Abstract
Objectives
To investigate the effect of short‐term statin treatment on impaired endothelium‐dependent vasodilatation and haemodynamic abnormalities typically occurring in chronic heart failure (CHF).
Methods
In a double‐blind, crossover study endothelium‐dependent vasodilatation was measured in conduit and resistance vessels of 23 patients with non‐ischaemic CHF after 6 weeks of placebo and 40 mg atorvastatin. The haemodynamic impact was assessed by cardioendocrine hormones, echocardiography and clinical indicators of CHF.
Results
Cholesterol concentrations were population average (low density lipoprotein 3.56 (SEM 0.16) mmol/l, triglycerides 1.70 (0.20) mmol/l and high density lipoprotein 1.17 (0.07) mmol/l). In resistance vessels, the area under the curve ratio during acetylcholine infusion increased from 9.2 (1.9) with placebo to 12.2 (2.1) with statin (p < 0.01). This improvement was reversed during co‐infusion with the nitric oxide antagonist NG‐monomethyl‐l‐arginine. In conduit arteries, flow‐mediated dilatation increased from 5.64 (SEM 0.88)% with placebo to 6.83 (0.97)% with statin (p < 0.05). Endothelium‐independent vasodilatation did not change (p = 0.68 for conduit and p = 0.45 for resistance vessels). Endothelin 1 and atrial natriuretic peptide (ANP) decreased from 1.57 (0.08) and 51.3 (1.0) with placebo to 1.42 (0.09) pg/ml (p < 0.05) and 42.1 (7.5) pmol/l (p < 0.05), respectively, with statin.
Conclusions
In patients with non‐ischaemic CHF and population‐average cholesterol concentrations, short‐term statin treatment improves endothelial function in conduit and resistance vessels and lowers plasma endothelin 1 and ANP concentrations.
The peripheral circulation is abnormal in chronic heart failure (CHF).1 The increase in total peripheral resistance adds to the burden of the struggling myocardium. Redistribution of arterial blood flow along with raised venous pressure compromises the functioning of various tissues and organs. Whereas it is well known that endothelial dysfunction contributes to these circulatory disturbances,2 how to reverse the underlying abnormalities is less evident.
Where coronary artery disease is present and the dominant cause of CHF, treatment with statins is logical. However, in a sizeable proportion of patients the cause of CHF is prior hypertension, idiopathic dilated cardiomyopathy or one of several other rarer disorders. In such clinical states a rational argument for benefit from statin treatment is controversial. Furthermore, concerns about potential adverse effects of statin in CHF have been raised.3,4
Endothelial dysfunction results from heart failure itself, irrespective of the presence of ischaemic heart disease,1 and has been recognised as a potential treatment target.2 As statins improve peripheral and coronary endothelial function through low density lipoprotein (LDL) lowering and pleiotropic mechanisms,5,6 we hypothesised that treatment with a statin would improve haemodynamic abnormalities in patients with cardiac failure even in the absence of underlying coronary artery disease. Accordingly we studied the effects of atorvastatin on endothelial function in patients with CHF in a double‐blind, placebo‐controlled crossover study. Presuming that any statin‐induced improvement in the regulation of peripheral circulation can be reflected by changes in central haemodynamic function, we measured plasma concentrations of the cardiac natriuretic peptides and echocardiographic indices of cardiac structure and function.
METHODS
Twenty‐four patients were recruited from the Cardiology Department outpatient clinic at Christchurch Hospital. All had symptomatic heart failure (New York Heart Association functional class II or III) and a reduced left ventricular ejection fraction (< 40%) on echocardiography. They were all receiving standard heart failure treatment with a loop diuretic, plus an angiotensin‐converting enzyme inhibitor or angiotensin II receptor antagonist with or without digoxin, a β adrenergic blocker and spironolactone in unchanged dosage for at least three months before enrolment. No patient was in atrial fibrillation or was receiving lipid‐modifying drugs, or had experienced a prior acute coronary event or revascularisation procedure. None had stenotic cardiac valve disease, impaired liver function, a glomerular filtration rate of < 100 ml/min (as calculated by the Cockcroft and Gault formula) or reduced pulmonary function (forced expiratory volume in 1 s of < 1 litre). The study size (n = 24) was determined from reported effects of statins on endothelial function under other clinical circumstances5,6 and assumed an increase in endothelium‐dependent blood flow by 30% with a standard deviation of 20%7 at p < 0.05 with a power of 80%. Informed, written consent was obtained from every participant. The Ethics Committee of Canterbury Health Limited approved the study protocol, which complied with the Declaration of Helsinki.
