Abstract
Objectives
To evaluate the clinical utility of near‐infrared spectroscopic (NIRS) monitoring of cerebral (Sco2) and splanchnic (Sso2) oxygen saturations for estimation of systemic oxygen transport after the Norwood procedure.
Methods
Sco2 and Sso2 were measured with NIRS cerebral and thoracolumbar probes (in humans). Respiratory mass spectrometry was used to measure systemic oxygen consumption (V̇o2). Arterial (Sao2), superior vena caval (Svo2) and pulmonary venous oxygen saturations were measured at 2 to 4 h intervals to derive pulmonary (Qp) and systemic blood flow (Qs), systemic oxygen delivery (Do2) and oxygen extraction ratio (ERo2). Mixed linear regression was used to test correlations. A study of 7 pigs after cardiopulmonary bypass (study 1) was followed by a study of 11 children after the Norwood procedure (study 2).
Results
Study 1. Sco2 moderately correlated with Svo2, mean arterial pressure, Qs, Do2 and ERo2 (slope 0.30, 0.64. 2.30, 0.017 and −32.5, p < 0.0001) but not with Sao2, arterial oxygen pressure (Pao2), haemoglobin and V̇o2. Study 2. Sco2 correlated well with Svo2, Sao2, Pao2 and mean arterial pressure (slope 0.43, 0.61, 0.99 and 0.52, p < 0.0001) but not with haemoglobin (slope 0.24, p > 0.05). Sco2 correlated weakly with V̇o2 (slope −0.07, p = 0.05) and moderately with Qs, Do2 and ERo2 (slope 3.2, 0.03, −33.2, p < 0.0001). Sso2 showed similar but weaker correlations.
Conclusions
Sco2 and Sso2 may reflect the influence of haemodynamic variables and oxygen transport after the Norwood procedure. However, the interpretation of NIRS data, in terms of both absolute values and trends, is difficult to rely on clinically.
Adequate systemic oxygen delivery (Do2) balanced with systemic oxygen consumption (V̇o2) is crucial to the care of any child requiring intensive care but is particularly difficult to assess after the Norwood procedure.1 Direct measurement of Do2 and V̇o2 is most desirable but is rarely performed outside of investigational protocols, and methods often use inappropriate surrogates. Consequently, indirect markers of oxygen balance, such as superior vena caval oxygen saturation (Svo2), arterial (Sao2) and venous oxygen saturation difference and blood lactate, are commonly used to estimate the adequacy of Do2 in these patients.2,3,4 The disadvantages of these techniques include the need for repeated blood sampling and the necessarily intermittent nature of the data accrual.
Near‐infrared spectroscopy (NIRS) provides a non‐invasive, continuous method to monitor regional tissue oxygenation.5,6 This technique depends on the transparency of biological tissue to light in the infrared region of the spectrum of tissue chromophores, such as haemoglobin and cytochrome aa3. Changes in absorption at several wavelengths can be converted into signals of oxyhaemoglobin, deoxyhaemoglobin and oxidised cytochrome aa3. Furthermore, newer generations of NIRS devices permit quantitative measurement of the ratio of oxyhaemoglobin to total haemoglobin, representing the tissue oxygenation index as an absolute term, independent of a tissue path length factor.7,8
NIRS has been extensively evaluated in the cerebral6,9,10,11,12,13 and splanchnic circulations of newborn infants.14,15,16 Because of its relative ease of use, it is also increasingly used in intensive care units as surrogates of Svo2 and systemic oxygenation.17,18,19 Good correlations have been generally reported between splanchnic (Sso2) or cerebral oxygen saturation (Sco2) and Svo2 in various patient groups.17,18,19 But there are few data in children with congenital heart disease, either preoperatively or postoperatively. Importantly, all the previous studies have used interindividual single‐point assessments in a relatively large population, and NIRS has not been validated against directly measured systemic haemodynamic variables and oxygen transport. Thus, we performed two studies. Study 1 examined pigs after cardiopulmonary bypass (CPB) with normal circulation. Study 2 was a clinical study of neonates during the 72 h after the Norwood procedure. The Norwood group was chosen because it is a particularly challenging subset of patients in which adequate Do2 is difficult to assess and would be highly advantageous to assess non‐invasively. Our original hypothesis was that NIRS would accurately reflect systemic oxygen transport when compared with direct measurements. We therefore obtained continuous NIRS measurements of Sco2 and Sso2, and continuous measurement of V̇o2 and, in combination with blood gases, derived repeated and quantitative measurements of pulmonary (Qp) and systemic blood flows (Qs), Do2 and oxygen extraction ratio (ERo2). We examined the correlation of Sco2 and Sso2 with each of the elements, as well as interindividual and intraindividual variability, to determine the clinical usefulness of NIRS for monitoring systemic haemodynamic function and oxygen transport in patients after the Norwood procedure.
