Abstract
Objective
To evaluate the prognostic value of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) in patients with aortic stenosis being treated conservatively or undergoing aortic valve replacement (AVR).
Methods
159 patients were followed up for a median of 902 days. 102 patients underwent AVR and 57 were treated conservatively. NT‐proBNP at baseline was raised in association with the degree of severity and of functional status.
Results
During follow up 21 patients (13%) died of cardiac causes or required rehospitalisation for decompensated heart failure. NT‐proBNP at baseline was higher in patients with an adverse outcome than in event‐free survivors (median 623 (interquartile range 204–1854) pg/ml v 1054 (687–2960) pg/ml, p = 0.028). This difference was even more obvious in conservatively treated patients (331 (129–881) pg/ml v 1102 (796–2960) pg/ml, p = 0.002). Baseline NT‐proBNP independently predicted an adverse outcome in the entire study group and in particular in conservatively treated patients (area under the curve (AUC) = 0.65, p = 0.028 and AUC = 0.82, p = 0.002, respectively) but not in patients undergoing AVR (AUC = 0.544). At a cut‐off value of 640 pg/ml, baseline NT‐proBNP was discriminative for an adverse outcome.
Conclusion
NT‐proBNP concentration is related to severity of aortic stenosis and provides independent prognostic information for an adverse outcome. However, this predictive value is limited to conservatively treated patients. Thus, the data suggest that assessing NT‐proBNP may have incremental value for selecting the optimal timing of valve replacement.
Aortic valve diseases, namely aortic stenosis (AS) and aortic regurgitation, are common in developed countries, with AS being the most common valvular heart disease.1,2 Symptomatic status and echocardiography are the most important clinical factors used to confirm the diagnosis, asses severity and monitor progression of AS. Aortic valve replacement (AVR) is indicated for symptomatic patients with severe AS3,4 but whether asymptomatic patients should undergo AVR or should be treated conservatively is controversial.5,6 For patients with moderate AS surgical treatment is generally not recommended.
B‐type natriuretic peptide (BNP) and its N‐terminal fragment (NT‐proBNP) are neurohormones synthesised and secreted mainly by the ventricular myocardium. Their release is stimulated by an increase in ventricular wall stress.7 In patients with AS, BNP and NT‐proBNP rise in correlation with severity and functional status as assessed by the New York Heart Association (NYHA) classification.8,9,10,11 Furthermore, a correlation with the progression of AS has been documented.12 Recently, two studies have been published showing a predictive value for BNP and NT‐proBNP in patients with severe AS, most of whom underwent AVR.13,14 Both studies showed that BNP and NT‐proBNP provided independent prognostic information for postoperative outcome.
Therefore, we aimed at evaluating the prognostic value of NT‐proBNP in patients with valvular AS who are treated conservatively compared with patients undergoing valve replacement.
METHODS
Patients
We included 159 consecutive patients who were referred to our institution for further evaluation of valvular AS from April 2002 to November 2003. All patients had an ejection fraction above 45% documented by echocardiography. Patients with concomitant relevant aortic regurgitation (> II°) and mitral regurgitation (> II°) were not included in this study. Functional status was evaluated and graded according to the NYHA classification by physicians blinded to NT‐proBNP values. The medical history was assessed as the patients reported it or based on their medical records, if available. The indication for valve surgery was left to the discretion of the treating physicians, who were blinded to NT‐proBNP values and who were encouraged to decide in accordance with the current guidelines.3,4 A first follow up visit was attended personally by 109 patients 329 (219–347) days after study entry and a second follow up by 123 patients 902 (861–952) days after study entry. The follow up time did not differ between surgically treated and conservatively treated patients (901 (98) v 892 (95), p = 0.551). At study entry and at follow up, medical history and functional status were assessed and echocardiography and blood sampling were performed. The remaining 36 patients who did not attend a follow up visit were interviewed by telephone or their treating home physicians were contacted. Cardiac death, rehospitalisation for acute decompensated heart failure and the combination of cardiac death and rehospitalisation for acute decompensated heart failure were regarded as adverse events. Death was considered to be cardiovascular if no other obvious causes of death had been identified.