Study protocol
All patients received atorvastatin 40 mg or placebo once daily in the evening for six weeks in a randomised, placebo‐controlled, double‐blind crossover study. The crossover design was chosen to minimise interindividual variability. Between treatments there was a two‐week washout period. Statin has been shown to reversibly improve endothelial function within two weeks in conduit8 and resistance vessels.9 All heart failure drugs were required to be continued in unchanged dosage for the duration of the study and the prior three months. After the six‐week treatment with statin or placebo, patients attended the research centre at 07 30 after fasting overnight for a set sequence of tests: brachial ultrasound, venepuncture, forearm plethysmography, 6 min walk test,10 echocardiography and the Minnesota Living with Heart Failure Quality of Life Questionnaire.11 All tests were carried out in a quiet, air‐conditioned room with a stable temperature of 22°C, with the exception of the 6 min walk, which took place in a designated hospital corridor.
Brachial ultrasound
Conduit artery function was assessed by brachial ultrasound based on the protocol described by Celermajer et al.12 The assessment adhered to guidelines published by the International Brachial Reactivity Task Force13 and used a fully digitised ultrasound system (Logiq 700 Expert Series; GE Medical Systems) with a high‐resolution, broad‐spectrum transducer (6–13 MHz, LA39; GE Medical Systems). Briefly, the participant was kept in a recumbent position for 15 min before measurements were started, with the non‐dominant arm being held in a specially designed cradle and the ultrasound transducer fixed in position with a stereotactic clamp. The brachial artery was imaged in the longitudinal plane during the baseline resting phase, after 5 min of forearm ischaemia to assess endothelium‐dependent flow‐mediated dilatation (FMD) and after 800 µg sublingual glyceryl trinitrate was given to assess endothelium‐independent dilatation (EID). Recordings were made only when well‐defined, double‐line patterns14 were present proximally and distally throughout the entire area of interest. Measurements from fixed anatomical landmarks eliminated positional variations between study visits. All recordings were made by the same investigator and frame analyses were completed before unblinding of the study drug sequence. Scans were recorded on sVHS tapes and digitised (Pinnacle DV500 Plus; Pinnacle Systems). Diastolic frames were identified by gating simultaneously recorded Doppler curves and were measured frame by frame with specially developed edge detection software. Vessel diameter was defined as the distance between the distal and proximal lumen–intima interface. FMD and EID were expressed as the percentage increase of vessel diameter from baseline—that is, [(maximum diameter after ischaemia or glyceryl trinitrate administration − baseline diameter)/baseline diameter] × 100. The coefficient of variation (CV) was 3% for measurement of baseline diameters, 22% for FMD and 11% for EID.
Forearm strain gauge plethysmography
Resistance vessel function was assessed by invasive venous occlusion plethysmography (Hokanson, Bellevue, Washington, USA) based on the protocol described by Watts et al.15 The brachial artery in the non‐dominant arm was cannulated with a 27 gauge needle. Blood flow was measured after the following intra‐arterial infusions: (A) 0.9% saline for 30 min (baseline); (B) acetylcholine at 7.5, 15 and 30 µg/min for 3 min each; (C) sodium nitroprusside at 1.5, 3.0 and 10 µg/min for 3 min each; and (D) NG‐monomethyl‐l‐arginine (l‐NMMA) (Clinalfa AG, Kilsyth, Victoria, Australia) at 4.0 µg/min alone and co‐infused with each incremental acetylcholine infusion for 3 min each. There was a break of 15 min between different infusions during which the saline infusion continued. Control blood flow was measured in the dominant (non‐infused) arm. The forearm was distended by inflating and deflating brachial cuffs to supravenous pressure levels (45 mm Hg) with a rapid cuff inflator (E20; Hokanson) for 5 and 10 s, respectively. Ten inflation–deflation cycles were recorded for each stage of the protocol and results were averaged. Forearm blood flow was expressed as the area under the curve according to the trapezoid rule, by using specially developed software. To correct for systemic changes the ratio between the area under the curve in the infused and the non‐infused arm was calculated.16 The intraobserver (between weeks) CV for this method was 19% in the resting state and 17% after 5 min of ischaemia.
Biochemical markers
Venous blood was drawn from a forearm vein at the conclusion of the brachial artery ultrasound study after the patients had been supine for 45 min, just before arterial cannulation, for measurements of plasma atrial natriuretic peptide (ANP),17 B‐type natriuretic peptide18 (BNP), N–terminal BNP,19 the endothelial markers C‐type natriuretic peptide (CNP), N–terminal CNP,20 endothelin 1,21 vascular cell adhesion molecule 1 (R&D Systems, Minneapolis, Minnesota, USA; enzyme‐linked immunosorbent assay intra‐assay CV 5%) and von Willebrand factor, adrenomedullin,22 aldosterone,22 epinephrine and norepinephrine.23 All samples from an individual patient were measured in the same assay. Intra‐assay and interassay CV varied between 5% and 9% for these tests. Also measured were plasma lipid concentrations, high‐sensitivity C reactive protein, fibrinogen, plasma glucose, urea, creatinine and liver function.