MATERIALS AND METHODS
Study 1
After review and approval by the Institutional Animal Care and Use Committee of the Research Institute in The Hospital for Sick Children, Toronto, Canada, seven Yorkshire pigs weighing 18.5 (1.6) kg were studied. Techniques for anaesthesia, CPB and postoperative management were as described elsewhere.20 Briefly, the animals were studied during general anaesthesia, with inhaled isoflurene (2%) and intravenous infusion of pancuronium (0.8 μg/kg/min). After median sternotomy the pigs underwent a total of 3 h of CPB at 32°C. After rewarming the animal were weaned from CPB, with continuous infusion of dopamine 5–10 μg/kg/min when necessary.
Study 2
Patients
This study was approved by the institutional Research Ethics Board. Written informed consent was obtained from the parents of 11 children (10 boys, aged from 4 to 92 days, median 7 days) undergoing the Norwood procedure between April and October 2004. Table 1 shows the patients' demographics.
Table 1 Clinical data for the 11 patients.
| Patient | Age (days) | Weight (kg) | BSA (m2) | CPB (min) | ACC (min) | Circulatory arrest (min) | Cerebral perfusion (min) | Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 3.5 | 0.23 | 108 | 47 | 12 | 35 | HLHS, AS, MS |
| 2 | 4 | 3.7 | 0.25 | 151 | 100 | 35 | 53 | HLHS, AS, MS |
| 3 | 7 | 4 | 0.26 | 105 | 47 | 3 | 44 | HLHS, AS, MS, |
| 4 | 16 | 3.5 | 0.24 | 133 | 39 | 34 | 0 | HLHS, endocardial fibroelastosis of LV, AS, MS |
| 5 | 7 | 4.2 | 0.27 | 122 | 62 | 3 | 60 | HLHS, AS, MS |
| 6 | 12 | 3.5 | 0.23 | 165 | 75 | 13 | 59 | DILV, TGA |
| 7 | 6 | 3.5 | 0.23 | 172 | 82 | 9 | 70 | HLHS, AA, MA |
| 8 | 92 | 4.1 | 0.26 | 105 | 58 | 30 | 17 | DILV, TGA, |
| 9 | 7 | 4 | 0.25 | 167 | 64 | 17 | 44 | HLHS, AS, MS |
| 10 | 6 | 2.9 | 0.2 | 142 | 62 | 1 | 62 | HLHS, AS, MS |
| 11 | 9 | 3.6 | 0.24 | 109 | 50 | 4 | 44 | HLHS, AS, MS |
AA, aortic atresia; AS, aortic stenosis; ACC, aortic cross clamp time; BSA, body surface area; CPB, cardiopulmonary bypass; DILV, double inlet left ventricle; HLHS, hypoplastic left heart syndrome; LV, left ventricle; MA, mitral atresia; MS, mitral stenosis; TGA, transposition of great arteries.
Intraoperative procedures
All patients were intubated with cuffed endotracheal tubes (microcuff Heidelberg paediatric; Microcuff GmbH, Weinheim, Germany). General anaesthesia was maintained with inhaled isoflurane, intravenous fentanyl and pancuronium bromide. Low‐flow CPB and selective cerebral perfusion was used in 10 of 11 patients. A standard Norwood procedure with 3.5 mm right modified Blalock–Taussig shunt was used.21 Phenoxybenzamine 0.25 mg/kg was given at initiation of CPB. Milrinone (100 μg/kg) was given before termination of CPB. Dopamine (5 μg/kg/min) was initiated for the immediate time around cessation of CPB and was subsequently discontinued if the haemodynamic and ventricular functions were good. A pulmonary venous line was inserted into the orifice of the right upper pulmonary vein.
Postoperative management
The central temperature (oesophageal) was maintained at 36–37°C. Postoperative monitoring included arterial, superior vena caval and pulmonary venous pressures and heart rate. Sedation was maintained by a continuous intravenous infusion of morphine and intermittent injections of a muscle relaxant (pancuronium) and lorazepam. Infants were ventilated with volume control and pressure support. Ventilation volume and rate were adjusted to maintain Paco2 between 40–50 mm Hg. Inotropic agents, vasoactive drugs (milrinone, dopamine, phenoxybenzamine and vasopressin) and volume infusions (5% albumin or blood) were given according to our standard protocol.22
Methods of measurement
Sco2 and Sso2
NIRS probes consist of a near‐infrared light emitter optode and a receiver optode with a distance of 5 cm. In pigs, they were placed on the right and left sides of the forehead. In patients, we chose to replicate previously published techniques of probe placement in this group of patients.13 Briefly, the probes were placed on the patient's forehead in the midline (Sco2) and slightly to the right of the midline on the thoracic–lumbar flank (Sso2). The probes were monitored by a dual‐detector device (INVOS 5100A; Somanetics, Troy, Michigan, USA) and recordings were made at 1 min intervals.
