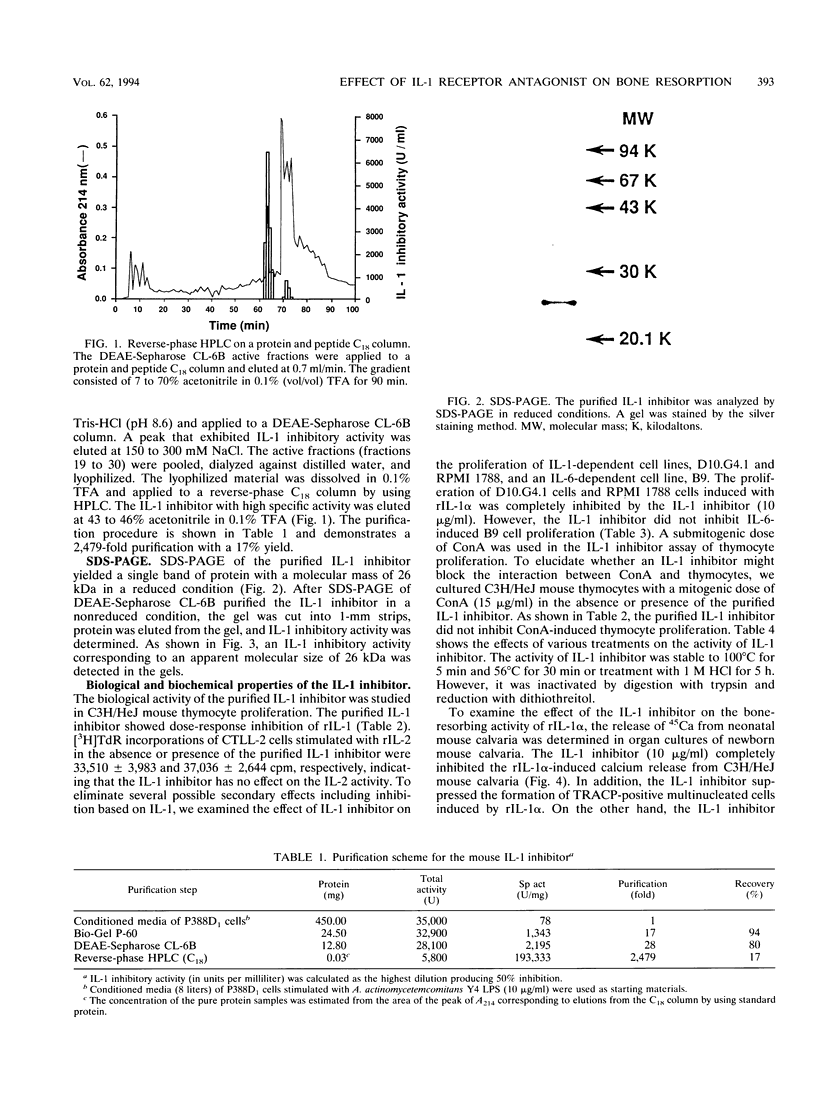
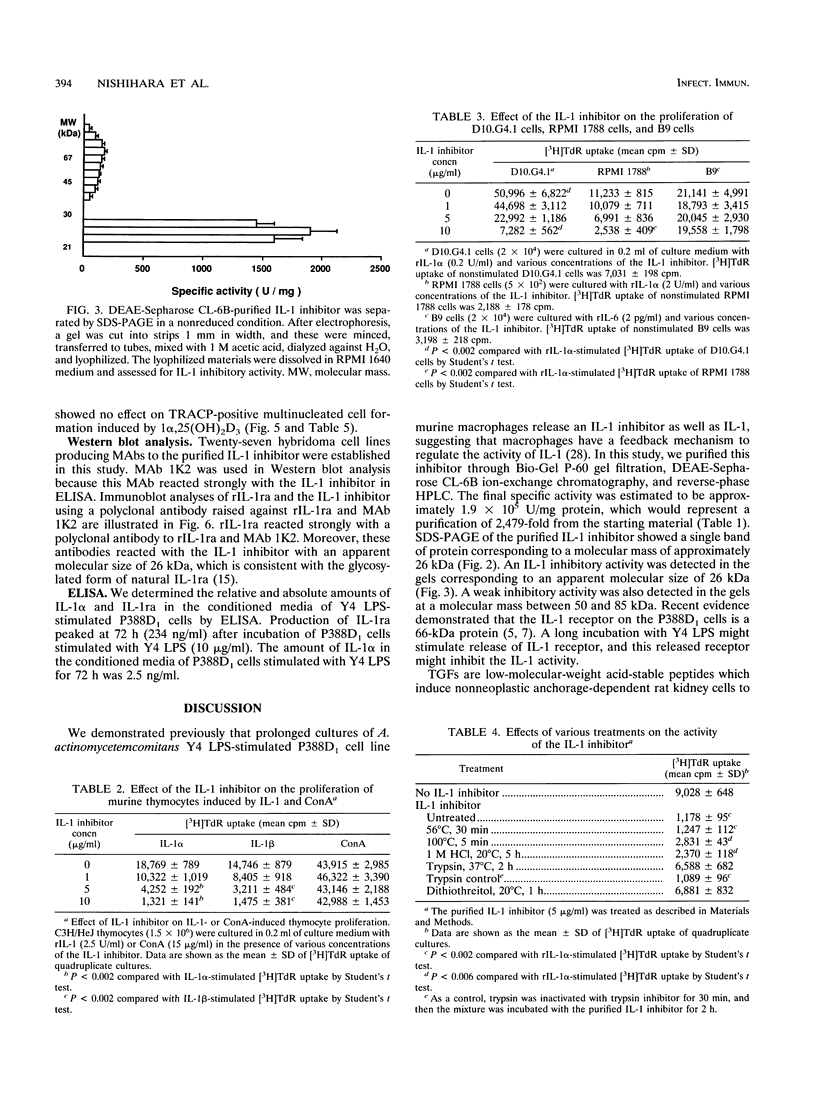
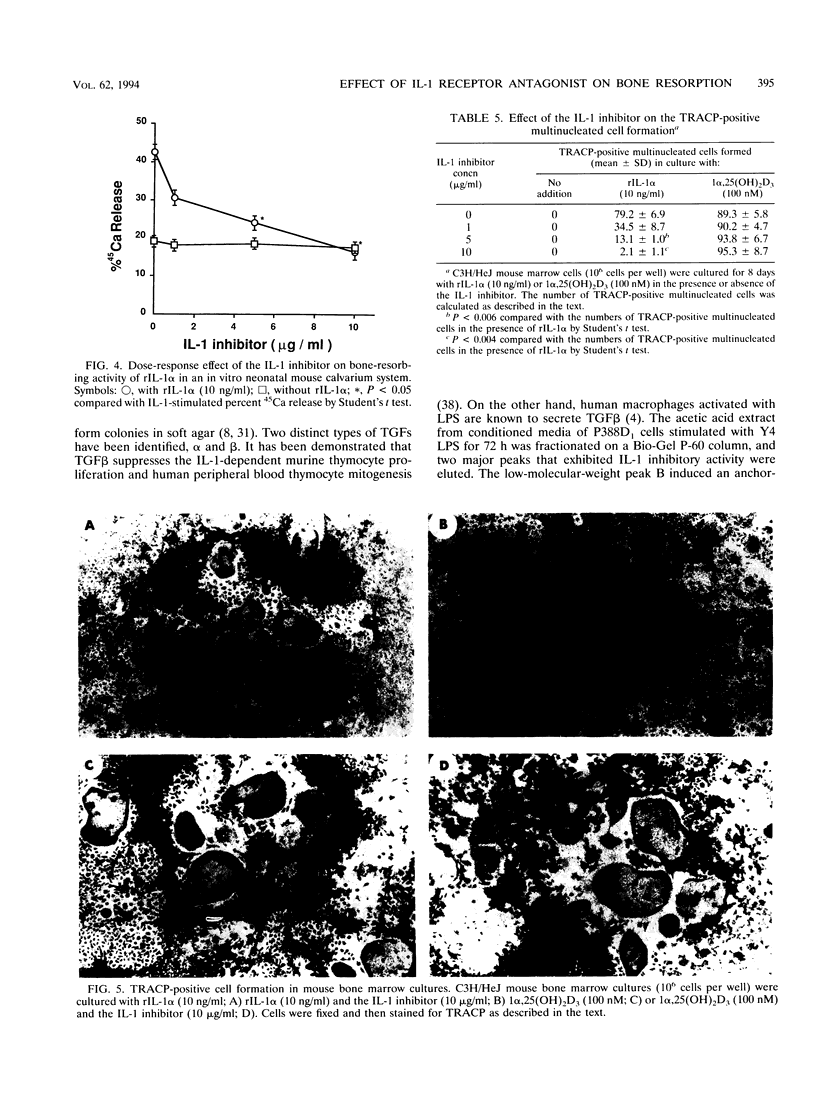
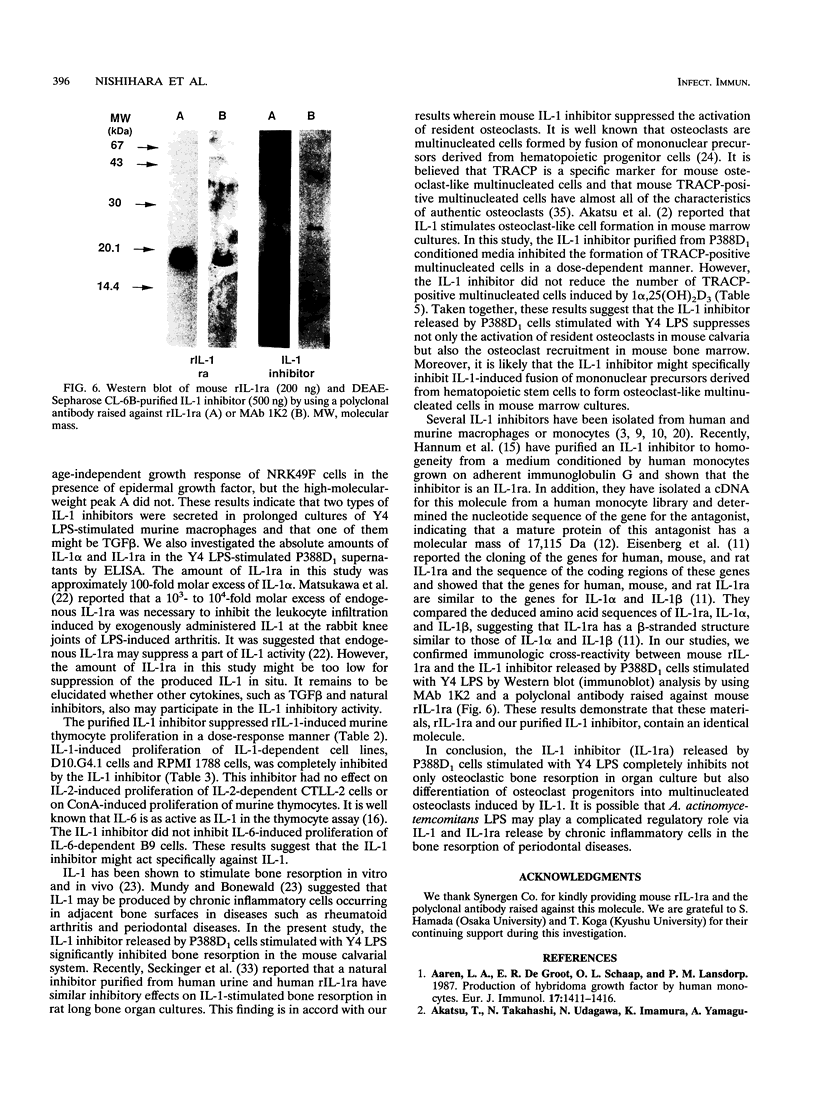

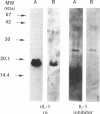
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Akatsu T., Takahashi N., Udagawa N., Imamura K., Yamaguchi A., Sato K., Nagata N., Suda T. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J Bone Miner Res. 1991 Feb;6(2):183–189. doi: 10.1002/jbmr.5650060212. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Thompson R. C., Hannum C. H. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. 1989 Sep 15;143(6):1851–1858. [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsztyk K., Sims J. E., Stanton T. H., Slack J., McMahan C. J., Valentine M. A., Dower S. K. Evidence for different interleukin 1 receptors in murine B- and T-cell lines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8034–8038. doi: 10.1073/pnas.86.20.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss B. D. An improved in vitro immunization procedure for the production of monoclonal antibodies against neural and other antigens. Brain Res. 1984 Jan 16;291(1):193–196. doi: 10.1016/0006-8993(84)90671-1. [DOI] [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Kilian P. L., Stern A. S., Nunes P., Parker K. P., Kaffka K. L., Chua A. O., Lugg D. K., Gubler U. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Brewer M. T., Verderber E., Heimdal P., Brandhuber B. J., Thompson R. C. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: evolution of a cytokine control mechanism. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5232–5236. doi: 10.1073/pnas.88.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Henderson B., Thompson R. C., Hardingham T., Lewthwaite J. Inhibition of interleukin-1-induced synovitis and articular cartilage proteoglycan loss in the rabbit knee by recombinant human interleukin-1 receptor antagonist. Cytokine. 1991 May;3(3):246–249. doi: 10.1016/1043-4666(91)90023-7. [DOI] [PubMed] [Google Scholar]
- Ishihara Y., Nishihara T., Maki E., Noguchi T., Koga T. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J Periodontal Res. 1991 May;26(3 Pt 1):155–160. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W. Native interleukin 1 inhibitors. Immunol Today. 1989 Feb;10(2):61–66. doi: 10.1016/0167-5699(89)90308-3. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa A., Ohkawara S., Maeda T., Takagi K., Yoshinaga M. Production of IL-1 and IL-1 receptor antagonist and the pathological significance in lipopolysaccharide-induced arthritis in rabbits. Clin Exp Immunol. 1993 Aug;93(2):206–211. doi: 10.1111/j.1365-2249.1993.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T., Fujiwara T., Koga T., Hamada S. Chemical composition and immunobiological properties of lipopolysaccharide and lipid-associated proteoglycan from Actinobacillus actinomycetemcomitans. J Periodontal Res. 1986 Sep;21(5):521–530. doi: 10.1111/j.1600-0765.1986.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Nishihara T., Ishihara Y., Noguchi T., Koga T. Membrane IL-1 induces bone resorption in organ culture. J Immunol. 1989 Sep 15;143(6):1881–1886. [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Production of an interleukin-1 inhibitor by cell line P388D1 murine macrophages stimulated with Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988 Nov;56(11):2801–2807. doi: 10.1128/iai.56.11.2801-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Suppression of murine macrophage interleukin-1 release by the polysaccharide portion of Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988 Mar;56(3):619–625. doi: 10.1128/iai.56.3.619-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Seckinger P., Klein-Nulend J., Alander C., Thompson R. C., Dayer J. M., Raisz L. G. Natural and recombinant human IL-1 receptor antagonists block the effects of IL-1 on bone resorption and prostaglandin production. J Immunol. 1990 Dec 15;145(12):4181–4184. [PubMed] [Google Scholar]
- Sims T. J., Moncla B. J., Darveau R. P., Page R. C. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally normal subjects. Infect Immun. 1991 Mar;59(3):913–924. doi: 10.1128/iai.59.3.913-924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P., Declercq W., Libert C., Fiers W. Development of a simple, sensitive and specific bioassay for interleukin-1 based on the proliferation of RPMI 1788 cells. Comparison with other bioassays for IL-1. J Immunol Methods. 1990 Dec 31;135(1-2):25–32. doi: 10.1016/0022-1759(90)90252-q. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Zambon J. J., Christersson L. A., Genco R. J. Diagnosis and treatment of localized juvenile periodontitis. J Am Dent Assoc. 1986 Aug;113(2):295–299. doi: 10.14219/jada.archive.1986.0152. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]