Abstract
Objective
To determine, through a systematic review and meta‐analysis, the magnitude of the survival deficit associated with a restrictive filling pattern after acute myocardial infarction (AMI).
Methods
Online databases were searched for prospective echocardiography outcome studies of patients after AMI. All authors were contacted to seek confirmation of their data. Restrictive filling was compared with all non‐restrictive filling patterns. Review Manager Version 4.2.7 software was used for analysis.
Results
3855 patients in 16 studies were identified. Follow up varied from two weeks to five years (> 1 year, 10 studies; and > 4 years, four studies). 776 (20%) of patients had a restrictive filling pattern at baseline. 580 patients died (247 in the restrictive group), and the overall odds ratio for death (restrictive filling worse) was 4.10 (95% confidence interval 3.38 to 4.99).
Conclusions
Mortality is about four times higher in patients with a restrictive filling pattern than in those with non‐restrictive filling patterns after AMI. Echocardiographic assessment of diastolic filling pattern is an important part of the echocardiographic assessment of patients after myocardial infarction and provides important prognostic information about such patients.
Echocardiographic Doppler indices of diastolic function have been widely used to identify subgroups of patients with different risk among patients with systolic dysfunction and heart failure (HF).1 In particular, the presence of restrictive mitral diastolic filling (high E velocity, low A velocity and shortened deceleration time) is associated with pronounced increases in mortality in patients with HF.1 This technique has also been widely applied in the acute coronary setting. The presence of restrictive filling, which is a sign of raised left atrial pressure, is also associated with worse survival after acute myocardial infarction (AMI).2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 As in the HF cohorts, many of these studies showed significant increases in mortality with advanced filling patterns. However, these studies are somewhat heterogeneous in comparison with the HF cohorts in that baseline clinical and functional status vary greatly, in particular left ventricular (LV) function. Importantly, both systolic and diastolic dysfunction are less prevalent in this group of patients than in HF populations, as are the overall event rates.
The objective of this systematic review and meta‐analysis was to combine the results of all studies investigating the relationship between prognosis and the presence of restrictive diastolic filling after myocardial infarction to estimate accurately the risk associated with this advanced diastolic filling abnormality. The hypothesis was that patients with evidence of restrictive filling after AMI may have worse long‐term survival.
METHODS
Identification of studies
Published studies were identified through online searches of several medical databases: Biological Abstracts, Clinical Evidence, Current Contents, Embase, Medline, Medline In‐progress and PubMed. The search terms “incidence”, “prognosis”, “outcome”, “mortality”, “clinical trials”, “echocardiography”, “ventricle”, “systolic”, “diastolic” and “myocardial infarction” were used. Papers published up until September 2005 and not restricted to the English language were searched. The citation lists of the identified papers were also reviewed. All authors were contacted and asked to provide further data or studies (published or unpublished). All three authors developed the strategy for database searching and one reviewer primarily performed the actual searches. One other reviewer implemented some targeted review. All papers were reviewed by two reviewers, both of whom extracted and confirmed the data. Ambiguities were reconciled through consensus reached through further discussion, evaluation and confirmation by the original authors. In all but five cases, the original authors subsequently reviewed these data.
Criteria for study inclusion
From the online searches, we selected any study that included echocardiography, prognosis and AMI. In addition, studies were required to have clearly stated diagnostic thresholds for definition of AMI. Each study was then reviewed according to a predetermined protocol, which included information about patients' recruitment and follow up (prospective, retrospective, consecutive recruitment, exclusions and reason), co‐morbidity, loss to follow up and completeness of data (that is, how many patients actually underwent the echocardiographic measurements). Only prospective studies that recruited patients after reaching a predetermined diagnostic threshold for AMI, with an end point of death, were included (fig 1).
Figure 1 Review process for identification of studies of prospective echocardiography outcomes of patients after acute myocardial infarction.
Definition of a prospective study
For these analyses, we determined a prospective study to be one into which patients were enrolled and then followed up. The diastolic analysis may have been retrospectively applied, but the patients needed to be recruited at the time of their acute coronary event. Retrospective cohort studies that identified patients at the end of the follow‐up period were excluded. Most studies recruited consecutive patients, although this was not an inclusion criterion in order to allow the exclusion of patients with incomplete data—for example, atrial fibrillation or suboptimal imaging.