V̇o2
V̇o2 was measured continuously with an AMIS2000 mass spectrometer (Innovision A/S, Odense, Denmark). This is a sensitive and accurate method that permits simultaneous measurements of multiple gas fractions. We have described the details elsewhere.23
Calculations of systemic haemodynamic variables and oxygen transport
Blood samples were taken from the arterial, superior vena cava and pulmonary vein lines for the measurements of blood gases. Qp and Qs were then calculated by the direct Fick method: Qp = V̇o2/(Cpvo2 − Cao2) and Qs = V̇o2/(Cao2 −Cvo2), where Cao2, Cpvo2 and Cvo2 indicate systemic arterial (equal to pulmonary arterial), pulmonary venous and superior vena caval oxygen contents, respectively. Do2 and ERo2 were calculated by standard equations: Do2 = Qs × Cao2 and ERo2 = V̇o2/Do2. All values in patients were indexed to body surface area and in pigs, to body weight.
Study protocols
The animal study (study 1) was performed during the first 6 h after CPB. Six sets of measurements were obtained at 1, 3 and 6 h, with a 10 min interval between each set of measurements. The clinical study (study 2) was performed during the first 72 h after the patient's arrival in the cardiac intensive care unit. Values of haemodynamic function, oxygen transport and central body temperature were collected at 2 h intervals during the first 24 h and at 4 h intervals during hours 25 through 72. Sampling was avoided if sedation, paralysis and ventilatory or haemodynamic treatment were changed within the prior 15 min.
Data analysis
Data are expressed as mean (SD). Interrelationships between the measures were sought by using mixed linear regression analysis for repeated measures without regard to time. When a significant correlation was found (p < 0.05), interindividual differences were further analysed. The extent of the correlation was indicated by the intercept and slope values. All data were analysed with SAS statistical software V.8 (SAS Institute, Inc, Cary, North Carolina, USA).
RESULTS
Pigs
Two pigs died before the end of the 6 h study period. As the NIRS measures of Sco2 on the two sides were similar, the mean values were used for analysis.
Patients
Sco2 was obtained in all the patients and Sso2 in five patients, in three of whom measurements were stopped at 28 and 48 h, respectively, due to technical issues in two patients (patients 1 and 3) and extubation in the other (patient 11). There was no incidence of circulatory collapse or death during the study period. All patients survived to hospital discharge. Extubation was done between 2–16 days (median seven days) after the procedure except in one child who had vocal cord complications. Extubation for that infant was done at 90 days, after a bidirectional cavopulmonary anastomosis (table 1).
Correlations of Sco2 and Sso2 with systemic haemodynamic variables and oxygen transport
Table 2 and figs 1–3 detail the results of the correlations of Sco2 and Sso2 with haemodynamic variables and oxygen transport in the animal and clinical studies.
Table 2 Correlations (mixed linear regression) of Sco2, Sso2 and Svo2 with haemodynamic and oxygen transport variables for the entire group and individually in 7 pigs and 11 patients.
