Abstract
Objective
To investigate the prognostic value of peak oxygen pulse, which is the amount of oxygen consumed per heart beat during exercise, and to compare the prognostic value of peak oxygen pulse and maximum oxygen uptake (V̇o2max) with respect to coronary heart disease (CHD) and overall death.
Design
Prospective population‐based study based on 1596 men without CHD or the use of β blockers at baseline.
Results
The risk of CHD was 2.45 (95% CI 1.10 to 5.45) times higher in men with low peak oxygen pulse (< 13.5 ml/beat) than in those with high peak oxygen pulse (> 17.8 ml/beat) after adjustment for age, alcohol consumption, smoking, body mass index, blood pressure, serum lipids, diabetes, family history of CHD and ischaemic ST changes during exercise. During an average follow up of 14 years, 267 men died, 67 of them due to CHD. The respective risk for overall death was 1.79 (95% CI 1.21 to 2.65). The continuous variable V̇o2max was a stronger risk predictor than peak oxygen pulse for CHD and overall death.
Conclusions
Assessment of oxygen pulse provides no complementary information to V̇o2max about cardiorespiratory fitness and prognosis for CHD. The analysis of respiratory gas exchange including the assessment of oxygen pulse during exercise does, however, provide an additional means for defining prognosis for patients with CHD.
In its current form, modern exercise testing is not limited to the observation of ECG changes, heart rate (HR) and blood pressure responses to exercise for screening coronary heart disease (CHD) because important prognostic information is derived from cardiorespiratory fitness in various populations when decisions about interventions are required.1,2,3,4,5,6,7,8,9 Cardiopulmonary exercise testing is a useful non‐invasive tool but its clinical potential is not fully known.1,10 Cardiopulmonary exercise testing with measures such as oxygen uptake and oxygen pulse provides objective and reproducible indices of cardiorespiratory fitness that can be applied to the management of cardiovascular disease (CVD).10,11 Exercise testing with a ventilatory gas analyser, which permits simultaneous assessment of circulatory and ventilatory reserves, has been shown to be a valuable prognostic measure for CVD in unhealthy12,13 and apparently healthy6,9,13 people.
Oxygen pulse is a measure of oxygen consumed per heart beat and may provide adjunctive information about the prognostic importance of exercise capacity with respect to CVD and all cause death.14,15 It is a measure for stroke volume and peripheral oxygen extraction during exercise. Although maximum oxygen uptake (V̇o2max) may be a stronger predictor of death than oxygen pulse in patients with heart failure,14 very limited data are available on the predictive power of oxygen pulse in men without clinical symptoms of CHD. Thus, the principal objective of this study was to investigate the prognostic value of oxygen pulse and V̇o2max with regard to CHD‐related and overall mortality in a population‐based sample of men. We also studied whether oxygen pulse has prognostic value in addition to V̇o2max, which is a well‐defined prognostic marker, and explored whether body mass index (BMI) may modify this association.
METHODS
Participants
The present study population consisted of participants in the KIHD (Kuopio Ischaemic Heart Disease Risk Factor Study), an ongoing population‐based study designed to investigate risk factors for CVD, atherosclerotic vascular diseases and related outcomes. This study is examining men from eastern Finland, an area known for its high prevalence and incidence of atherosclerotic CVD.6,13 The study population is a representative random sample of men living in the town of Kuopio or its surrounding rural communities. These men were 42–61 years old at the baseline examinations between March 1984 and December 1989. Of 3235 eligible men, 2682 (83%) participated in the present study.
Men who had prevalent CHD (n = 677) or who were taking β blockers (n = 485) at baseline were excluded from the study. Prevalent CHD was defined as a history of myocardial infarction or angina pectoris, angina pectoris on effort and the use of glyceryl trinitrate for chest pain at least once a week at baseline. Sixty eight men did not do the exercise test because of the presence of severe cardiovascular or other disease, and 314 men had missing values of V̇o2max. Thus, the present study is based on 1596 men who had complete data on respiratory gas analysis and ECG recordings during exercise. The KIHD was approved by the research ethics committee of the University of Kuopio, Kuopio, Finland. Each participant provided written informed consent.