Data collection
For many studies, the publication identified the numbers of patients and events in each filling pattern group. Every investigator was asked by letter or email to confirm the data we had extracted or to provide data where the paper's content was insufficient. Authors were also asked to confirm that patients were included in one publication only and to identify sources of potential patient overlap. We also sought any additional references to either published or unpublished studies. One author supplied unpublished data from a doctoral thesis29 to supplement already published data.27 Not all studies reported diastolic parameters as a primary end point. We contacted authors of any publications that reported outcome data and comprehensive echocardiographic examinations at baseline to determine whether patients were able to be stratified according to diastolic filling grade (fig 1).
Differentiation of restrictive filling
Restrictive filling was determined by the individual authors and clearly stated in the papers' methods. These criteria were reviewed and considered to be acceptable and in accordance with internationally accepted standards, allowing for slight regional and institutional variation. One paper reported pseudonormal and restrictive filling pattern together, and these deaths were attributed equally between the two groups.30
Statistical methods
The Cochrane Collaboration Program Review Manager V.4.2.7 (http://www.cc‐ims.net/RevMan) was used for analysis. For each study, patients were stratified according to the individual study criteria as having restrictive or non‐restrictive filling. The number of patients and the number of events allocated to each group were recorded. The odds ratio in a fixed effects model is presented, but a random effects model was also evaluated. As the random effects model was not different from the fixed effects model, we present only the fixed effects model. Each study was weighted in the model according to sample size. Standard tests for heterogeneity were used including χ2 (presented). Funnel plots were examined for evidence of publication bias and none was observed. All‐cause mortality was the primary end point.
RESULTS
Thirty‐one potential studies were identified,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 of which we analysed 162,3,4,5,6,7,12,13,17,18,19,22,23,24,25,26,27 (table 1). One author provided additional data published in thesis form only,29 which were merged with another publication27 to alleviate patient overlap. Initially, 12 studies were reviewed and subsequently excluded5,8,9,10,11,15,16,20,21,28,31,32: one study was a retrospective cohort study; two studies reported other outcomes than death; and nine studies reported on patients who were included in other patient cohorts. Study data (number of patients and events) were confirmed by 12 of the authors. Despite repeated attempts, confirmation was not received for four studies. Of these, three studies clearly reported numbers in the publication and were included, leaving only one study that may have been eligible but was excluded.14 Thus, 16 studies and 3855 patients were included in the final analysis.2,3,4,6,7,12,13,17,18,19,22,23,24,25,26,27 The average follow‐up time varied between two weeks and five years: follow up was six months or less in two studies; one year in five studies; 1–3 years in six studies; and 4–5 years in the remaining four studies (table 1).
Table 1 Prognosis studies on restrictive filling pattern classification after myocardial infarction.
| Reference (publication year) | Author confirmed data | Country | No | FU (years) | Events | Echocardiographic definition of restrictive filling |
|---|---|---|---|---|---|---|
| Garcia‐Rubira3 (1997) | Numbers in paper | Spain | 133 | In hospital | 20 deaths | Non‐restrictive, restrictive |
| Nijland2 (1997) | Numbers in paper | The Netherlands | 95 | 3 | 8 deaths | E:A >2 or DT <140 ms |
| Sakata4 (1997) | Numbers in paper | Japan | 206 | 5 | 33 deaths | Low mitral A velocity |
| Poulsen17 (1999) | Yes | Denmark | 58 | 1 | 6 deaths (MFP 2/3) | DT <140 ms |
| Burgess6 (2000) | Yes | UK | 102 | 1 | 9 deaths | Non‐restrictive, restrictive |
| Møller7 (2000) | Yes | Denmark | 125 | 1 | 33 deaths | DT <140 ms |
| Cerisano13 (2001) | Yes | Italy | 104 | 2.7 | 9 deaths | DT <130 ms |
| Otasevic12 (2001) | Yes | Yugoslavia | 106 | 4.9 | 14 deaths | DT <150 ms |
| Møller18 (2003) | Yes | Denmark multicentre | 799 | 2.8 | 197 deaths | DT <140 ms |
| Møller19 (2003) | Yes | USA | 288 | 1.25 | 46 deaths | DT <140 ms |
| Beinart25 (2004) | Yes | Israel | 371 | 5 | 63 deaths | Non‐restrictive, restrictive* |
| Kinova27 (2004) | Yes | Bulgaria | 119 | 0.5 | 12 deaths | DT <140 ms |
| Karvounis24 (2004) | Yes | Greece | 33 | 1 | 3 deaths | E:A >2 |
| Møller23 (2004) | Yes | Europe multicentre | 225 | 2.3 | 23 deaths | DT <140 ms |
| Quintana26 (2004) | Yes | Sweden | 520 | 2.6 | 57 deaths | DT <140 ms |
| Temporelli22 (2004) | Yes | Italy | 571 | 4 | 47 deaths | DT <130 ms |
*Based on age‐dependent E:A ratio and deceleration time.