| Dependent variable | Independent variable | Intercept | Group slope | p Value | Range of individual slopes | p Value for interindividual slope difference |
|---|---|---|---|---|---|---|
| Pig data | ||||||
| Sco2 | Svo2 | 28.1 | 0.30 | <0.0001 | 0.14–0.67 | 0.0005 |
| Sao2 | 57.6 | −0.11 | 0.82 | |||
| Pao2 | 48.0 | −0.01 | 0.60 | |||
| Haemoglobin | 52.4 | −0.36 | 0.28 | |||
| MAP | 18.6 | 0.64 | <0.0001 | −0.71–1.16 | <0.0001 | |
| CO | 39.7 | 2.30 | <0.0001 | 1.56–6.42 | 0.0006 | |
| Do2 | 39.0 | 0.017 | <0.0001 | 0.02–0.07 | 0.006 | |
| V̇o2 | 39.9 | 0.01 | 0.48 | |||
| ERo2 | 59.7 | −32.5 | <0.0001 | −65.4–16.4 | 0.002 | |
| Patient data | ||||||
| Sco2 | Svo2 | 28.5 | 0.43 | <0.0001 | 0.17–0.97 | <0.0001 |
| Sao2 | 4.6 | 0.61 | <0.0001 | 0.14–2.13 | 0.0001 | |
| Pao2 | 12.1 | 0.99 | <0.0001 | 0.36–1.93 | <0.0001 | |
| Haemoglobin | 47.5 | 0.24 | 0.54 | |||
| MAP | 26.0 | 0.52 | <0.0001 | 0.14–1.11 | 0.21 | |
| Qp | 43.6 | 3.72 | 0.001 | 0.86–6.72 | 0.03 | |
| Qs | 42.1 | 3.22 | <0.0001 | 0.27–13.64 | 0.002 | |
| Do2 | 42.4 | 0.03 | <0.0001 | 0.01–0.10 | 0.001 | |
| V̇o2 | 56.8 | −0.07 | 0.046 | −0.33–0.39 | <0.0001 | |
| ERo2 | 61.1 | −33.2 | <0.0001 | −91.7–−5.3 | <0.0001 | |
| Sso2 | Svo2 | 49.2 | 0.26 | <0.0001 | 0.11–0.54 | 0.32 |
| Sao2 | 33.9 | 0.39 | 0.0001 | Infinite likelihood | ||
| Pao2 | 46.9 | 0.41 | 0.002 | 0.19–0.83 | 0.22 | |
| Haemoglobin | 58.2 | 0.30 | 0.48 | |||
| MAP | 51.7 | 0.22 | 0.03 | Infinite likelihood | ||
| Qp | 60.0 | 1.48 | 0.23 | |||
| Qs | 59.3 | 1.73 | 0.03 | 0.46–10.3 | 0.28 | |
| Do2 | 58.2 | 0.015 | 0.004 | 0.006–0.066 | 0.11 | |
| V̇o2 | 63.3 | −0.007 | 0.84 | |||
| ERo2 | 68.9 | −20.3 | 0.0002 | Infinite likelihood | ||
| Svo2 | Qp | 47.1 | 2.49 | 0.02 | −9.3–10.8 | 0.04 |
| Qs | 40.5 | 5.82 | <0.0001 | 3.1–11.3 | 0.02 | |
| Do2 | 36.4 | 0.05 | <0.0001 | 0.032–0.097 | 0.001 | |
| V̇o2 | 69.7 | −0.20 | <0.0001 | −0.45–0.028 | 0.008 | |
| ERo2 | 77.9 | −83.2 | <0.0001 | −91.9–−69.4 | 0.18 | |
Do2, systemic oxygen delivery; ERo2, oxygen extraction ratio; MAP, mean arterial pressure; Pao2, arterial oxygen pressure; Qp, pulmonary blood flow; Qs, systemic blood flow; Sao2, arterial oxygen saturation; Sco2, cerebral oxygen saturation; Sso2, splanchnic oxygen saturation; Svo2, superior vena caval oxygen saturation; V̇o2, systemic oxygen consumption.
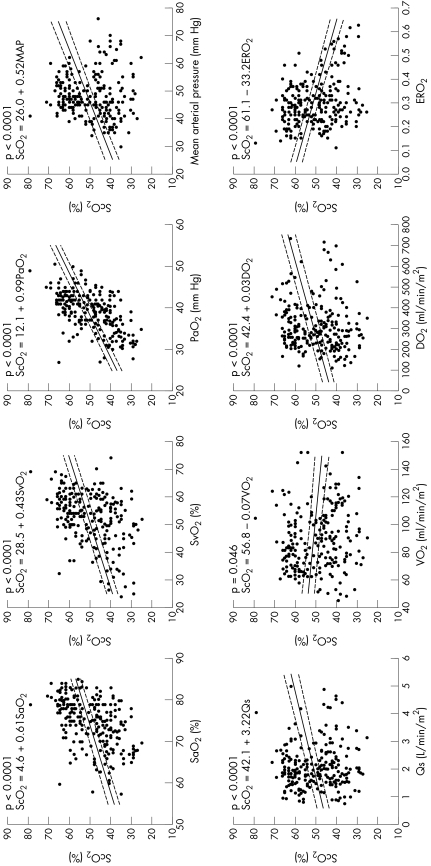
Figure 1 Correlations between cerebral oxygen saturation (Sco2) and systemic haemodynamic and oxygen transport variables of arterial oxygen saturation (Sao2), superior vena caval oxygen saturation (Svo2), arterial partial oxygen pressure (Pao2), mean arterial pressure (MAP), pulmonary (Qp) and systemic blood flows (Qs), systemic oxygen consumption (V̇o2), oxygen delivery (Do2) and oxygen extraction ratio (ERo2) in patients during the first 72 h after arrival in the cardiac intensive care unit.
