Abstract
Objective
To examine whether raised plasma brain natriuretic peptide (BNP) concentrations decrease after successful pulmonary vein isolation (PVI) in patients with atrial fibrillation (AF).
Methods
53 patients (mean age 53 years) with drug‐refractory, paroxysmal lone AF underwent segmental ostial PVI. Blood samples were collected before and after PVI. BNP concentrations were determined by immunoassays.
Results
Median plasma BNP concentrations were significantly higher in patients with lone AF than in controls (patients with supraventricular tachyarrhythmias, n = 21) (64.6 (71.9) v 13.9 (7.8) pg/ml, p < 0.01). AF recurred in 21 patients after the initial PVI procedure (recurrent AF group), and the others were free from AF without antiarrhythmic drugs (non‐recurrent AF group). BNP concentrations were significantly decreased by PVI in the non‐recurrent AF group (38.9 (39.1) to 18.3 (16.1) pg/ml, p < 0.01) but not in the recurrent AF group.
Conclusions
Raised plasma BNP concentrations decreased after successful segmental ostial PVI in patients with AF.
Brain natriuretic peptide (BNP) is a member of the natriuretic peptide family.1 This natriuretic peptide has a compensatory action in the cardiovascular systems including systemic arterial dilatation, natriuresis, diuresis and renin inhibition. Plasma BNP concentration has been used as an efficient tool for identifying patients with various cardiovascular diseases.2,3,4 Atrial fibrillation (AF) is the most frequently observed sustained arrhythmia in patients with either impaired or normal ventricular function. Recently, several lines of evidence have shown that BNP concentrations are raised in patients with AF as well as with idiopathic bilateral atrial dilatation.5,6,7,8,9,10,11 Ellinor et al10 reported that BNP concentrations were significantly higher in patients with lone AF than in their control group. In addition, plasma BNP concentrations have been shown to decrease after successful cardioversion, indicating that AF affects BNP secretion in patients with AF.5,6,11,12
Pulmonary veins (PVs) are well recognised to play an important part in both the initiation and maintenance of AF.13,14,15,16,17 Segmental ostial pulmonary vein isolation (PVI) procedures with radiofrequency currents have become more common for the treatment of AF that is unresponsive to drug treatment.13,14,15,16,17 However, the influence of PVI on plasma BNP concentration has yet to be clarified. We therefore examined the impact of successful PVI on BNP concentrations in patients with drug‐refractory AF.
METHODS
Patients
Fifty‐nine consecutive patients with frequent attacks of drug‐refractory paroxysmal AF, who were referred to us to undergo catheter ablation, were enrolled in this study. Patients were excluded if they had a left ventricular ejection fraction of less than 55% on an echocardiogram, a history of myocardial infarction, cardiomyopathy, rheumatic heart disease, significant valvular disease, renal failure or hyperthyroidism. Patients who had hypertension before the onset of AF episodes were also excluded. Only patients with lone AF who did not meet any exclusion criteria were enrolled in this study. All antiarrhythmic drugs were discontinued for at least five half lives before the study. Control participants (n = 21), matched for age and sex, were selected from patients with supraventricular tachyarrhythmias (13 patients with atrioventricular nodal re‐entrant tachycardia and eight with atrioventricular re‐entrant tachycardia; patients with atrial tachycardia and atrial flutter were excluded from analysis) who underwent catheter ablation, with no other cardiovascular disease.
Electrophysiological study
All patients were informed of the nature of catheter ablation and its possible complications and gave their informed consent to participate in the study protocol, which was approved by the Human Research Committee at this institution. The study was performed as described previously.13,15,16,17 The left atrium (LA) and PVs were explored through either a patent foramen ovale or transseptal catheterisation with two catheters: one for circumferential PV mapping and a quadripolar mapping/ablation catheter. All four PVs were directly visualised by selective venography, which was performed during mid‐expiration by hand injection of contrast media (10–20 ml) in biplane views. Findings were displayed during the procedure to show the venous anatomy and the location of the LA–PV junction. The PV was mapped with a steerable circular catheter measuring 15–30 mm in diameter (according to the PV diameter as determined by venography) equipped with twenty 1 mm electrodes in a loop made of shape‐retaining material (Lasso; Biosense Webster, Diamond Bar, California, USA) orthogonal to the shaft. The PV muscle potentials were defined as described previously15 and were recorded in a bipolar mode from 10 bipoles through the band pass filters of 30–500 Hz and an amplification of 1 to 2 cm/mV on polygraphy (EP Med System; Century Medical, Tokyo, Japan).