DT, deceleration time of passive mitral filling velocity (E); E:A, ratio of early to late mitral filling; FU, follow up; HF, heart failure; MFP, mitral filling pattern.
Restrictive filling versus non‐restrictive filling
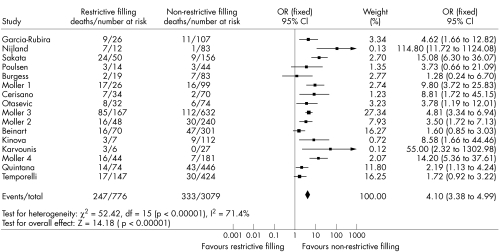
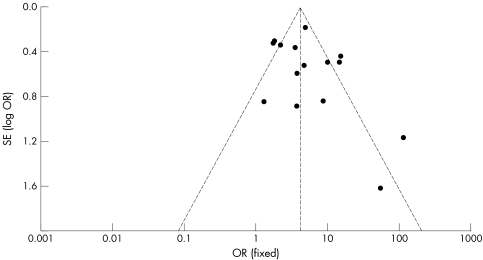
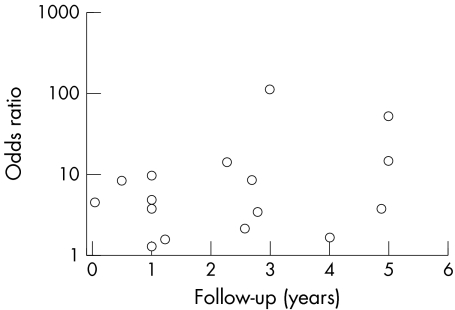
Of the 3855 patients, 776 (20%) had a restrictive filling pattern and the remaining 3079 (80%) had a non‐restrictive pattern. There were 580 deaths in total: 247 (43%) in the restrictive group and 333 (57%) in the non‐restrictive group. The event rate in the whole group was 15.1%: 31.8% in the restrictive filling group and 10.8% in the non‐restrictive group. The odds ratio for death associated with restrictive filling pattern was 4.10 (95% confidence interval 3.38 to 4.99, p < 0.00001) (fig 2). The group was significantly heterogeneous (p < 0.00001) but this was driven by two studies2,24 with unusually high odds ratio due to lower event rates in the non‐restrictive group. When these studies were excluded from the analysis heterogeneity was reduced, but not eliminated. This is confirmed by inspection of the funnel plots (fig 3) and Egger test (p = 0.023). The odds ratio was not related to length of follow up (fig 4).
Figure 2 Meta‐analysis of the restrictive filling pattern after myocardial infarction. Fixed effects model, studies weighted according to sample size. CI, confidence interval; OR, odds ratio.
Figure 3 Funnel plot of odds ratios (OR) (fixed effects model).
Figure 4 Odds ratio according to follow‐up time of each study.
Criteria for detection of restrictive filling
The criteria for restrictive filling varied between studies and usually included a shortened deceleration time (cut off ranged from 130–150 ms) (table 1). The observed odds ratio for the individual study and the deceleration time cut off used were not related.
DISCUSSION
This analysis combined the results of several studies to compare the prognostic relevance of the presence of a restrictive filling pattern with all other non‐restrictive filling patterns after AMI. The presence of a restrictive filling pattern documented soon after the initial ischaemic injury was associated with four times the risk of death in this meta‐analysis, which represents 3855 patients and 580 deaths, providing a robust and significant result. This risk was observed in a variety of patients with differing baseline clinical status and degrees of underlying LV systolic dysfunction. Assessment of diastolic filling is a relatively easy addition to the clinical echocardiographic examination of patients after AMI and is an important part of the information provided by echocardiography in this situation.