Echocardiography
A comprehensive transthoracic echocardiography study was performed with an Agilent Sonos 1.75–3.5 MHz scanner (Phillips Medical Ultrasound) with the use of harmonic imaging at study entry and at follow up. All examinations were done by an experienced echocardiographer, blinded to NT‐proBNP measurements. Left ventricular diameter was assessed by M mode in the left parasternal view. Left ventricular function was visually assessed and quantified as shortening fraction, and ejection fraction was calculated according to Teichholz. Maximum and mean aortic velocities were measured by continuous‐wave Doppler echocardiography from the apical or right parasternal view. In sinus rhythm transaortic velocity was averaged over three cycles and in patients with atrial fibrillation over seven cycles. Maximum and mean pressure gradients were calculated by the built‐in software. Aortic valve area was calculated from mean velocities. Severity of AS was graded according to the mean transvalvular pressure gradient obtained echocardiographically. A mean transvalvular pressure gradient below 30 mm Hg was considered mild AS (AS I), from 30 and 50 mm Hg moderate AS (AS II) and above 50 mm Hg severe AS (AS III). Left ventricular mass was calculated by the formula of Devereux.15
NT‐proBNP measurement
From all patients blood samples were taken at study entry and at follow up from an antecubital vein in gel‐filled tubes without anticoagulants. The specimens were centrifuged within 1 h and serum was frozen at −80°C until analysis. NT‐proBNP was measured by an electrochemiluminescence immunoassay (Elecsys proBNP; Roche Diagnostics, Mannheim, Germany).
Statistics
Values of NT‐proBNP are expressed as median and interquartile range and of all other variables as mean (SD). For statistical comparison of NT‐proBNP values the Mann–Whitney test (two groups) and Kruskal–Wallis test (n groups) were applied. For analyses of patients' baseline characteristics Student's t test (two groups) or analysis of variance (n groups) was used for continuous variables and the χ2 test or Fischer's exact test for categorical variables. The correlation between NT‐proBNP and clinical parameters was analysed by Spearmen's correlation coefficient (rs). Receiver operating characteristic (ROC) curve analysis was performed for NT‐proBNP at baseline as a predictor for an adverse event and the area under the curve (AUC) from the ROC curves was calculated. An optimised cut‐off value was derived from the ROC curve as the value providing the optimal test accuracy. Event rates according to NT‐proBNP values were plotted as Kaplan–Meier curves and for statistical analysis the log rank test was applied. For multivariate estimation of the hazard ratio for the occurrence of the end points Cox regression analysis was performed. All tests were performed two sided and p < 0.05 was considered to indicate significance. For all statistical analysis the statistical software SPSS V.10.0 (SPSS Inc, Chicago, Illinois, USA) for Windows was used.
RESULTS
A total of 159 patients were enrolled. According to the above mentioned definitions, 26 patients were classified as having mild AS (AS I), 31 patients as having moderate AS (AS II) and 102 patients as having severe AS (AS III). Aortic valves were replaced in 102 patients and 57 patients were treated conservatively. Table 1 details the patients' baseline characteristics. Conservatively treated and surgical patients did not differ in sex distribution, age, serum creatinine and frequency of atrial fibrillation. Coronary artery disease defined as a history of coronary artery bypass grafting, percutaneous intervention, acute myocardial infarction or presence of relevant coronary artery stenosis at angiography was present in 49 patients. There was no difference in frequency of coronary artery disease between conservatively treated patients and patients undergoing valve replacement. Patients scheduled for valve replacement had lower ejection fraction, higher left ventricular mass index, smaller aortic valve area and higher mean pressure gradient than patients for whom a conservative strategy was recommended.
Table 1 Baseline characteristics of all patients according to therapeutic strategy.