Ablation procedure
In each case, all four PVs were targeted to be electrically disconnected from the LA. The segments of the PV perimeter showing the earliest activation with electrogram polarity reversal were preferentially targeted. The ablation strategy was anatomically directed to the atrial side of the PV–atrial junction (segmental ostial PVI). After PV muscle conduction distal to the ablation sites was eliminated, according to either the abolition or dissociation of the distal PV muscle potentials, the absence of conduction from the PV to the LA was also confirmed by pacing inside the PV with either a mapping catheter or the Lasso catheter. Radiofrequency energy was delivered at the distal electrode (8 mm tip) of the thermocouple‐equipped ablation catheter (target 50°C) with a power limit of 30–35 W for 60 s. The end point was the establishment of bidirectional block between the LA and PV, as described previously.15,16,17
Blood sampling and natriuretic hormone assays
Blood samples were obtained in EDTA from each participant. Samples were centrifuged, and plasma was extracted, aliquoted and stored at −80°C until analysis. Plasma BNP concentration was measured by an immunoradiometric assay specific for human BNP in a commercial kit (Shionoria; Shionogi Co Ltd, Osaka, Japan). The minimum detectable quantity of human BNP is 4 pg/ml. This assay system does not cross react with human atrial natriuretic peptide (ANP). Plasma BNP concentrations were determined two days before the PVI procedure and at a three‐month follow up after the PVI procedure in an outpatient clinic. For patients who had recurrent AF after an initial PVI and underwent second PVI, BNP concentrations were also measured at a three‐month follow up after the second PVI procedure. At enrolment into this study, 33 patients with lone paroxysmal AF were in sinus rhythm and the rest were in AF; all control participants were in sinus rhythm.
Patient follow up
After the procedure, all patients were followed up once a month at an outpatient clinic. The recurrence of AF was evaluated by symptoms, ECG recordings and 24 h ambulatory monitoring (one, three and six months after the procedure). When sustained AF recurred after an early unstable period (usually within one week after the procedure), an antiarrhythmic drug, which had previously been ineffective, was restarted either temporarily or continuously. Freedom from AF recurrence was defined as no detected episode of AF, without antiarrhythmic drug treatment, beyond 30 days after the initial procedure. A repeat ablation session was recommended for patients who were judged to have had an unsuccessful procedure at three months after the initial procedure.
All patients underwent two‐dimensional echocardiography in the left lateral decubitus position with an ultrasonic device equipped with a 2.5 MHz transducer. Left ventricular diastolic and systolic dimensions were measured in the parasternal long‐axis view according to standard recommendations.18
Statistical analysis
Quantitative data are expressed as mean (SD), and they were compared by paired and unpaired t tests. Categorical variables, including the frequency of baseline characteristics, were compared by the 2 × 2 χ2 test. A value of p < 0.05 was considered to be significant.
RESULTS
Plasma BNP concentrations in patients with lone AF
Table 1 shows the baseline characteristics of the patients with lone AF and the echocardiographic data.
Table 1 Baseline characteristics of patients with lone AF and controls.
| Lone AF group (n = 53) | Control group (n = 21)* | |
|---|---|---|
| Baseline characteristics | ||
| Men | 47 (89%) | 18 (86%) |
| Age (years) | 53.1 | 53.9 |
| Duration of AF (years) | 5.2 (4.6) | NA |
| HTN developed after AF | 3 (6%) | NA |
| Echocardiogram | ||
| Ejection fraction (%) | 64.4 (6.1) | NA |
| LVDd (mm) | 48.8 (5.9) | NA |
| Left atrial size (mm) | 38.2 (4.9) | NA |
Data are presented as the number (%) or the mean (SD).
*Patients with supraventricular tachyarrhythmias without structural heart disease.
AF, atrial fibrillation; HTN, hypertension; LVDd, left ventricular diastolic dimension.