Restrictive filling pattern is associated with higher left atrial pressure,33,34,35,36,37,38,39 higher neurohormone concentration40,41 and higher New York Heart Association functional class.42 As neurohormonal concentration, in particular B‐type natriuretic peptide, is also linked to prognosis after acute coronary events43,44 it may not be so surprising that patients with evidence of restrictive filling have poor prognosis, as both most likely reflect a degree of haemodynamic stress.
LV systolic impairment and clinical HF are common complications of AMI and undoubtedly explain some of the risk observed in the patients with restrictive filling pattern. Excluding the patients who are admitted with shock, HF either is present at admission or develops during hospitalisation in 20–30% of patients after AMI.45,46,47,48,49 This number continues to rise after hospital discharge,45 with about 40% of patients developing HF by six years.49 LV systolic dysfunction has been considered to be one of the most important predictors of outcome after AMI and thus has been the focus of echocardiography in this setting. When LV ejection fraction is measured, patients with a low ejection fraction experience higher mortality rates than those with preserved ejection fraction.49,50 But, interestingly, patients whose LV ejection fraction is not measured (most patients in many acute coronary studies) experience similar mortality to those with depressed ejection fraction.49,50 While many of the patients who develop HF have depressed LV systolic function, the relationship between ejection fraction and outcome is biphasic, with increased risk in patients with hyperdynamic systolic function.47 Both of these groups of high risk patients with differing systolic function may have advanced diastolic filling abnormalities, which may be associated with outcome. Patients with angiographically documented coronary artery disease but no detectable resting systolic dysfunction almost always have diastolic filling abnormalities when assessed by radionuclide techniques.51 Often patients with advanced diastolic filling abnormalities and HF have small LV cavities that appear to contract normally but, because of the reduced volume, the stroke volume is inadequate. In such patients, systolic function may be wrongly assumed to be normal. This hypothesis is supported by the many studies, and the current meta‐analysis, showing increased risk associated with restrictive filling pattern.
Nevertheless, many of the patients with restrictive filling pattern also have significant systolic impairment in addition to their diastolic abnormalities. In multivariate analyses of patients with HF the presence of restrictive filling pattern, characterised by high E velocity, low A velocity and shortened deceleration time, remains one of the only independent predictors of cardiovascular outcome.52,53,54 Several of the larger studies in this systematic review and meta‐analysis showed that when both diastolic and systolic measurements were available systolic measurements offered little or no additional prognostic information to diastolic filling pattern.18,22,25 In a multicentre study of 799 patients, wall motion score index, diastolic filling pattern and the Tei index (a measure of global systolic and diastolic LV function) all predicted outcome but, in a multivariate model, wall motion score index had no prognostic value.18 In another study of 571 patients, predischarge restrictive filling pattern was a very strong predictor of death and LV end diastolic index was a weaker, but still significant, predictor of death in a Cox proportional hazards model.22 These findings were confirmed in another smaller study (n = 371) where restrictive filling pattern predicted death alongside other clinical factors and left atrial size, but not systolic function.25 In contrast, in a multicentre study of 520 patients, although restrictive filling pattern predicted death, it did not reach significance in a multivariate model where wall motion score index, age, history of hypertension and diabetes did.26 Thus, there is lack of consensus regarding the prognostic significance of individual echocardiographic variables among the previously published studies.
It is difficult to truly evaluate the independent role and combined effects of systolic and diastolic dysfunction, and it was not within the realm of this systematic review and meta‐analysis to evaluate the individual and multivariate contributions of all echocardiographic and clinical variables to overall risk. This would require a meta‐analysis incorporating individual patient data from each study. Because of the similarity between the studies' designs and the total number of patients, this approach may be able to discern the relative weighting of systolic and diastolic parameters and overall risk. An individual patient meta‐analysis or large individual study (sample size 1000–2000 patients, 300–500 events) would potentially have the power to discriminate between the individual echocardiographic parameters. This would be an important study that may lead to understanding of both the development of HF and the risk factors for death in patients having acute coronary events. In turn, this may lead to enhanced medical management of these patients. Nevertheless, this systematic review and meta‐analysis has collated the results of 16 studies, from a wide variety of settings and more than a dozen countries, and provides an average size of the mortality risk associated with the presence of restrictive filling after AMI. Some of the larger studies provided similar results, but their effect sizes were inconsistent (with odds ratio ranging from about 2 to 15 for the five largest studies).