Exercise test
A maximal symptom‐limited exercise test was performed between 08 00 and 10 00 on an electrically braked cycle ergometer as described previously.6,16 The standardised testing protocol comprised an increase in the workload of 20 W/min, and the starting workload was 50 W for the first 226 men and 20 W for the other 1370 men. For safety reasons, and to obtain reliable information about exercise test variables, the test was supervised by an experienced physician with the assistance of a trained nurse. The ECG was registered continuously during the exercise test. The criterion for myocardial ischaemia in ECG during exercise was horizontal or downsloping ST depression ⩾ 1.0 mm at 80 ms after the J point. Maximum systolic blood pressure (SBP) was defined as the highest value achieved during the exercise test.
Assessment of V̇o2max, peak oxygen pulse and HR
Respiratory gas exchange was measured for the first 226 men by the mixing chamber method and for the other 1370 men by a breath‐by‐breath method.6 V̇o2max was defined as the highest value for or the plateau of oxygen uptake. If a plateau in oxygen uptake could not be reached despite an increase in the exercise workload, the highest value of oxygen uptake was used as V̇o2max. The mixing chamber method measured oxygen uptake as an average value over 30 s, whereas the breath‐by‐breath method analyser measured an average value over 8 s. Pearson's correlation coefficient between the simultaneous mixing chamber and breath‐by‐breath methods was 0.97 in 13 men, indicating a close correlation. Peak oxygen pulse was calculated by dividing derived V̇o2max by the maximum HR during exercise and was expressed in millilitres per beat. HR was recorded by ECG at rest, during the 20 W change at each stage of exercise and at peak exercise. Maximum HR was recorded immediately before termination of the test. Exercise HR response was defined as an actual increase in HR from rest to peak exercise (HR reserve).
Assessment of risk factors
Smoking, alcohol consumption, blood pressure and leisure‐time physical activity were assessed as described previously.6,17 The collection of blood specimens and the measurement of serum lipids and lipoproteins have been described elsewhere.17 The cholesterol content of serum lipoprotein fractions and triglycerides was measured enzymatically (Boehringer Mannheim, Mannheim, Germany). Serum high density lipoprotein cholesterol was separated from fresh serum samples by ultracentrifugation and precipitation. BMI was computed as weight in kilograms divided by the square of height in meters. Medical history, the use of drugs and family history of diseases were assessed by self‐administered questionnaires. Information about medical history and the use of drugs was checked during a medical examination. Family history of CHD was defined as CHD in parents or in the first‐degree relatives before the age of 55 in men and before the age of 65 in women.
Echocardiography
Echocardiographic studies were performed with an ATL Ultramark IV system (Advanced Technology Laboratories, Bothell, Washington, USA) and two‐dimensional guided M mode measurements with a 3.0 or 3.5 MHz transducer. Left ventricular (LV) end diastolic and end systolic internal short‐axis dimensions were among the measures collected.18 All the echocardiographic assessments were performed and interpreted by one cardiologist. Echocardiography was not available for the all men included in the prospective KIHD study, which was focused on exercise testing. A total of 669 men were studied by both echocardiography and exercise testing at baseline.
Ascertainment of follow‐up events
Deaths were ascertained by computer linkage to the National Death Registry by means of the Finnish personal identification codes. No patients were lost to follow up. All the deaths that occurred between study enrolment (from 29 March 1984 to 5 December 1989) and 31 December 2002 were included. CVD and CHD deaths were coded according to the International classification of diseases, ninth revision (codes 390–459 and 410–414, respectively) or 10th revision (codes I00–I99 and I20–I25, respectively).
Statistical analysis
For descriptive purposes, the associations of peak oxygen pulse with the clinical and other exercise characteristics were examined by analysis of variance. In the analysis, peak oxygen pulse was divided into quartiles. Peak oxygen pulse was entered as quartiles into forced Cox proportional hazards regression models, with the first quartile serving as the reference group. Two different sets of covariates were used: (1) age and examination year (1985–1989); and (2) age, examination year, alcohol consumption, smoking, BMI, SBP, diastolic blood pressure, serum lipids (triglycerides, and serum low and high density lipoprotein cholesterol), diabetes mellitus, family history of CHD and ischaemic ST changes during exercise. Peak oxygen pulse and V̇o2max were analysed as continuous variables when the predictive value of these variables was compared.