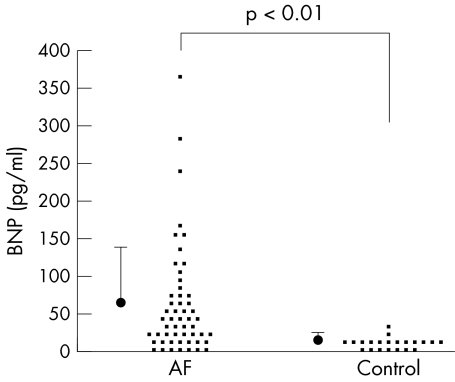
Six patients with cardiovascular disease (four with a diagnosis of hypertension before the onset of AF, one with hypertrophic cardiomyopathy and one with dilated cardiomyopathy) were excluded from analysis. As a result, the data from 53 patients (47 men, six women; mean age 53 (10) years, range 30–71 years) with frequent attacks of paroxysmal lone AF were analysed in this study. Symptomatic AF had first occurred 5.2 (4.6) years before enrolment in the study. Three patients in this study who had hypertension that developed after the onset of AF had a normal ejection fraction. The mean values of the echocardiographic parameters of the patients with lone AF, including chamber dimensions and left ventricular ejection fraction, were all normal. Baseline characteristics did not differ significantly between the lone AF group and the control group. Patients with lone AF did not have any other known factors that can increase the BNP concentrations, including impaired cardiac performance, ventricular hypertrophy and renal failure. Baseline BNP concentration was significantly higher in patients with lone AF than in the controls (64.6 (71.9) v 13.9 (7.8) pg/ml, p < 0.01) (fig 1). Plasma BNP concentration exceeded normal (< 20 pg/ml) in 39 of 53 patients with lone AF (74%), whereas only two control patients had an abnormal BNP concentration. In the patients with lone AF, the heart rhythm (sinus rhythm or AF) at the time of blood sampling did not affect baseline BNP concentrations (sinus rhythm: 65.3 (77.1) pg/ml, n = 33; AF: 62.3 (63.7) pg/ml, n = 20).
Figure 1 Baseline plasma brain natriuretic peptide (BNP) concentrations in patients with lone atrial fibrillation (AF) and controls (patients with supraventricular tachyarrhythmia without structural heart disease). Averaged data are presented as mean (SD).
Ablation results
All 212 targeted PVs were successfully isolated from the LA in 53 patients. No complications related to these procedures were observed. After a mean follow up of 14 months, 60.4% (32 of 53) of the patients were free from AF without antiarrhythmic drug treatment after the initial PVI (non‐recurrent AF group, n = 32). AF recurred in the remaining patients (recurrent AF group, n = 21). Twelve patients underwent a second PVI procedure after a follow up of at least three months. All of them were free from AF without antiarrhythmic drug treatment after a mean follow up of 10 months. The overall success rate of PVI was 83% (44 of 53). We compared the clinical, echocardiographic and electrophysiological parameters between the non‐recurrent AF group and the recurrent AF group. Patients in the recurrent AF group were significantly older than those in the non‐recurrent AF group (56 (9) v 50 (10) years, p < 0.05), although they did not differ significantly in left atrial dimension, left ventricular diastolic dimension, history of AF, sex and the number of radiofrequency applications.
Effects of PVI on plasma BNP concentration
In the non‐recurrent AF group, plasma BNP concentration decreased significantly after successful PVI (from 38.9 (39.1) to 18.3 (16.1), p < 0.01) (fig 2). In contrast, in the recurrent AF group, BNP concentration did not change significantly after an unsuccessful initial PVI (from 103.5 (91.8) to 86.6 (83.6), NS) (fig 2). However, among patients who underwent a second PVI procedure after an unsuccessful initial PVI (n = 12), plasma BNP concentration decreased significantly after a successful second PVI, but not after an unsuccessful initial PVI (107.4 (107.4), 91.5 (94.0) and 23.7 (16.4) at baseline, after an initial unsuccessful PVI and after a second successful PVI, respectively; p < 0.05 after initial PVI versus after the second PVI) (fig 3), thus suggesting that cessation of AF episodes by successful PVI procedures may be associated with a reduction in plasma BNP concentration.
Figure 2 Plasma brain natriuretic peptide (BNP) concentrations decreased in patients without recurrent atrial fibrillation (AF) after pulmonary vein isolation (PVI) but not in patients with recurrent AF after PVI. Averaged data are presented as mean (SD).
Figure 3 Plasma brain natriuretic peptide (BNP) concentrations decreased after a second pulmonary vein isolation (PVI) procedure was successful in patients with recurrent atrial fibrillation (n = 12) after an unsuccessful initial PVI. Averaged data are presented as mean value (SD).
DISCUSSION
In this study, we showed that (1) plasma BNP concentration was significantly higher in patients with lone AF than in the control participants; and (2) raised BNP concentrations significantly declined after successful PVI procedures, but not after an unsuccessful initial PVI procedure.