| Conservative treatment | Aortic valve replacement | p Value | |
|---|---|---|---|
| Number | 57 | 102 | |
| Women | 29 (51%) | 50 (50%) | 0.822 |
| Age (years) | 69 (13) | 69 (10) | 0.971 |
| NYHA class | |||
| I | 22 | 12 | |
| II | 26 | 34 | <0.001 |
| III | 7 | 41 | |
| IV | 2 | 15 | |
| Atrial fibrillation | 6 (11%) | 15 (15%) | 0.455 |
| Coronary artery disease | 19 (33%) | 30 (29%) | 0.608 |
| Creatinine (μmol/l) | 84 (17) | 80 (19) | 0.265 |
| Body mass index (kg/m2) | 28.4 (3.2) | 27.3 (3.6) | 0.073 |
| Ejection fraction (%) | 59 (4) | 56 (9) | 0.022 |
| LVMI (g/m2) | 118 (38) | 140 (37) | 0.002 |
| Mean pressure gradient (mm Hg) | 32 (21) | 60 (17) | <0.001 |
| Aortic valve area (cm2) | 0.96 (0.33) | 0.69 (0.17) | <0.001 |
| NT‐proBNP (pg/ml) | |||
| Baseline | 423 (163–1145) | 832 (364–2752) | 0.001 |
| (n = 57) | (n = 102) | ||
| First follow up | 456 (166–1664) | 501 (229–892) | 0.940 |
| (n = 39) | (n = 70) | ||
| Second follow up | 498 (165–1918) | 493 (225–996) | 0.842 |
| (n = 40) | (n = 83) |
Values are expressed as number (%), mean (SD) or median (interquartile range).
LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
NT‐proBNP at baseline
NT‐proBNP at baseline was raised in association with the degree of severity (AS I, 301 (135–472) pg/ml; II, 466 (145–1054) pg/ml; and III, 1035 (473–2980) pg/ml; p < 0.001) and functional status (NYHA I, 235 (114–1187) pg/ml; II, 623 (308–1425) pg/ml; III, 733 (313–3033) pg/ml; and IV, 4269 (1446–6857) pg/ml; p < 0.001). Baseline NT‐proBNP in female patients was not different from that in male patients (715 (293–1872) v 673 (225–2207) pg/ml, p = 0.589). Patients with atrial fibrillation (n = 21) had higher values than patients in sinus rhythm (2100 (920–5782) v 608 (215–1615) pg/ml, p = 0.001). NT‐proBNP at baseline was moderately correlated with ejection fraction (−0.419, p < 0.001) and left ventricular mass index (0.434, p < 0.001) and was weakly but significantly correlated with mean pressure gradient (0.351, p < 0.001), aortic valve area (rs = 0.380, p < 0.001), age (rs = 0.302, p < 0.001) and creatinine (rs = 0.171, p = 0.037). Baseline NT‐proBNP was higher in patients undergoing AVR than in patients who were treated conservatively.
Association between NT‐proBNP and clinical outcome
In the entire study population 18 patients (11%) died during the follow up period. Thirteen patients (8%) died of cardiac causes and five of non‐cardiac causes (Wegner's disease with glomerulonephritis, ileus, pulmonary embolism, cerebral stroke and gastric cancer). Ten patients (6%) required rehospitalisation for acute decompensated heart failure. The combined end point of cardiac death and rehospitalisation for acute heart failure was reached by 21 patients (13%). There was a slight trend towards a higher rate of rehospitalisation in the conservative group than in the surgical group (6 (11%) v 4 (4%), p = 0.169). However, this difference was not significant.