Relative hazards, adjusted for risk factors, were estimated as antilogarithms of coefficients from multivariable models. The fit of the proportional hazards models was examined by plotting the hazard functions in different categories of risk factors over time. The results indicated that the application of the models was appropriate. In the interaction analyses, oxygen pulse was dichotomised according to the lowest 25 centile (13.5 ml/beat) and body mass was divided according to BMI (< 25 kg/m2 or ⩾ 25 kg/m2). The cumulative incidence of death by peak oxygen pulse was calculated by the Kaplan–Meier method. Data were statistically analysed with SPSS V.11.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Characteristics
At the beginning of the follow up, the mean age of the participants was 52.1 (SD 5.3) years (range 42.0–61.0). In this sample of men, 267 died during an average follow‐up period of 14.4 years (range 1.0–18.0 years). There were 99 CVD deaths, 67 of which were a consequence of CHD. Mean peak oxygen pulse was 15.7 (SD 3.3) ml/beat (range 4.7–31.3) and mean V̇o2max was 32.4 (SD 7.4) ml/kg/min (range 10.3–65.4 ml/kg/min).
Peak oxygen pulse was directly associated with BMI, LV diastolic and systolic diameters (table 1) and exercise characteristics such as ml/kg/min o2max, exercise duration, HR reserve, maximum SBP, energy expenditure and mean intensity of leisure‐time physical activity (table 2). Peak oxygen pulse was inversely associated with age, smoking, resting SBP, serum low density lipoprotein cholesterol, resting HR, respiratory gas exchange ratio and dyspnoea or angina pectoris as the reasons for stopping the exercise test (tables 1 and 2). Peak oxygen pulse was not related to the use of drugs for hypertension or dyslipidaemia, and prevalent hypertension, claudication, stroke or pulmonary diseases at baseline.
Table 1 Clinical characteristics according to quartiles of peak oxygen pulse in men without CHD or the regular use of β blockers.
| Characteristic | All men | Peak oxygen pulse quartile (ml/beat) | p Value for difference* | |||
|---|---|---|---|---|---|---|
| 1st (>17.8) (n = 399) | 2nd (15.8–17.8) (n = 399) | 3rd (13.5–15.7) (n = 399) | 4th (<13.5) (n = 399) | |||
| Age (years) | 52.1 (5.3) | 51.1 (5.2) | 51.7 (5.1) | 52.0 (5.3) | 53.5 (5.3) | <0.001 |
| Alcohol consumption (g/week) | 72.8 (110.9) | 69.9 (97.9) | 71.0 (103.7) | 73.0 (106.2) | 77.1 (131.3) | 0.81 |
| Body mass index (kg/m2) | 26.5 (3.3) | 27.7 (3.5) | 26.7 (3.1) | 26.4 (3.2) | 25.3 (3.2) | <0.001 |
| Cigarette smoking (pack‐years)† | 7.9 (16.0) | 4.8 (12.7) | 6.4 (12.8) | 8.3 (16.3) | 11.8 (19.9) | <0.001 |
| Resting heart rate (beats/min) | 62 (11) | 59 (10) | 61 (9) | 64 (10) | 67 (12) | <0.001 |
| Resting SBP (mm Hg) | 133 (18) | 132 (15) | 132 (15) | 134 (16) | 135 (18) | 0.03 |
| Resting DBP (mm Hg) | 88 (10) | 88 (10) | 88 (10) | 89 (10) | 89 (11) | 0.37 |
| Diabetes‡ | 3.4% | 3.3% | 2.5% | 3.5% | 4.5% | 0.48 |
| Serum LDL cholesterol (mmol/l) | 4.02 (0.99) | 3.88 (0.93) | 3.99 (0.97) | 4.07 (0.97) | 4.11 (1.08) | 0.005 |
| Serum HDL cholesterol (mmol/l) | 1.31 (0.29) | 1.33 (0.28) | 1.33 (0.31) | 1.29 (0.26) | 1.31 (0.03) | 0.74 |
| Serum triglycerides (mmol/l) | 1.20 (0.72) | 1.12 (0.61) | 1.19 (0.81) | 1.23 (0.72) | 1.25 (0.73) | 0.06 |
| Family history of CHD | 46.4% | 45.7% | 44.6% | 47.2% | 48.0% | 0.78 |
| LVEDD (mm)§ | 51.3 (4.3) | 53.1 (3.9) | 51.8 (3.9) | 50.6 (4.2) | 49.8 (4.3) | <0.001 |
| LVESD (mm)§ | 34.0 (4.6) | 35.1 (4.0) | 34.3 (4.2) | 33.4 (4.8) | 33.1 (4.8) | <0.001 |
Data are mean (SD) or percentage.
*One‐way analysis of variance; †lifelong exposure to smoking, which was estimated as the product of years smoked and the number of tobacco products smoked daily at the time of examination12; ‡defined as a history of taking drugs for diabetes or fasting glucose ⩾6.7 mmol/l; §echocardiographic data were available for 668 men (175 in the 1st quartile, 173 in the 2nd, 147 in the 3rd, 173 in the 4th).