The importance of assessing diastolic filling pattern in the setting of AMI is often underrated. In this situation, the diagnostic role of echocardiography is of paramount importance: quantification of regional wall motion, infarct size, viable myocardium and structural trauma constitute the first goal of echocardiography in patients with an acute coronary event. However, the consistency of the findings and the size of the effect observed in this systematic review and meta‐analysis suggest that it may be equally important for prognostic purposes to identify diastolic filling grades accurately, allowing a means of identifying patients who are at highest risk of death after their acute coronary event. A similar systematic review and meta‐analysis of observational data evaluating the prognosis associated with higher natriuretic peptides in patients with acute coronary syndrome55 showed that patients with higher natriuretic peptide concentrations (above the median) experienced higher mortality rates than those with lower concentrations. The odds ratio was of similar order of magnitude to that observed in the present review of restrictive mitral inflow. Both higher neurohormone concentrations and the presence of restrictive filling appear to be important cardiovascular markers that offer further prognostic information above simple clinical parameters, but their independence remains uncertain.
Most of the patients included in this analysis underwent echocardiography during their hospital admission. The timing of the echocardiography may be important. One recent study has shown that, although the filling pattern close to the ischaemic injury (1–2 days) predicts outcome, the persistence of a restrictive filling pattern (10–12 days later) is even more ominous.22 This poses a potential conflict, given that the optimal timing of diagnostic echocardiography may be earlier in the course of the patient's admission.
Many of the studies included in this review were performed and reported before the recent redefinition of myocardial infarction and as a result may not apply to all of the patients who would now have acute coronary syndrome diagnosed. Further evaluation of the prognostic role of echocardiographic, clinical and neurohormonal factors within this new diagnostic framework is required.
Limitations
The process of meta‐analysis contains many inherent biases. The first of these is publication bias. We may have omitted some unpublished data, which may or may not be in agreement with our results. Unpublished studies are often negative and their omission may lead to exaggeration of the difference between the two groups. We did contact all identified authors to request any unpublished data and did identify one further study in this way. To minimise this risk we identified all studies that included prognosis and echocardiography, but not necessarily restrictive filing pattern. These authors were contacted and asked whether their data could be broken down by restrictive versus non‐restrictive filling.
A further bias may be patient overlap between studies. Because of the nature of our search strategy outlined above, we did identify several publications that reported on the same patients but were using different echocardiographic variables. In consultation with all authors, we were able to identify several studies based on the same patients and thus minimise this effect. We believe this rigorous methodological approach has minimised these potential sources of bias and error.
Lastly, the criteria used by individual investigators for classification of restrictive filling varied slightly. In most cases, the investigators predetermined this to be the best cut off for detecting at‐risk patients. This may have influenced the results but we do not think this was the case for two reasons. Firstly, the deceleration time used varied little and, secondly, the analysis of odds ratio as a function of deceleration cut off showed no relationship and thus implies no bias.
The size of the risk estimates and confidence intervals around the risk estimates in the main results of this systematic review and meta‐analysis, in conjunction with the sample size, suggest that these potential sources of error, although possible, are likely to have minimal effect on the overall risk estimates.
Conclusion
The assessment of diastolic filling grade confers important prognostic information about patients after AMI. In this study, about 20% of patients who had an AMI displayed a restrictive filling pattern, which was associated with a four times higher mortality. This prognostically important finding is thus not a rare phenomenon in this patient group. The findings of this study support the comprehensive echocardiographic assessment of diastolic filling pattern in patients close to the time of their ischaemic event. This important prognostic information should be considered alongside other important echocardiographic and clinical findings when managing such patients.
ACKNOWLEDGEMENTS
GW was the recipient of a National Heart Foundation of New Zealand PhD Scholarship and is the National Heart Foundation of New Zealand Senior Fellow. The authors gratefully acknowledge the assistance of James Aoina, who helped with data collection, and the following authors, who confirmed their published data or provided additional data for use in this publication: Dr Malcolm Burgess, Brisbane, Australia; Dr Giampaolo Cerisano, Florence, Italy; Dr Haralambos Karvounis, Thessaloniki, Greece; Dr Elena Kinova, Sofia, Bulgaria; Dr Jacob Møller, Odense, Denmark; Dr Aleksandar Neskovic, Belgrade, Yugoslavia; Dr Ioannis Nouskas, Thessaloniki, Greece; Dr Patricia Pellikka, Rochester, USA; Dr Miguel Quintana, Stockholm, Sweden and Dr Pier Temporelli, Veruno, Italy.