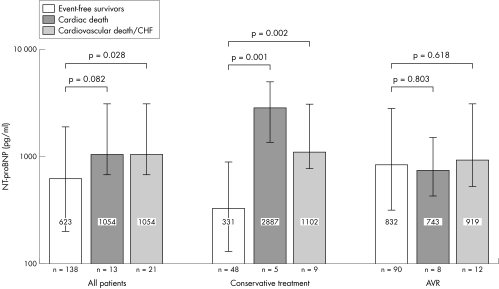
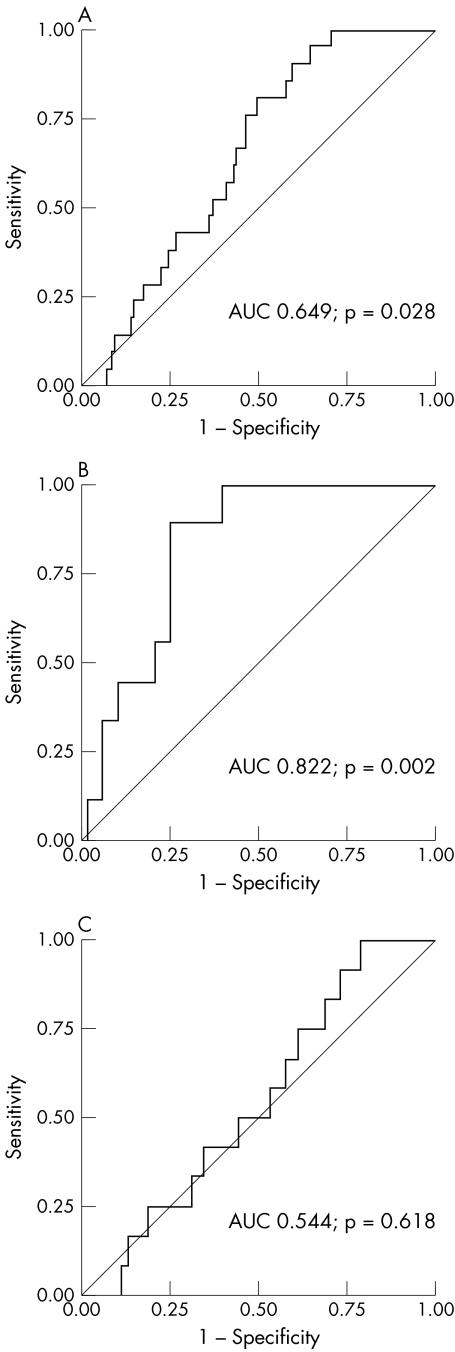
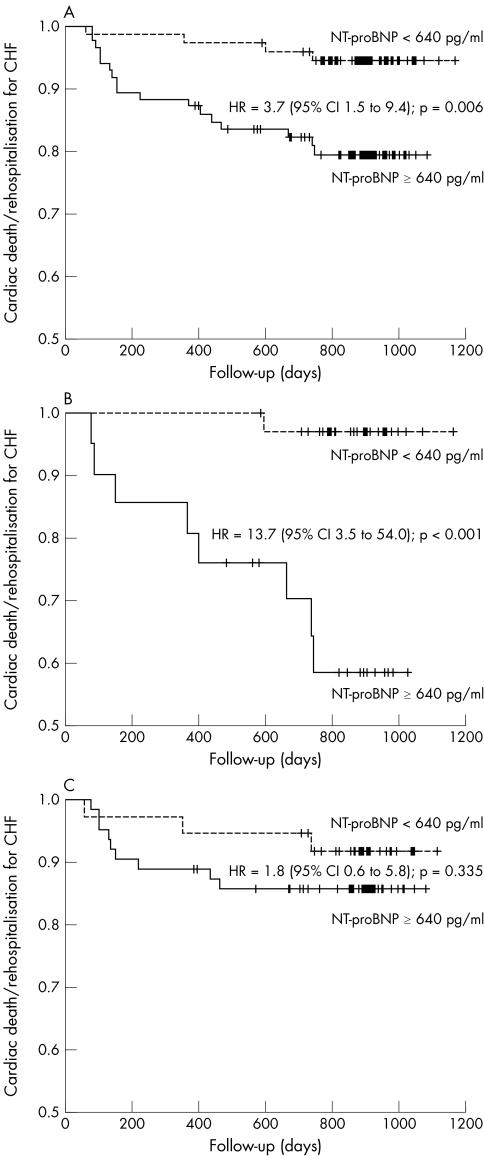
In the entire study population baseline NT‐proBNP concentrations were lower in patients without an adverse event during the follow up period than in patients who died of cardiac causes or who were readmitted for decompensated heart failure (623 (204–1854) v 1054 (687–2960) pg/ml, p = 0.028). If only patients with cardiac death were compared with survivors, there was a strong trend towards higher baseline NT‐proBNP values, but this difference did not reach significance (1054 (687–2969) v 646 (218–1930) pg/ml, p = 0.082). In the conservatively treated group, baseline NT‐proBNP concentrations differed notably between survivors and those who died of cardiac causes (362 (133–913) v 2887 (1617–4032) pg/ml, p = 0.001) and between event‐free survivors and patients experiencing the combined end point of cardiac death or rehospitalisation for decompensated heart failure (331 (129–881) v 1102 (796–2960) pg/ml, p = 0.002). In contrast, among patients who underwent AVR baseline NT‐proBNP values did not differ between event‐free survivors and those with an adverse outcome (865 (331–2840) v 743 (434–1482) pg/ml, p = 0.803 for patients with cardiac death and 832 (318–2752) v 919 (519–2998) pg/ml, p = 0.618 for the combined end point) (fig 1). The AUC of the ROC curve for baseline NT‐proBNP as a predictor for the combination of cardiac death or rehospitalisation for decompensated heart failure was 0.649 (p = 0.028) for the entire study group. If the ROC curves were plotted separately for conservatively treated patients and for patients undergoing valve surgery, test accuracy improved for the conservatively treated patients with an AUC of 0.822 (p = 0.002), whereas baseline NT‐proBNP had no predictive value for patients undergoing valve surgery (AUC = 0.544, p = 0.618) (fig 2). From the ROC analysis a cut‐off value providing optimal test accuracy for the prediction of cardiac death or rehospitalisation for decompensated heart failure of 640 pg/ml was calculated. In applying this cut‐off value to the entire study population, sensitivity was 81%, specificity 51%, positive predictive value 20% and negative predictive value 95%. However, for the subgroup of conservatively treated patients test accuracy was noticeably better, with a sensitivity of 89%, specificity 73%, positive predictive value 38% and negative predictive value 97%. Consequently, in Kaplan–Meier analysis baseline NT‐proBNP values dichotomised at this cut off discriminated patients with an adverse outcome in the entire study group (hazard ratio (HR) 3.70, 95% confidence interval (CI) 1.46 to 9.36, log rank test p = 0.006) and even better in the conservative group (HR 13.71, 95% CI 3.48 to 54.03, log rank test p < 0.001). However, for surgically treated patients baseline NT‐proBNP did no discriminate patients at high risk (HR 1.78, 95% CI 0.55 to 5.76, log rank test p = 0.335) (fig 3).