CHD, coronary heart disease; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; SBP, systolic blood pressure.
Table 2 Exercise characteristics according to quartiles of peak oxygen pulse in men without CHD or the regular use of β blockers.
| Characteristic | All men | Peak oxygen pulse quartile (ml/beat) | p Value for difference* | |||
|---|---|---|---|---|---|---|
| 1st (>17.8) (n = 399) | 2nd (15.8–17.8) (n = 399) | 3rd (13.5–15.7) (n = 399) | 4th (<13.5) (n = 399) | |||
| Maximum V̇o2 (ml/kg/min) | 32.4 (7.4) | 38.3 (7.3) | 33.8 (5.8) | 30.9 (5.3) | 26.4 (5.4) | <0.001 |
| Exercise test duration (min) | 9.6 (2.4) | 11.7 (2.2) | 10.2 (1.6) | 9.2 (1.7) | 7.6 (1.9) | <0.001 |
| Peak RER (V̇co2/V̇o2) | 1.11 (0.13) | 1.09 (0.11) | 1.11 (0.13) | 1.11 (0.14) | 1.12 (0.15) | 0.03 |
| Heart rate reserve (beats/min)† | 101 (21) | 102 (24) | 102 (18) | 101 (19) | 97 (20) | <0.001 |
| Maximum pulmonary ventilation (l/min) | 88.3 (28.2) | 106.3 (28.9) | 92.3 (24.6) | 84.3 (23.3) | 69.6 (21.8) | <0.001 |
| Maximum SBP during exercise (mm Hg) | 207 (26) | 210 (24) | 206 (25) | 206 (27) | 205 (28) | 0.03 |
| Myocardial ischaemia during exercise‡ | 6.8% | 4.9% | 7.9% | 6.4% | 7.6% | 0.31 |
| Dyspnoea or angina pectoris during exercise§ | 3.1% | 1.6% | 2.7% | 3.0% | 5.1% | 0.04 |
| Delayed blood pressure response at exercise¶ | 1.9% | 1.3% | 1.2% | 1.7% | 3.4% | 0.10 |
| Energy expenditure of LTPA (kJ/week) | 4057 (4998) | 5939 (6703) | 3737 (4175) | 3469 (4145) | 3116 (3851) | <0.001 |
| Mean intensity of LTPA (METs) | 6.0 (1.8) | 6.6 (1.8) | 6.1 (1.8) | 5.7 (1.7) | 5.5 (1.7) | <0.001 |
Data are mean (SD) or percentage.
*One‐way analysis of variance; †defined as maximum heart rate minus resting heart rate; ‡the criterion for myocardial ischaemia on the ECG during exercise was horizontal or downsloping ST depression ⩾1.0 mm at 80 ms after the J point; §dyspnoea or typical angina pectoris as the reasons for termination of the exercise test; ¶systolic or diastolic blood pressure did not increase or decreased in three consecutive blood pressure measurements during exercise.
LTPA, leisure‐time physical activity; METs, metabolic equivalents of oxygen consumption (a metabolic units is the ratio of metabolic rate during exercise to the metabolic rate at rest); RER, respiratory gas exchange ratio; SBP, systolic blood pressure; V̇co2, carbon dioxide production; V̇o2, oxygen uptake.
Peak oxygen pulse, CHD and overall mortality
Peak oxygen pulse was inversely associated with the risk of CHD death after adjustment for age and examination year (table 3). The risk was 2.45 times higher for CHD death in men with a low peak oxygen pulse (< 13.5 ml/beat) than in men with a high peak oxygen pulse (> 17.8 ml/beat) after adjustment for risk factors (age, alcohol consumption, smoking, BMI, blood pressure, serum lipids, diabetes, family history of CHD and ischaemic ST changes during exercise). Further adjustment for leisure‐time physical activity did not notably change the observed association between peak oxygen pulse and death.