AF and plasma BNP concentrations
Recently, Silvet et al7 reported higher plasma BNP concentrations in outpatients with chronic AF than in a control group without cardiovascular disease. Other investigators have also reported high BNP concentrations in patients with both paroxysmal and persistent AF as well as idiopathic bilateral atrial dilatation,5,6,8,9,10,11 although conflicting evidence has been reported in heterogeneous populations including patients with left ventricular dysfunction.19 Ellinor et al10 reported a discordant pattern of plasma BNP and ANP concentrations, with a significant rise of pro‐BNP in the presence of normal pro‐ANP concentrations in patients with a history of lone AF without structural heart disease. They also showed that this rise is present even during sinus rhythm. In this study, we also studied patients with lone AF without structural heart disease as suggested by echocardiographic data and medical history. We identified significantly higher plasma BNP concentrations in patients with lone AF undergoing PVI than in the control group with supraventricular tachyarrhythmias in the absence of structural heart disease. We also found that the rhythm of the study participants at the time of blood sampling (AF or sinus rhythm) was not associated with plasma BNP concentration.
Effects of PVI on plasma BNP concentration
Various investigations have shown that sinus rhythm restored by direct current cardioversion leads to a decrease in plasma BNP concentration.5,6,11,12 We evaluated BNP concentration before and after PVI in patients with drug‐refractory AF. To our knowledge, this is the first study showing that plasma BNP concentration decreased dramatically after successful PVI. In the non‐recurrent AF group, plasma BNP decreased significantly to concentrations similar to those of the control group measured three months after the elimination of AF by successful PVI. In the recurrent AF group, however, BNP concentration remained high as long as AF recurred even after unsuccessful PVI. These results suggest that the change in plasma BNP concentration did not result from the PVI procedure itself. Although the causes of the raised BNP concentrations in patients with AF remain to be clarified,20 studies with human atrial tissue have shown that the atrium can also produce BNP.21 A rise of plasma BNP concentration in patients with chronic AF has recently been reported to be due to augmented BNP secretion from the atrium.6 Histological examination of atrial biopsy specimens from patients with lone AF showed a variety of changes, such as severe hypertrophy, fibrosis and inflammation.22 All of these pathological changes have been reported to augment production of BNP. An irregular ventricular rhythm in patients with acute AF may also explain the high BNP concentrations in this group of patients. It is well known that AF itself can lead to heart failure, or so‐called tachycardia‐induced cardiomyopathy.23 Atrial production of BNP possibly is enhanced by the atrial overload caused by an unfavourable haemodynamic profile and altered left ventricular filling pattern in patients with AF. If frequent irregular atrial tachycardia itself increases BNP production in the atria, it seems quite reasonable to assume that elimination of AF by PVI is the main cause of reduced plasma BNP concentration. Common pathophysiological changes related to both AF and increased BNP concentration may also exist, including age, left atrial pressure, left ventricular hypertrophy and increased left ventricular wall stress. In fact, patients in the recurrent AF group were older than those in the non‐recurrent AF group in this study, which may explain why patients with recurrent AF had a higher BNP concentration. A recent report suggested that AF and congestive heart failure are distinct manifestations of a common aetiology characterised by abnormal BNP concentrations.24 In patients with congestive heart failure and AF, the restoration and maintenance of sinus rhythm by catheter ablation without the use of drugs has been reported to improve cardiac function.25 Given that plasma BNP decreased dramatically to concentrations similar to those of the control group after only three months, it seems unlikely that PVI relatively rapidly alleviates these common pathophysiological conditions as the causes of both AF and an increase in BNP concentration. However, because we did not examine cardiac function after PVI, the disappearance of AF episodes after PVI may have improved mild cardiac dysfunction, which was not detectable by echocardiography. Further studies are needed to answer these issues.
Study limitations
Firstly, heart rhythm at the time of baseline blood sampling was heterogeneous. Although the heart rhythm at blood sampling did not affect baseline BNP concentrations in patients with lone AF, BNP production may have increased in the heart during AF. Secondly, the number of patients enrolled in the study was relatively small. A larger patient population should be studied to validate our findings. Thirdly, we did not examine the time course of the change in BNP concentration after PVI. BNP concentration possibly decreases over time even after unsuccessful PVI.
Conclusions
Plasma BNP concentration was significantly higher in patients with lone AF than in the control group, and the raised BNP concentrations declined significantly only after successful PVI. A large‐scale study is needed to fully address whether BNP concentration is a useful marker for assessing the recurrence of AF after PVI procedures.
ACKNOWLEDGEMENTS
We thank Mr Brian Quinn for checking the English of our manuscript. We also thank Masato Matsushima MD for assistance with the statistical analyses.
Abbreviations
AF - atrial fibrillation
ANP - atrial natriuretic peptide
BNP - brain natriuretic peptide
LA - left atrium
PV - pulmonary vein
PVI - pulmonary vein isolation
Footnotes
Competing interests: None declared.