Figure 1 N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) at study entry (baseline) in relation to clinical outcome and therapeutic strategy. Values are expressed as median (interquartile range). AVR, aortic valve replacement; CHF, decompensated heart failure.
Figure 2 Receiver operating characteristic curves for N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) as a predictor of cardiac death or rehospitalisation for decompensated heart failure. (A) All patients. (B) Only conservatively treated patients. (C) Only surgically treated patients.
Figure 3 Kaplan–Meier curves of event‐free survival (freedom from cardiac death or rehospitalisation for decompensated heart failure (CHF)) of patients according to N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) values above (solid line) and below (dotted line) a cut‐off value of 640 pg/ml. (A) All patients. (B) Only conservatively treated patients. (C) Only surgically treated patients. CI, confidence interval; HR, hazard ratio.
Conservatively treated patients with an adverse outcome had a lower left ventricular ejection fraction (53 (9)% v 60 (4)%, p = 0.049) than the event‐free survivors. Other parameters such as age, left ventricular mass, valve area and pressure gradient, however, were not different between the two groups (table 2). In multivariate Cox regression analysis for the entire study population and for the subgroup of conservatively treated patients, baseline NT‐proBNP was an independent predictor for cardiac death or rehospitalisation for decompensated heart failure. On the contrary, in surgically treated patients only the presence of atrial fibrillation, but not baseline NT‐proBNP, was an independent predictor for an adverse outcome (table 3).
Table 2 Clinical data of conservatively treated patients.
| Event‐free survivors | Cardiovascular death/readmission for CHF | p Value | |
|---|---|---|---|
| Number | 48 | 9 | |
| Women | 25 (52%) | 4 (44%) | 0.730 |
| Atrial fibrillation | 4 (8%) | 2 (22%) | 0.237 |
| Coronary artery disease | 14 (30%) | 5 (56%) | 0.143 |
| Moderate aortic stenosis | 38 (79%) | 6 (67%) | 0.412 |
| Severe aortic stenosis | 9 (21%) | 3 (33%) | |
| Age (years) | 69 (13) | 73 (8) | 0.416 |
| Ejection fraction (%) | 60 (4) | 53 (9) | 0.049 |
| LVMI (g/m2) | 116 (37) | 131 (45) | 0.317 |
| Mean pressure gradient (mm Hg) | 31 (22) | 38 (13) | 0.385 |
| Aortic valve area (cm2) | 0.98 (0.33) | 0.87 (0.07) | 0.140 |
| NT‐proBNP at baseline (pg/ml) | 331 (129–881) | 1102 (796–2966) | 0.002 |
Values are expressed as number (%), mean (SD) or median (interquartile range).