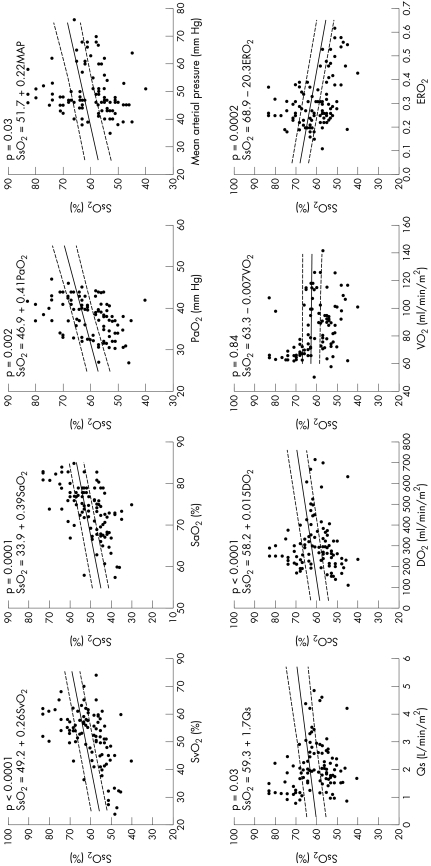
Figure 2 Correlations between splanchnic oxygen saturation (Sso2) and systemic haemodynamic and oxygen transport variables of superior vena caval oxygen saturation (Svo2), arterial oxygen saturation (Sao2), arterial partial oxygen pressure (Pao2), mean arterial pressure (MAP), pulmonary (Qp) and systemic blood flows (Qs), oxygen delivery (Do2), systemic oxygen consumption (V̇o2) and oxygen extraction ratio (ERo2) in patients during the first 72 h after arrival in the cardiac intensive care unit.
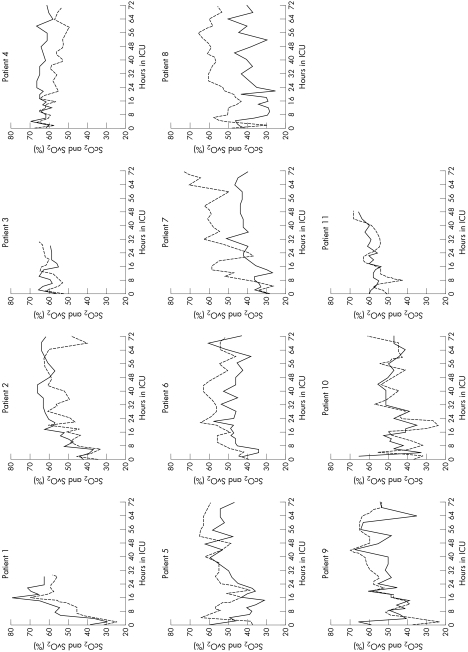
Figure 3 Trends for cerebral oxygen saturation (Sco2, solid line) and superior vena caval oxygen saturation (Svo2, dotted line) for each of the 11 patients during the first 72 h after arrival in the cardiac intensive care unit (ICU).
Study 1
We obtained 106 sets of measurements in the seven pigs. Sco2 ranged from 30–59%, Svo2 from 46.6–86.1%, Sao2 from 91.1–100% and arterial oxygen pressure (Pao2) from 60–237 mm Hg.
Sco2 was not correlated with Sao2 (slope −0.11, p = 0.82), Pao2 (slope −0.01, p = 0.60) or haemoglobin (slope −0.36, p = 0.28). Sco2 was correlated with Svo2 (slope 0.30, p < 0.0001) but with large interindividual variations (slope 0.14 to 0.67, p = 0.0005). Sco2 was also correlated with mean arterial pressure (slope 0.64, p < 0.0001) with large interindividual variations (slope −0.71 to 1.16, p < 0.0001) and moderately correlated with Qs, Do2 and ERo2 (slope 2.3, 0.017, −32.5, respectively, p < 0.0001 for all) with significant interindividual variations (table 2). Sco2 was not correlated with V̇o2 (slope 0.01, p = 0.48) (table 2).
Study 2
We obtained 247 sets of measurements for Sco2 and 103 sets for Sso2. For the entire group, Sco2 ranged from 25–79% and Sso2 from 40–83%; Svo2 ranged from 24–74%, Sao2 from 55–86% and Pao2 from 25.0–50.6 mm Hg.