Abbreviations
AMI - acute myocardial infarction
HF - heart failure
LV - left ventricular
Footnotes
Competing interests: None declared.
References
- 1.Whalley G A, Wasywich C A, Walsh H J.et al Role of echocardiography in the contemporary management of chronic heart failure. Expert Rev Cardiovasc Ther 2005351–70. [DOI] [PubMed] [Google Scholar]
- 2.Nijland F, Kamp O, Karreman A J.et al Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial Doppler echocardiographic study. J Am Coll Cardiol 1997301618–1624. [DOI] [PubMed] [Google Scholar]
- 3.Garcia‐Rubira J, Molano F, Espina A.et al Abnormal filling pattern of the left ventricle and outcome in acute myocardial infarction. Int J Cardiol 199761143–149. [DOI] [PubMed] [Google Scholar]
- 4.Sakata K, Kashiro S, Hirata S.et al Prognostic value of Doppler transmitral flow velocity patterns in acute myocardial infarction. Am J Cardiol 1997791165–1169. [DOI] [PubMed] [Google Scholar]
- 5.Tsai W C, Tsai L M, Teng J K.et al Prognostic value of Doppler‐derived mitral deceleration time in post infarction patients with left ventricular ejection fractions of 35% or more. J Formos Med Assoc 19999870–72. [PubMed] [Google Scholar]
- 6.Burgess M, Atkinson P, Ray S. Restrictive left ventricular filling pattern after myocardial infarction: significance of concomitant preserved systolic function. Echocardiography 200017659–664. [DOI] [PubMed] [Google Scholar]
- 7.Møller J E, Søndergaard E, Poulsen S H.et al Pseudonormal and restrictive filling patterns predict left ventricular dilatation and cardiac death after a first myocardial infarction: a serial color m‐mode Doppler echocardiographic study. J Am Coll Cardiol 2000361841–1846. [DOI] [PubMed] [Google Scholar]
- 8.Møller J E, Søndergaard E, Seward J B.et al Ratio of left ventricular peak E‐wave velocity to flow propagation velocity assessed by color m‐mode Doppler echocardiography in first myocardial infarction. J Am Coll Cardiol 200035363–370. [DOI] [PubMed] [Google Scholar]
- 9.Poulsen S H, Jensen S E, Nielsen J C.et al Serial changes and prognostic implications of a Doppler‐derived index of combined left ventricular systolic and diastolic myocardial performance in acute myocardial infarction. Am J Cardiol 20008519–25. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen S H, Møller J E, Norager B.et al Prognostic implications of left ventricular diastolic dysfunction with preserved systolic function following acute myocardial infarction. Cardiology 200195190–197. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen S H, Jensen S E, Møller J E.et al Prognostic value of left ventricular diastolic function and association with heart rate variability after the first acute myocardial infarction. Heart 200186376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otasevic P, Neskovic A N, Popovic Z.et al Short early filling deceleration time on day 1 after acute myocardial infarction is associated with short and long‐term left ventricular remodelling. Heart 200185527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerisano G, Bolognese L, Buonamici P.et al Prognostic implications of restrictive left ventricular filling in reperfused anterior acute myocardial infarction. J Am Coll Cardiol 200137793–799. [DOI] [PubMed] [Google Scholar]
- 14.Szymanski P, Rezler J, Stec S.et al Long‐term prognostic value of an index of myocardial performance in patients with myocardial infarction. Clin Cardiol 200225378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hole T, Vegsundvag J A, Morstol T H.et al Early changes in left ventricular volume and function are predictors for long‐term remodelling in patients with acute transmural myocardial infarction and preserved systolic function. J Am Soc Echocardiogr 200316630–637. [DOI] [PubMed] [Google Scholar]
- 16.Garcia‐Rubira J, Garcia‐Martinez J T, Hidalgo R.et al Doppler transmitral flow pattern is an independent prognostic factor in acute myocardial infarction. Cardiology 199788203–206. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen S H, Jensen S E, Egstrup K. Longitudinal changes and prognostic implications of left ventricular diastolic function in first acute myocardial infarction. Am Heart J 1999137910–918. [DOI] [PubMed] [Google Scholar]