CHF, decompensated heart failure; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 3 Multivariate analysis of predictors of an adverse outcome.
| All patients | Conservative group | AVR | ||||
|---|---|---|---|---|---|---|
| Wald | p Value | Wald | p Value | Wald | p Value | |
| NT‐proBNP ⩾640 pg/ml | 4.45* | 0.035* | 5.24* | 0.022* | 0.09 | 0.766 |
| Rhythm | 3.83* | 0.050* | 0.82 | 0.364 | 6.37* | 0.012* |
| Age | 1.08 | 0.299 | 1.99 | 0.158 | 2.48 | 0.116 |
| Mean pressure gradient | 0.96 | 0.326 | 0.00 | 0.949 | 0.11 | 0.741 |
| Sex | 0.39 | 0.533 | 0.19 | 0.659 | 0.16 | 0.685 |
| NYHA class III or IV | 0.39 | 0.533 | 0.05 | 0.823 | 0.89 | 0.345 |
| Ejection fraction | 0.28 | 0.596 | 7.27* | 0.007* | 0.00 | 0.964 |
| Coronary artery disease | 0.15 | 0.697 | 1.91 | 0.167 | 0.45 | 0.501 |
*Significant predictor.
Multivariate Cox regression analysis for predictors of an adverse outcome (cardiac death and rehospitalisation for decompensated heart failure) for the entire study group, conservatively treated patients and patients who underwent valve surgery.
AVR, aortic valve replacement; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Among conservatively treated patients, 11 underwent unscheduled AVR because of disease progression. NT‐proBNP values at baseline did not differ between patients who progressed to AVR and those who did not (463 (114–715) v 409 (172–1145) pg/ml, p = 0.994). The frequency of adverse events was not different between patients who underwent unscheduled AVR and those who did not (p = 0.354).
DISCUSSION
In this study we aimed at analysing the diagnostic value of NT‐proBNP for patients with valvular AS of all degrees of severity. Patients were either treated conservatively or underwent AVR if indicated. The major finding was that NT‐proBNP value was related to disease severity and provided independent prognostic information for an adverse outcome in the entire study population. However, subgroup analyses showed that the prognostic value of NT‐proBNP was limited to patients who were treated conservatively. For patients undergoing valve surgery NT‐proBNP had no prognostic value.
To date two studies have investigated the predictive value of BNP or NT‐proBNP in AS. Bergler‐Klein and colleagues13 reported an independent prognostic value of NT‐proBNP for postoperative survival in 79 symptomatic patients with severe AS. Furthermore, they found a predictive value of BNP and NT‐proBNP for symptom deterioration of 43 initially asymptomatic patients with severe AS. In another study Lim et al14 described their finding of a predictive value of BNP for survival in 70 patients with severe AS. Although both studies included patients who did not undergo valve surgery and thus were treated conservatively, nothing has been reported on the predictive value of BNP or NT‐proBNP in this subgroup. Thus, our data confirm these previous reports and, more importantly, they extend present knowledge. In contrast to the above mentioned studies in which only patients with severe AS were included, we also included patients with mild and moderate AS. In this cohort we obtained a predictive value for an unfavourable outcome for the entire study population. However, and this finding is new, in our study the predictive value of NT‐proBNP was limited to the group of patients who were treated conservatively. This finding is of clinical interest, as our data suggest that NT‐proBNP assessment provides important additional information about patients not undergoing valve replacement. From our data it can be concluded that NT‐proBNP assessment improves risk stratification and may contribute to deciding on the optimal timing of valve replacement. However, this hypothesis needs to be tested in further prospective studies. Conversely, NT‐proBNP value does not provide any incremental prognostic information about patients undergoing valve replacement. Therefore, NT‐proBNP assessment is not of diagnostic value in this setting.
We were able to calculate an optimised cut‐off value of NT‐proBNP for the prediction of an adverse outcome of 640 pg/ml. Applying this cut‐off, NT‐proBNP values discriminate patients at high risk. Notably, the calculated cut‐off value in our study was almost identical to the cut‐off value for NT‐proBNP of 80 pmol/ml (or 592 pg/ml) that Bergler‐Klein et al13 applied in their study.