Table 3 Relative risk (RR) of death in the quartiles (each n = 399) of peak oxygen pulse in 1596 men with no history of CHD and who were not taking β blockers at baseline.
| CHD death (n = 67) | Overall deaths (n = 267) | |||||
|---|---|---|---|---|---|---|
| RR (95% CI)* | RR (95% CI)† | No of cases | RR (95% CI)* | RR (95% CI)† | No of cases | |
| Peak oxygen pulse (ml/beat) | ||||||
| >17.8 | 1.00 (reference) | 1.00 (reference) | 10 | 1.00 (reference) | 1.00 (reference) | 47 |
| 15.8–17.8 | 1.40 (0.62 to 3.17); p = 0.417 | 1.32 (0.59 to 3.01); p = 0.497 | 14 | 1.24 (0.85 to 1.83); p = 0.265 | 1.19 (0.81 to 1.75); p = 0.395 | 59 |
| 13.5–15.7 | 1.38 (0.61 to 3.13); p = 0.438 | 1.21 (0.52 to 2.82); p = 0.657 | 14 | 1.34 (0.91 to 1.96); p = 0.135 | 1.18 (0.80 to 1.75); p = 0.427 | 63 |
| <13.5 | 2.87 (1.37 to 6.00); p = 0.005 | 2.45 (1.10 to 5.45); p = 0.028 | 29 | 2.08 (1.45 to 2.99); p<0.001 | 1.79 (1.21 to 2.65); p = 0.003 | 98 |
*Adjusted for age and examination year (1985–9); †adjusted for age, examination year, alcohol consumption, smoking, body mass index, systolic and diastolic blood pressures, serum lipids (triglycerides, serum low density lipoprotein and high density lipoprotein cholesterol), diabetes, family history of coronary heart disease (CHD) and ischaemic ST changes during exercise.
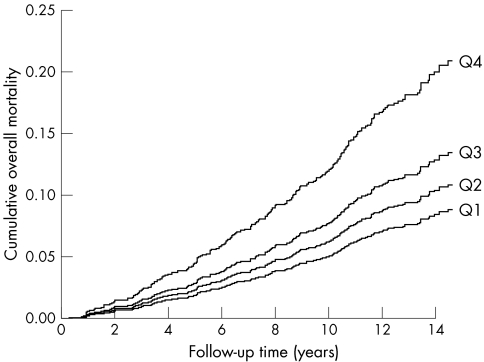
The risk of overall death was 2.08 times higher in men with low peak oxygen pulse (< 13.5 ml/beat) than in men with high peak oxygen pulse (> 17.8 ml/beat) after adjustment for age and examination year, and the risk remained 1.79‐fold after additional adjustment for risk factors (table 3). The use of hypertension drugs, lipid‐lowering drugs or aspirin did not change the observed associations. Figure 1 shows the cumulative risk for death according to the quartiles of peak oxygen pulse.
Figure 1 Multivariable‐adjusted cumulative curves for overall mortality in men without coronary heart disease or the regular use of β blockers according to quartiles (Q) of peak oxygen pulse during an average follow up of 14 years (Q1, > 17.8 ml/beat; Q2, 15.7–17.8 ml/beat; Q3, 13.5–15.7 ml/beat; Q4, < 13.5 ml/beat).
Comparison between peak oxygen pulse and cardiorespiratory fitness
A 1 SD increment in V̇o2max (7.4 ml/kg/min) was related to the risk of overall death (relative risk (RR) 0.63, 95% confidence interval (CI) 0.55 to 0.74, p < 0.001). A 1 SD increment in peak oxygen pulse (3.3 ml/beat) was also associated with the risk of death (RR 0.72, 95% CI 0.62 to 0.84, p < 0.001) after adjustment for age, examination year and risk factors. Similarly, V̇o2max (RR 0.56, 95% CI 0.40 to 0.79, p = 0.001) was a slightly stronger predictor for CHD death than was peak oxygen pulse (RR 0.66, 95% CI 0.48 to 0.89, p = 0.007) when they were entered separately as continuous variables (per 1 SD change) in the multivariable model. In stepwise models adjusted for other risk factors, V̇o2max was selected before peak oxygen pulse for the risk predictor for both overall and CHD death, indicating stronger predictive power. After both exercise test duration and peak oxygen pulse were included with all other confounders in the multivariable model, the risk of all cause death was 0.68 (95% CI 0.60 to 0.79, p < 0.001) and the risk for CHD death was 0.62 (95% CI 0.47 to 0.82, p = 0.001) per 1 SD increment (142 s change) in exercise test duration.