Unlike in pigs, in patients Sco2 was correlated with both Sao2 and Pao2 (slope 0.61 and 0.99, respectively, p < 0.0001 for both) but with large interindividual variations (slope 0.17–0.97 for Sao2; 0.36–1.93 for Pao2; p < 0.0001 for both). Sco2 was not correlated with haemoglobin (slope 0.24, p = 0.54). Similarly to the data in pigs, Sco2 was correlated with Svo2 (slope 0.43, p < 0.0001) but with large interindividual variations (slope 0.17–0.97 for Svo2; 0.14–2.13 for Sao2; p < 0.0001 for both). It was also correlated with mean arterial pressure (slope 0.52, p < 0.0001) but, interestingly, interindividual variations were insignificant (slope 0.14–1.11, p = 0.21). Sco2 was weakly correlated with V̇o2 (slope −0.07, p = 0.05) and moderately correlated with Qp, Qs, Do2 and ERo2 (slope 3.1, 3.2, 0.03, −33.2, respectively, p < 0.0001 for all except for Qp, p = 0.001) with significant interindividual variations (table 2, fig 1).
Sso2 was similarly, although more weakly, correlated with haemodynamic and oxygen transport variables, with the coefficients being about half those with Sco2. Interindividual variations were also large, although they did not achieve significance, probably due to the smaller sample size. Sso2 was not correlated with V̇o2 (slope −0.007, p = 0.84) (table 2, fig 2).
The correlations of Sco2 and Sso2 with Qs, Do2, V̇o2 and ERo2 were much weaker than those of Svo2 with these variables (table 2).
Lastly, examples of individual patient plots (fig 3) of the NIRS and the directly derived data show notably variable intraindividual relationships that changed with time and at individual time points.
DISCUSSION
This is the first comprehensive investigation of the relationship between NIRS measurements of Sco2 and Sso2, as a non‐invasive clinical haemodynamic monitor of systemic oxygenation, and the directly measured systemic haemodynamic and oxygen transport variables. We assessed the utility of NIRS after CPB in two different circulations—namely, the normal biventricular circulation in an animal model and the more complex parallel circulation, with residual arterial desaturation, in children after the Norwood procedure. Our data showed that, in patients with varied and relatively low values of Pao2 and Sao2, Sco2 was closely correlated with both of these values, but not in pigs with fully saturated arterial oxygenation. Most important, Sco2 was, similarly in both groups, closely correlated with mean arterial pressure, loosely correlated with Svo2, Sao2, Qp, Qs, Do2 and ERo2, poorly correlated with V̇o2 and not significantly correlated with either haemoglobin or Qp. Whereas the correlation between NIRS‐derived oxygen saturations and many of the directly measured indices were highly significant, the relatively loose correlations at an absolute level, combined with wide interindividual variability, cast doubt on the potential clinical utility of such measurements.
Previous studies have evaluated single‐point comparisons between Sco2 or Sso2 and Svo2 in individual children in a larger population.17,18,19 Good correlations have generally been reported, but large interindividual differences are apparent, even in these studies. Our study was the first to evaluate NIRS in the setting of postoperative complex congenital heart disease, comparing the non‐invasive data with directly measured indices of oxygen transport during the first three days after the Norwood procedure, and confirmed the previous findings. Our data showed that an increase in Sco2 or Sso2 of 1% explained 0.43% or 0.26%, respectively, of the increase in Svo2. However, interindividual variations were large, ranging from 0.17–0.97% for Sco2 and from 0.11–0.54% for Sso2. Furthermore, the individual trends shown in fig 3 show an inconsistent relationship between Sco2 and Svo2 throughout the measurement period. Although differences in absolute values and even wide interindividual variability may be tolerated if NIRS is used for trend analysis, the second issue of an unreliable intraindividual relationship between NIRS and Svo2, for example, casts doubt on its potential utility as a precise tool for monitoring haemodynamic trends.
This is, however, not surprising. NIRS measures the equilibrium of oxyhaemoglobin and deoxyhaemoglobin in a mixture of veins, arteries and capillaries in the underlying tissue and reflects a regional state of oxygenation. Although NIRS has been extensively used to monitor cerebral and splanchnic oxygenation in various clinical situations including during CPB, deep hypothermic circulatory arrest13,24 and in other high risk newborns,6,15,19,25 and has been found to be helpful in predicting cerebrovascular dysfunction24,25 and splanchnic ischaemia,15 it has rarely been rigorously examined for its validity, particularly in the setting of complex parallel circulations such as those that exist after the Norwood procedure. However, whereas the venous portion predominantly determines NIRS measurement of the underlying tissue oxygenation, direct measurements, such as jugular bulb oxygen saturation and hepatic venous oxygen saturation, are well known to correlate variably with NIRS measurement of that organ.11,14 This may be a particular problem in children with complex congenital heart disease, where the contribution of the venous portion has been shown to range from 60–100% of Sco2 with varied systemic oxygen saturation as seen in our patients after the Norwood procedure.12 It must also be remembered that NIRS measures oxygenation in a small part of the target organ, and the regional venous oxygen saturation reflects the balance of oxygen delivery and consumption of the whole organ. Svo2, conversely, reflects the balance of systemic oxygen transport, presumably explaining the discrepancy that Yeh T Jr et al26 observed between jugular bulb oxygen saturation and Svo2 in children undergoing CPB. Extracerebral tissue factors, such as ischaemia,27 oedema and the location of the probe,28 may also affect the robustness of NIRS signals.