Limitations
Although this study included a high number of patients relative to previously published studies, it is limited by the small number of patients in the conservatively treated group. Our study must therefore be regarded as a pilot study. Although our data suggest that NT‐proBNP may contribute to deciding on the optimal timing of valve replacement, admittedly our data do not provide evidence that patients with high NT‐proBNP would have benefited from valve surgery. NT‐proBNP was assessed at baseline and at follow up while patients were taking standard drugs including β blocker, angiotensin‐converting enzyme inhibitors and diuretics. All of these drugs are known to influence natriuretic peptide concentration and an impact of NT‐proBNP in the present study has to be considered. We did not perform exercise testing in this study. Especially in asymptomatic patients with severe AS, exercise testing might have distinguished more patients with an indication for valve surgery.
Conclusion
NT‐proBNP is related to severity of valvular AS and provides independent prognostic information for an adverse outcome. However, this predictive value is limited to conservatively treated patients. Thus, our data suggest that NT‐proBNP assessment may be of incremental value in deciding on the optimal timing of valve replacement. In contrast, NT‐proBNP assessment provides no additional prognostic information for patients undergoing valve replacement.
ACKNOWLEDGEMENTS
This study has been made possible by a grant from the Kühl‐Stiftung. We are indebted to Roche Diagnostics Germany, which supported this study by providing assays for NT‐proBNP free of charge. Special thanks to B Rabenau and S Vogt for the tremendous work they have done in the laboratory.
Abbreviations
AS - aortic stenosis
AUC - area under the curve
AVR - aortic valve replacement
BNP - B‐type natriuretic peptide
HR - hazard ratio
NT‐proBNP - N‐terminal pro‐B‐type natriuretic peptide
NYHA - New York Heart Association
ROC - receiver operating characteristic
References
- 1.Aronow W S, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am J Cardiol 199167776–777. [DOI] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkila J.et al Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993211220–1225. [DOI] [PubMed] [Google Scholar]
- 3.Bonow R O, Carabello B, de Leon A C., Jret al Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998981949–1984. [DOI] [PubMed] [Google Scholar]
- 4.Iung B, Gohlke‐Barwolf C, Tornos P.et al Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J 2002231252–1266. [DOI] [PubMed] [Google Scholar]
- 5.Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J 2002231417–1421. [DOI] [PubMed] [Google Scholar]
- 6.Carabello B A. Clinical practice: aortic stenosis. N Engl J Med 2002346677–682. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T, Matsuda K, Itoh H.et al Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end‐systolic wall stress in patients with aortic stenosis. Am Heart J 1997133307–314. [DOI] [PubMed] [Google Scholar]
- 8.Talwar S, Downie P F, Squire I B.et al Plasma N‐terminal pro BNP and cardiotrophin‐1 are elevated in aortic stenosis. Eur J Heart Fail 2001315–19. [DOI] [PubMed] [Google Scholar]
- 9.Qi W, Mathisen P, Kjekshus J.et al Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001142725–732. [DOI] [PubMed] [Google Scholar]
- 10.Gerber I L, Stewart R A, Legget M E.et al Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation 20031071884–1890. [DOI] [PubMed] [Google Scholar]
- 11.Weber M, Arnold R, Rau M.et al N‐terminal pro brain‐type natriuretic peptide (NT‐proBNP) is a highly sensitive biochemical marker for surgical therapy in patients with aortic stenosis. Circulation 2003108(17 Suppl)IV513 [Google Scholar]
- 12.Weber M, Arnold R, Rau M.et al Relation of N‐terminal pro B‐type natriuretic peptide to progression of aortic valve disease. Eur Heart J 2005261023–1030. [DOI] [PubMed] [Google Scholar]
- 13.Bergler‐Klein J, Klaar U, Heger M.et al Natriuretic peptides predict symptom‐free survival and postoperative outcome in severe aortic stenosis. Circulation 20041092302–2308. [DOI] [PubMed] [Google Scholar]
- 14.Lim P, Monin J L, Monchi M.et al Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B‐type natriuretic peptide. Eur Heart J 2004252048–2053. [DOI] [PubMed] [Google Scholar]
- 15.Devereux R B, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation 197755613–618. [DOI] [PubMed] [Google Scholar]