- 18.Møller J E, Egstrup K, Kober L.et al Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J 2003145147–153. [DOI] [PubMed] [Google Scholar]
- 19.Møller J E, Hillis G S, Oh J K.et al Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 20031072207–2212. [DOI] [PubMed] [Google Scholar]
- 20.Schwammenthal E, Adler Y, Amichai K.et al Prognostic value of global myocardial performance indices in acute myocardial infarction. Chest 20031241645–1651. [DOI] [PubMed] [Google Scholar]
- 21.Møller J E, Poulsen S H, Sondergaard E.et al Impact of early changes in left ventricular filling pattern on long‐term outcome after acute myocardial infarction. Int J Cardiol 200389207–215. [DOI] [PubMed] [Google Scholar]
- 22.Temporelli P L, Giannuzzi P, Nicolosi G L.et al Doppler‐derived mitral deceleration time as a strong prognostic marker of left ventricular remodeling and survival after acute myocardial infarction: Results of the GISSI‐3 Echo substudy. J Am Coll Cardiol 2004431646–1653. [DOI] [PubMed] [Google Scholar]
- 23.Møller J E, Dahlstrom U, Gotzsche O.et al Effects of losartan and captopril on left ventricular systolic and diastolic function after acute myocardial infarction: results of the Optimal Trial in Myocardial Infarction (OPTIMAAL) echocardiographic substudy. Am Heart J 2004147494–501. [DOI] [PubMed] [Google Scholar]
- 24.Karvounis H I, Nouskaaaas I G, Farmakis T M.et al Evaluation of a Doppler‐derived index combining systolic and diastolic left ventricular function in acute myocardial infarction. Angiology 20045521–28. [DOI] [PubMed] [Google Scholar]
- 25.Beinart R, Boyko V, Schwammenthal E.et al Long‐term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol 200444327–334. [DOI] [PubMed] [Google Scholar]
- 26.Quintana M, Edner M, Kahan T.et al Is left ventricular diastolic function an independent marker of prognosis after acute myocardial infarction? Int J Cardiol 200496183–189. [DOI] [PubMed] [Google Scholar]
- 27.Kinova E, Kozhuharov H. Left ventricular diastolic filling patterns as predictors of heart failure after myocardial infarction: a colour M‐mode Doppler study. Hellenic J Cardiol 20044523–31. [Google Scholar]
- 28.Hillis G, Møller J E, Pellikka P A.et al Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 200443360–367. [DOI] [PubMed] [Google Scholar]
- 29.Kinova E. “Left ventricular function in acute coronary syndromes: clinical and echocardiographic assessment. ” PhD dissertation, Sophia University 2003
- 30.Poulsen S H, Jensen S E, Gøtzsche O.et al Evaluation and prognostic significance of left ventricular diastolic function assessed by Doppler echocardiography in the early phase of a first acute myocardial infarction. Eur Heart J 1997181882–1889. [DOI] [PubMed] [Google Scholar]
- 31.Møller J E, Søndergaard E, Poulsen S H.et al The Doppler echocardiographic myocardial performance index predicts left ventricular dilatation and cardiac death after myocardial infarction. Cardiology 200195105–111. [DOI] [PubMed] [Google Scholar]
- 32.Boccalandro F, Loghin C, Darwood S.et al Restrictive left ventricular filling pattern with preserved systolic function assessed by Doppler echocardiography: clinical, echocardiographic and prognostic implications. Cardiology 200298148–153. [DOI] [PubMed] [Google Scholar]
- 33.Pozzoli M, Capomolla S, Pinna G.et al Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure with and without mitral regurgitation. J Am Coll Cardiol 199627883–893. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura R A, Appleton C P, Redfield M M.et al Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol 1996281226–1233. [DOI] [PubMed] [Google Scholar]
- 35.Nagueh S F, Kopelen H, Quinones M. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation 1996942138–2145. [DOI] [PubMed] [Google Scholar]