Limitations
The superior vena cava was used to measure systemic venous saturation for the calculations of Qs and Do2. This measure does not account for potential differences in inferior vena cava saturation29,30 and may at least partly account for the poorer correlations with Sso2. Conversely, it could be argued that, by sampling upper body venous saturation, we have a more representative measurement, incorporating cerebral blood flow. Nonetheless, jugular bulb saturation would have been better in this regard but was not appropriate in this clinical protocol.31
Furthermore, we chose the usual position (posterior flank) to assess Sso2 by NIRS. Recently, Fortune et al15 used the site of just below the umbilicus to monitor splanchnic oxygenation and found a sensitivity of 90% to detect splanchnic ischaemia in neonates during apnoeic episodes. Sso2 measured in such a way may reflect more Do2 and V̇o2, and thus warrants further investigation in children with heart disease.
Conclusions
NIRS measurement of Sco2 and Sso2 reflects the changes in haemodynamic variables and oxygen transport during the early postoperative period after the Norwood procedure. However, large interindividual differences and intraindividual temporal variability make interpretation difficult and may limit the utility of NIRS as a continuous monitor of systemic haemodynamic function and oxygen transport in critically ill patients.
Abbreviations
Cao2 - systemic arterial oxygen contents
CPB - cardiopulmonary bypass
Cpvo2 - systemic pulmonary venous oxygen contents, Cvo2, systemic superior vena caval oxygen contents
Do2 - systemic oxygen delivery
ERo2 - oxygen extraction ratio
NIRS - near‐infrared spectroscopy
Pao2 - arterial oxygen pressure
Qp - pulmonary blood flow
Qs - systemic blood flow
Sao2 - arterial oxygen saturation
Sco2 - cerebral oxygen saturation
Sso2 - splanchnic oxygen saturation
Svo2 - superior vena caval oxygen saturation
V̇o2 - systemic oxygen consumption
Footnotes
This work was supported by the Heart and Stroke Foundation of Canada (JL and ANR), and the Canadian Institute of Health Research (JL, ANR, CC and GSV).
References
- 1.Li J, Zhang G, Holtby H.et al Inclusion of oxygen consumption improves the accuracy of arterial and venous oxygen saturation interpretation after the Norwood procedure. J Thorac Cardiovasc Surg 20061311099–1107. [DOI] [PubMed] [Google Scholar]
- 2.Bradley S M, Atz A M, Simsic J M. Redefining the impact of oxygen and hyperventilation after the Norwood procedure. J Thorac Cardiovasc Surg 2004127473–480. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman G M, Ghanayem N S, Kampine J M.et al Venous saturation and the anaerobic threshold in neonates after the Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg 2000701515–1520. [DOI] [PubMed] [Google Scholar]
- 4.Tweddell J S, Hoffman G M, Fedderly R T.et al Patients at risk for low systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg 2000691893–1899. [DOI] [PubMed] [Google Scholar]
- 5.Jobsis F F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 19771981264–1267. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt J S, Cope M, Delpy D T.et al Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 1986ii1063–1066. [DOI] [PubMed]
- 7.Matcher S J, Kirkpatrick P, Nahid K.et al Absolute quantification methods in tissue near infrared spectroscopy. Proc SPIE 19952389486–495. [Google Scholar]
- 8.Suzuki S, Takasaki S, Ozaki T.et al A tissue oxygenation monitor using NIR spatially resolved spectroscopy. Proc SPIE 19993579144–146. [Google Scholar]
- 9.Hayashida M, Kin N, Tomioka T.et al Cerebral ischaemia during cardiac surgery in children detected by combined monitoring of BIS and near‐infrared spectroscopy. Br J Anaesth 200492662–669. [DOI] [PubMed] [Google Scholar]
- 10.Nollert G, Jonas R A, Reichart B. Optimizing cerebral oxygenation during cardiac surgery: a review of experimental and clinical investigations with near infrared spectrophotometry. Thorac Cardiovasc Surg 200048247–253. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitani K, Kawaguchi M, Iwata M.et al Comparison of changes in jugular venous bulb oxygen saturation and cerebral oxygen saturation during variations of haemoglobin concentration under propofol and sevoflurane anaesthesia. Br J Anaesth 200594341–346. [DOI] [PubMed] [Google Scholar]