References
- 1.Mukoyama M, Nakao K, Hosoda K.et al Brain natriuretic peptide as a novel cardiac hormone in humans: evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 1991971402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura M, Endo H, Nasu M.et al Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population. Heart 200287131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieczorek S J, Wu A H, Christenson R.et al A rapid B‐type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: a multicenter evaluation. Am Heart J 2002144834–839. [DOI] [PubMed] [Google Scholar]
- 4.Mueller T, Gegenhuber A, Poelz W.et al Diagnostic accuracy of B type natriuretic peptide and amino terminal pro BNP in the emergency diagnosis of heart failure. Heart 200591606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jourdain P, Bellorini M, Funck F.et al Short‐term effects of sinus rhythm restoration in patients with lone atrial fibrillation: a hormonal study. Eur J Heart Fail 20024263–267. [DOI] [PubMed] [Google Scholar]
- 6.Inoue S I, Murakami Y O, Sano K.et al Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Fail 2000692–96. [DOI] [PubMed] [Google Scholar]
- 7.Silvet H, Young‐Xu Y, Walleigh D.et al Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol 2003921124–1127. [DOI] [PubMed] [Google Scholar]
- 8.Arima M, Kanoh T, Kawano Y.et al Plasma levels of brain natriuretic peptide increase in patients with idiopathic bilateral atrial dilatation. Cardiology 20029712–17. [DOI] [PubMed] [Google Scholar]
- 9.Horie H, Tsutamoto T, Minai K.et al Brain natriuretic peptide predicts chronic atrial fibrillation after ventricular pacing in patients with sick sinus syndrome. Jpn Circ J 200064965–970. [DOI] [PubMed] [Google Scholar]
- 10.Ellinor P T, Low A F, Patton K K.et al Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol 20054582–86. [DOI] [PubMed] [Google Scholar]
- 11.Ohta Y, Shimada T, Yoshitomi H.et al Drop in plasma brain natriuretic peptide levels after successful direct current cardioversion in chronic atrial fibrillation. Can J Cardiol 200117415–420. [PubMed] [Google Scholar]
- 12.Mabuchi N, Tsutamoto T, Maeda K.et al Plasma cardiac natriuretic peptides as biochemical markers of the recurrence of atrial fibrillation in patients with congestive hearts failure. Jpn Circ J 200064765–771. [DOI] [PubMed] [Google Scholar]
- 13.Haissaguerre M, Jais P, Shah D C.et al Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998339659–666. [DOI] [PubMed] [Google Scholar]
- 14.Jais P, Sanders P, Hsu L F.et al Catheter ablation for atrial fibrillation. Heart . 2005;917–9. [DOI] [PMC free article] [PubMed]
- 15.Yamane T, Shah D C, Jais P.et al Electrogram polarity reversal as an additional indicator of breakthroughs from the left atrium to the pulmonary veins. J Am Coll Cardiol 2002391337–1344. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Shah D C, Jais P.et al Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation 20001022463–2465. [DOI] [PubMed] [Google Scholar]
- 17.Jais P, Hocini M, Macle L.et al Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 20021062479–2485. [DOI] [PubMed] [Google Scholar]
- 18.Schiller N B, Shah P M, Crawford M, American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A, Enriquez‐Sarano M, Burnett J C., Jret al Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler‐echocardiographic study. J Am Coll Cardiol 2000351256–1262. [DOI] [PubMed] [Google Scholar]
- 20.Rashidi A. Mechanism of high brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol 200493670. [DOI] [PubMed] [Google Scholar]
- 21.Tuinenburg A E, Brundel B J, Van Gelder I C.et al Gene expression of the natriuretic peptide system in atrial tissue of patients with paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol 199910827–835. [DOI] [PubMed] [Google Scholar]
- 22.Frustaci A, Chimenti C, Bellocci F.et al Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997961180–1184. [DOI] [PubMed] [Google Scholar]
- 23.Nerheim P, Birger‐Botkin S, Piracha L.et al Heart failure and sudden death in patients with tachycardia‐induced cardiomyopathy and recurrent tachycardia. Circulation 2004110247–252. [DOI] [PubMed] [Google Scholar]
- 24.Wang T J, Larson M G, Levy D.et al Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 20031072920–2925. [DOI] [PubMed] [Google Scholar]
- 25.Hsu L F, Jais P, Sanders P.et al Catheter ablation of atrial fibrillation in congestive heart failure. N Engl J Med 20043512373–2383. [DOI] [PubMed] [Google Scholar]