Peak oxygen pulse, BMI and all cause mortality
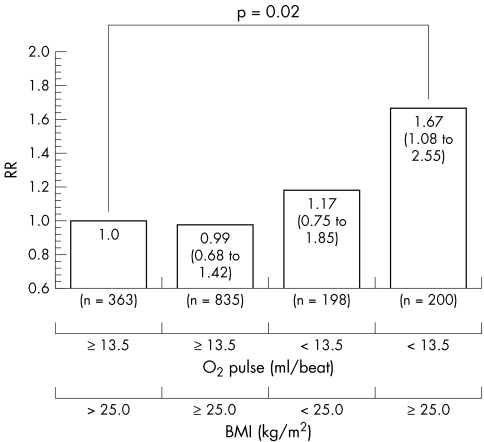
Overweight men (BMI ⩾ 25.0 kg/m2, mean peak oxygen pulse 16.4 ml/beat) had a slightly higher mean peak oxygen pulse than normal‐weight men (BMI < 25.0 kg/m2, mean peak oxygen pulse 15.3 ml/beat). Figure 2 presents the risk factor‐adjusted RRs of death in the four combinations of peak oxygen pulse and BMI. Furthermore, overweight men with low peak oxygen pulse (< 13.5 ml/beat, mean 11.9 ml/beat) had a 1.41‐fold (95% CI 1.05 to 1.93, p = 0.029) risk of death compared with all other overweight men who had moderate to high peak oxygen pulse (⩾ 13.5 ml/beat, mean 17.5 ml/beat) after adjustment for age, examination year and other risk factors except for BMI.
Figure 2 The multivariate‐adjusted relative risk (RR) of overall death during an average follow up of 14 years in 1596 men without coronary heart disease or the regular use of β blockers classified according to peak oxygen pulse (O2 pulse) and body mass index (BMI). The reference group comprised normal‐weight (BMI < 25.0 kg/m2) men with moderate to high peak oxygen pulse (⩾ 13.5 ml/beat).
DISCUSSION
This long‐term follow‐up study shows the prognostic value of oxygen pulse during exercise test with mortality from CHD and any causes in a population‐based sample of men. Peak oxygen pulse was inversely related to the risk of death among men with no history of CHD or the use of β blockers.
V̇o2max is one of the strongest prognostic measures among patients with previously documented CVD,10,12,14 as well as in those without known atherosclerotic CVD.6,16 Whereas V̇o2max indicates oxygen consumption per minute during exercise, oxygen pulse is primarily an indicator of oxygen consumption per heart beat. Oxygen pulse is the product of stroke volume and the arteriovenous oxygen difference during exercise, reflecting myocardial oxygen supply and cardiac functional reserve under physiological stress.19 Normally, oxygen pulse increases quite linearly until it approaches the highest value, whereas in circulatory disorders or cardiac dysfunction, oxygen pulse increases only slightly with increasing workloads in parallel with the increase of stroke volume.15 Thus, the rise of stroke volume in the case of underlying cardiac dysfunction reaches a plateau much earlier, and this leads to a decrease of the oxygen pulse during exercise.19,20 Peak oxygen pulse and oxygen uptake are commonly attributable to low stroke volume and only rarely to low peripheral oxygen utilisation, peripheral vascular disease, poor blood oxygenation in the lung or arrhythmias.21
A substantial proportion of patients with impaired oxygen uptake have been found to have LV dysfunction.11 Cardiac dysfunction may lead to decreased stroke volume due to exercise‐induced myocardial ischaemia. HR increase is relatively steep due to low stroke volume when adequate cardiac output is maintained by tachycardia during exercise.22 On the other hand, a slower increase in HR during exercise in a patient with narrow atherosclerotic coronary arteries would allow more time during diastole for myocardial perfusion. This can be viewed as a protective physiological mechanism,23 as more oxygen can be provided to the myocardium. Patients with decreased LV function who perform regular physical activities benefit from these cardiac adaptations, for example, through increased cardiac output and oxygen utilisation.22,24,25 Improved cardiorespiratory fitness is associated with decreased HR and increased stroke volume.26 Consistently, our study also showed that peak oxygen pulse was directly associated with physical activity, and thereby it emphasises the importance of physical exercise in maintaining good cardiovascular function.
In this study, peak oxygen pulse was related directly to LV dimensions and inversely to conventional CVD risk factors such as serum low density lipoprotein cholesterol, SBP and smoking. The direct association between peak oxygen pulse and BMI can be partly explained by a larger muscle mass in overweight men. Some of the overweight men with a large muscle mass may also have an increased stroke volume and oxygen consumption. It has been shown that the independent effect of fat mass on LV function is lower than that of fat‐free mass.27 Some other studies have suggested that cardiac adaptation to obesity includes LV dilatation and decreased cardiac function.28 The plausible explanation for our finding, therefore, is that the risk is considerably increased in overweight men with high fat mass who have a low peak oxygen pulse, an inappropriately raised stroke volume and decreased cardiac function during exercise.