- 12.Watzman H M, Kurth C D, Montenegro L M.et al Arterial and venous contributions to near‐infrared cerebral oximetry. Anesthesiology 200093947–953. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman G M, Stuth E A, Jaquiss R D.et al Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg 2004127223–233. [DOI] [PubMed] [Google Scholar]
- 14.Weiss M, Schulz G, Fasnacht M.et al Transcutaneously measured near‐infrared spectroscopic liver tissue oxygenation does not correlate with hepatic venous oxygenation in children. Can J Anaesth 200249824–829. [DOI] [PubMed] [Google Scholar]
- 15.Fortune P M, Wagstaff M, Petros A J. Cerebro‐splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med 2001271401–1407. [DOI] [PubMed] [Google Scholar]
- 16.Petros A J, Heys R, Tasker R C.et al Near infrared spectroscopy can detect changes in splanchnic oxygen delivery in neonates during apnoeic episodes. Eur J Pediatr 1999158173–174. [DOI] [PubMed] [Google Scholar]
- 17.Schulz G, Weiss M, Bauersfeld U.et al Liver tissue oxygenation as measured by near‐infrared spectroscopy in the critically ill child in correlation with central venous oxygen saturation. Intensive Care Med 200228184–189. [DOI] [PubMed] [Google Scholar]
- 18.Nagdyman N, Fleck T, Barth S.et al Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Med 200430468–471. [DOI] [PubMed] [Google Scholar]
- 19.Weiss M, Dullenkopf A, Kolarova A.et al Near‐infrared spectroscopic cerebral oxygenation reading in neonates and infants is associated with central venous oxygen saturation. Paediatr Anaesth 200515102–109. [DOI] [PubMed] [Google Scholar]
- 20.Kharbanda R K, Li J, Konstantinov I E.et al Remote ischaemic preconditioning protects against cardiopulmonary bypass‐induced tissue injury:a preclinical study. Heart (in press) [DOI] [PMC free article] [PubMed]
- 21.Azakie T, Merklinger S L, McCrindle B W.et al Evolving strategies and improving outcomes of the modified Norwood procedure: a 10‐year single‐institution experience. Ann Thorac Surg 2001721349–1353. [DOI] [PubMed] [Google Scholar]
- 22.De Oliveira N C, Van Arsdell G S. Practical use of alpha blockade strategy in the management of hypoplastic left heart syndrome following stage one palliation with a Blalock‐Taussig shunt. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004711–15. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Schulze‐Neick I, Lincoln C.et al Oxygen consumption after cardiopulmonary bypass surgery in children: determinants and implications. J Thorac Cardiovasc Surg 2000119525–533. [DOI] [PubMed] [Google Scholar]
- 24.Nollert G, Mohnle P, Tassani‐Prell P.et al Postoperative neuropsychological dysfunction and cerebral oxygenation during cardiac surgery. Thorac Cardiovasc Surg 199543260–264. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji M, Saul J P, du P A.et al Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 2000106625–632. [DOI] [PubMed] [Google Scholar]
- 26.Yeh T, Jr, Gouldman J, Auden S M.et al Mixed venous oxygen saturation does not adequately predict cerebral perfusion during pediatric cardiopulmonary bypass. J Thorac Cardiovasc Surg 2001122192–193. [DOI] [PubMed] [Google Scholar]
- 27.Germon T J, Kane N M, Manara A R.et al Near‐infrared spectroscopy in adults: effects of extracranial ischaemia and intracranial hypoxia on estimation of cerebral oxygenation. Br J Anaesth 199473503–506. [DOI] [PubMed] [Google Scholar]
- 28.Kishi K, Kawaguchi M, Yoshitani K.et al Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near‐infrared spectrophotometers. J Neurosurg Anesthesiol 200315302–306. [DOI] [PubMed] [Google Scholar]
- 29.Uusaro A, Ruokonen E, Takala J. Splanchnic oxygen transport after cardiac surgery: evidence for inadequate tissue perfusion after stabilization of hemodynamics. Intensive Care Med 19962226–33. [DOI] [PubMed] [Google Scholar]
- 30.Landow L, Phillips D A, Heard S O.et al Gastric tonometry and venous oximetry in cardiac surgery patients. Crit Care Med 1991191226–1233. [DOI] [PubMed] [Google Scholar]
- 31.Nollert G, Mohnle P, Tassani‐Prell P.et al Determinants of cerebral oxygenation during cardiac surgery. Circulation 199592(9 Suppl)II327–II333. [DOI] [PubMed] [Google Scholar]