- 36.Temporelli P, Scapellato F, Corrà U.et al Estimation of pulmonary wedge pressure by transmitral Doppler in patients with chronic heart failure and atrial fibrillation. Am J Cardiol 199983724–727. [DOI] [PubMed] [Google Scholar]
- 37.Chirrillo F, Brunazzi M, Barbiero M.et al Estimating mean pulmonary wedge pressure in patients with chronic atrial fibrillation from transthoracic Doppler indices of mitral and pulmonary venous flow velocity. J Am Coll Cardiol 19973019–26. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Oki T, Yamada H.et al Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J Am Soc Echocardiogr 200316333–339. [DOI] [PubMed] [Google Scholar]
- 39.Traversi E, Cobelli F, Pozzoli M. Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure even when atrial fibrillation is present. Eur J Heart Failure 20013173–181. [DOI] [PubMed] [Google Scholar]
- 40.Margulies K B, Jaffer S, Pollack P S.et al Physiological significance of early deceleration time prolongation in asymptomatic elderly subjects. J Cardiac Fail 1999592–99. [DOI] [PubMed] [Google Scholar]
- 41.Akioka K, Takeuchi K, Yanagi S.et al Prognostic value of Doppler transmittal flow patterns and cardiac natriuretic peptides in patients with chronic congestive heart failure admitted for episodes of acute decompensation. Heart Vessels 20001553–60. [DOI] [PubMed] [Google Scholar]
- 42.Xie G Y, Berk M R, Smith M D.et al Relation of Doppler transmitral flow patterns to functional status in congestive heart failure. Am Heart J 1996131766–771. [DOI] [PubMed] [Google Scholar]
- 43.De Lemos J A, Morrow D A, Bentley J H.et al The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 20013451014–1021. [DOI] [PubMed] [Google Scholar]
- 44.Sabatine M S, Morrow D A, de Lemos J A.et al Multimarker approach to risk stratification in non‐ST elevation acute coronary syndromes. Circulation 20021051760–1763. [DOI] [PubMed] [Google Scholar]
- 45.Hellerman J P, Jacobsen S J, Gersch B J.et al Heart failure after myocardial infarction: a review. Am J Med 2002113324–330. [DOI] [PubMed] [Google Scholar]
- 46.Spencer F A, Meyer T E, Gore J M.et al Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure. The National Registry of Myocardial Infarction. Circulation 20021052605–2610. [DOI] [PubMed] [Google Scholar]
- 47.Hasdai D, Topol E J, Kilaru R.et al Frequency, patient characteristics, and outcomes of mild‐to‐moderate heart failure complicating ST‐segment elevation acute myocardial infarction: lessons from 4 international fibrinolytic therapy trials. Am Heart J 200314573–79. [DOI] [PubMed] [Google Scholar]
- 48.Steg P G, Dabbous O, Feldman L J.et al Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004109494–499. [DOI] [PubMed] [Google Scholar]
- 49.Hellerman J P, Jacobsen S B, Redfield M M.et al Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Failure 20057119–125. [DOI] [PubMed] [Google Scholar]
- 50.Rott D, Behar S, Hod H.et al Improved survival of patients with acute myocardial infarction with significant left ventricular dysfunction undergoing invasive coronary procedures. Am Heart J 2001141267–276. [DOI] [PubMed] [Google Scholar]
- 51.Bonow R O, Bacharach S L, Green K M.et al Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation 198164315–323. [DOI] [PubMed] [Google Scholar]
- 52.Bettencourt P, Ferreira A, Dias P.et al Predictors of prognosis in patients with stable mild to moderate heart failure. J Cardiac Fail 20006306–313. [DOI] [PubMed] [Google Scholar]
- 53.Dini F L, Michelassi C, Micheli G.et al Prognostic value of pulmonary venous flow Doppler signal in left ventricular dysfunction. J Am Coll Cardiol 2000361295–1302. [DOI] [PubMed] [Google Scholar]
- 54.Hansen A, Haass M, Zugck C.et al Prognostic value of Doppler echocardiographic mitral inflow patterns: implications for risk stratification in patients with congestive heart failure. J Am Coll Cardiol 2001371049–1055. [DOI] [PubMed] [Google Scholar]
- 55.Galvani M, Ferrini D, Ottani F. Natriuretic peptides for risk stratification o of patients with acute coronary syndromes Eur J Heart Failure 20046327–333. [DOI] [PubMed] [Google Scholar]