The strengths of the current study are that we had a representative population‐based sample of middle‐aged men from Finland. Secondly, the participation rate was high and no patients were lost to follow up. Thirdly, we had reliable data on outcome events because deaths were ascertained from the Finnish National Death Registry through personal identification codes with the same procedures as in the FINMONICA (Finnish Multinational Monitoring of trends and determinants in Cardiovascular disease) study.13,16 Fourthly, we had comprehensive data on health habits and other risk predictors for death, which allowed us to control for many potential risk factors.
Few, if any, data based on population samples have shown the inverse relationship between V̇o2max by using ventilatory gas analyses and all cause mortality. On the basis of our findings, peak oxygen pulse was a strong predictive factor in men with no history of CHD or use of β blockers, but it did not provide superior prognostic power beyond that of V̇o2max. Our findings from this prospective study are consistent with a previous study of patients with heart failure showing that oxygen pulse has no additional prognostic value to V̇o2max.14
The limitations of this study are that it is impossible to know how peak oxygen pulse changed during the long follow‐up period, possibly due to changes in health habits, in the use of drugs or in body composition during the follow up. On the other hand, the interventions or any other changes during follow up, if anything, tend to weaken the observed associations.29 Whether genetic susceptibility among men with low peak oxygen pulse and V̇o2max accounted for our findings is not known, but it is unlikely to be a major reason for the results and we cannot show the role of genetic factors in this study. It should be noted that LV dimensions and function were not measured for the participants in the follow‐up study.
This population‐based study shows that peak oxygen pulse is inversely related to mortality in middle‐aged men. Peak oxygen pulse may help to distinguish those patients with increased risk of death, although it did not provide incremental prognostic value beyond that of V̇o2max. On the basis of this study, determining oxygen pulse has no additional value in predicting death from CHD in a population‐based study. Compared with a standard exercise testing, however, applying the available technology for the analysis of respiratory gas exchange during exercise testing will enable the clinician to make a better assessment of prognosis.
ACKNOWLEDGEMENTS
We thank the staff of the Research Institute of Public Health and the Kuopio Research Institute of Exercise Medicine, University of Kuopio, Kuopio, Finland, for data collection in the KIHD study.
Abbreviations
BMI - body mass index
CHD - coronary heart disease
CVD - cardiovascular disease
FINMONICA - Finnish Multinational Monitoring of trends and determinants in Cardiovascular disease
HR - heart rate
KIHD - Kuopio Ischaemic Heart Disease Risk Factor Study
LV - left ventricular
SBP - systolic blood pressure
V̇o2max - maximum oxygen uptake
Footnotes
This study was supported by the grants from National Heart, Lung, and Blood Institute (grant HL44199), Washington, DC, USA; Academy of Finland and the Finnish Ministry of Education, Helsinki, Finland; City of Kuopio, Kuopio, Finland; Juho Vainio Foundation, Helsinki, Finland; Finnish Cultural Foundation of Northern Savo, Kuopio, Finland; Finnish Medical Foundation, Helsinki, Finland; Maud Kuistila Foundation, Helsinki, Finland; Finnish Foundation for Cardiovascular Research, Helsinki, Finland, and Sigrid Juselius Foundation, Helsinki, Finland
Competing interests: None declared.
References
- 1.Myers J, Gullestad L, Vagelos R.et al Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med 1998129286–293. [DOI] [PubMed] [Google Scholar]
- 2.Roger V L, Jacobsen S J, Pellikka P A.et al Prognostic value of treadmill exercise testing: a population‐based study in Olmsted County, Minnesota. Circulation 1998982836–2841. [DOI] [PubMed] [Google Scholar]
- 3.Robbins M, Francis G, Pashkow F J.et al Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation 19991002411–2417. [DOI] [PubMed] [Google Scholar]
- 4.Goraya T Y, Jacobsen S J, Pellikka P A.et al Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000132862–870. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Prakash M, Froelicher V.et al Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002346793–801. [DOI] [PubMed] [Google Scholar]
- 6.Lakka T A, Venäläinen J M, Rauramaa R.et al Relation of physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med 19943301549–1554. [DOI] [PubMed] [Google Scholar]
- 7.Blair S N, Kohl HW I I I, Barlow C E.et al Changes in physical fitness and all‐cause mortality: a prospective study of healthy and unhealthy men. JAMA 19952731093–1098. [PubMed] [Google Scholar]
- 8.Wei M W, Kampert J B, Barlow C E.et al Relationship between low cardiorespiratory fitness and mortality in normal‐weight, overweight, and obese men. JAMA 19992821547–1553. [DOI] [PubMed] [Google Scholar]
- 9.Laukkanen J A, Lakka T A, Rauramaa R.et al Cardiovascular fitness as a predictor of mortality in men. Arch Intern Med 2001161825–831. [DOI] [PubMed] [Google Scholar]
- 10.Mancini D M, Eisen H, Kussmaul W.et al Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 199183778–786. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwland W, Berkhuysen M A, van Veldhuisen D J.et al Impairment of exercise capacity and peak oxygen consumption in patients with mild left ventricular dysfunction and coronary artery disease. Eur Heart J 1998191688–1695. [DOI] [PubMed] [Google Scholar]
- 12.Vanhees L, Fagard R, Thijs L.et al Prognostic significance of peak exercise capacity in patients with coronary artery disease. J Am Coll Cardiol 199423358–363. [DOI] [PubMed] [Google Scholar]
- 13.Laukkanen J A, Kurl S, Salonen R.et al The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population‐based cohort study. Eur Heart J 2004251428–1437. [DOI] [PubMed] [Google Scholar]
- 14.Cohen‐Solal A, Barnier P, Pessione F.et al Comparison of the long term prognostic value of peak exercise oxygen pulse and peak oxygen uptake in patients with chronic heart failure. Heart 199778572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stringer W W, Hansen J E, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol 199782908–912. [DOI] [PubMed] [Google Scholar]
- 16.Laukkanen J A, Kurl S, Lakka T A.et al Exercise‐induced silent myocardial ischemia and coronary morbidity and mortality in middle‐aged men. J Am Coll Cardiol 20013872–79. [DOI] [PubMed] [Google Scholar]
- 17.Salonen J T, Salonen R, Seppanen K.et al HDL, HDL2, HDL3 subfractions, and the risk of acute myocardial infarction: a prospective population study in eastern Finnish men. Circulation 199184129–139. [DOI] [PubMed] [Google Scholar]
- 18.Laukkanen J A, Kurl S, Eränen J.et al Left atrium size and the risk of cardiovascular death in middle‐aged men. Arch Intern Med 20051651788–1793. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman K. Diagnosing cardiovascular and lung pathophysiology from exercise gas exchange. Chest 19971121091–1101. [DOI] [PubMed] [Google Scholar]
- 20.Ferraro S, Perrone‐Filardi P, Desiderio A.et al Left ventricular systolic and diastolic function in severe obesity: a radionuclide study. Cardiology 199687347–353. [DOI] [PubMed] [Google Scholar]
- 21.Harrington D, Anker S D, Coats A J. Preservation of exercise capacity and lack of peripheral changes in asymptomatic patients with severely impaired left ventricular function. Eur Heart J 200122392–399. [DOI] [PubMed] [Google Scholar]
- 22.Koike A, Itoh H, Taniguchi K.et al Detecting abnormalities in left ventricular function during exercise by respiratory measurement. Circulation 1989801737–1746. [DOI] [PubMed] [Google Scholar]
- 23.Ellestad M H. Chronotropic incompetence: the implications of heart rate response to exercise (compensatory parasympathetic hyperactivity?). Circulation 1996931485–1487. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh T, Yacoub M H, Mertens D J.et al Cardiorespiratory responses to exercise training after orthotopic cardiac transplantation. Circulation 198877162–171. [DOI] [PubMed] [Google Scholar]
- 25.Hambrecht R, Niebauer J, Fihn E.et al Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 1995251239–1249. [DOI] [PubMed] [Google Scholar]
- 26.Seals D R, Hagberg J M, Spina R J.et al Enhanced left ventricular performance in endurance trained older men. Circulation 199489198–205. [DOI] [PubMed] [Google Scholar]
- 27.De Simone G, Devereux R B, Daniels S R.et al Stroke volume and cardiac output in normotensive children and adults: assessment of relations with body size and impact of overweight. Circulation 1997951837–1843. [DOI] [PubMed] [Google Scholar]
- 28.Messerli F H, Sundgaard‐Riise K, Reisin E D.et al Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med 198399757–761. [DOI] [PubMed] [Google Scholar]
- 29.MacMahon S, Peto R, Cutler J.et al Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias, Lancet 1990335765–774. [DOI] [PubMed] [Google Scholar]