Echocardiography
Transthoracic echocardiographic examinations were performed with a Vivid 3 echocardiograph (General Electric, Fairfield, Connecticut, USA) by a single operator who was blinded as to the treatment phase, with participants in the left lateral decubitus position. M mode echocardiographic variables were measured according to the recommendations of the American Society of Echocardiography.24 Left ventricular mass was calculated with the formula derived from the American Society of Echocardiography data.25 Left ventricular ejection fraction was calculated by the biplane disc summation method.26 The average of three measurements was used for all variables.
Statistical analysis
Statistical calculations were performed with SPSS Base V.10.0 (SPSS, Inc, Chicago, Illinois, USA). The paired t test or Wilcoxon signed rank test was used for comparisons between placebo and statin groups. The treatment effect on resistance vessel function during drug infusion was analysed by repeated measures analysis of variance (general linear model). The influence of the treatment sequence and the severity of heart failure on statin‐induced changes were analysed with repeated measures analysis of variance by using the in‐between group function. If influences were found, respective subgroups were analysed for the difference of the mean by the independent t test or Mann–Whitney U test. Correlation was analysed with Pearson's or Spearman's correlation coefficient. Significance was accepted at the 95% confidence interval (p < 0.05).
LDL cholesterol and triglycerides were reduced by 50% and 32%. High density lipoprotein cholesterol remained unchanged.
RESULTS
Twenty‐three patients completed the study, as one was excluded with worsening heart failure necessitating alteration of drugs. One patient with myotonic dystrophy developed mild proximal myalgia without a rise in muscle enzymes. Compliance rates based on tablet counts were 97% during placebo administration and 98% during statin treatment.
Baseline characteristics
Table 1 snows baseline characteristics of the patients.
Table 1 Baseline characteristics of study participants.
| Total number of participants | 23* |
| Age (years) | 60.7 (10.4) |
| Men/women | 16/7 |
| Smokers | 3 |
| NYHA class II/III | 14/9 |
| LVEF (%) | 30.1 (7.7) |
| Time from first diagnosis (months) | 46.7 (54.6) |
| Normal coronary angiogram | 15† |
| Abnormal coronary angiogram | 4§ |
| Type 2 diabetes | 4 |
| Hypertension | 8 |
| Body mass index (kg/m2) | 27.2 (4.9) |
| Systolic blood pressure (mm Hg) | 120.8 (16.5) |
| Diastolic blood pressure (mm Hg) | 77.8 (8.9) |
| MAP (mm Hg) | 92.1 (11.1) |
| Serum urea (mmol/l) | 7.5 (3.0) |
| Serum creatinine (µmol/l) | 0.09 (0.02) |
| Plasma glucose (mmol/l) | 5.5 (2.5) |
| Serum GGT (mmol/l) | 47.9 (63.3) |
| Serum AST (mmol/l) | 23.5 (7.4) |
| hsCRP (mg/l) | 2.02 (0.33) |
| Fibrinogen (g/l) | 3.3 (0.1) |
| UACR (g/mol) | 0.95 (0.12) |
| Concomitant drugs | |
| Furosemide | 18 |
| Furosemide dose (mg/day) | 142.2 (341.5) |
| Enalapril or equivalent | 18 |
| Enalapril dose (mg/day) | 13.9 (7.4) |
| ATIIRB | 4 |
| β blocker | 13 |
| Digitalis | 2 |
| Spironolactone | 4 |
| Aspirin | 14 |
| Aspirin dose (mg/day) | 167.9 (75.0) |
Data presented as mean (SD) or number.
*Heart failure causes: idiopathic (12), hypertension (4), viral (4), familial (2), myotonic dystrophy (1), Marfan's syndrome (1); †coronary angiogram not performed in four participants with a low probability of ischaemic heart disease; ‡some degree of coronary artery disease in patients without angina pectoris and normal thallium and sestamibi stress test.
AST, aspartate amino transferase; ATIIRB, angiotensin II receptor blocker; GGT, γ glutamyl transferase; hsCRP, high sensitivity C reactive protein; LVEF, left ventricular ejection fraction; MAP, mean arterial blood pressure; NYHA, New York Heart Association; UACR, urinary albumin creatinine ratio.
Nineteen patients underwent coronary angiography; 15 of the angiograms were normal and four had some features of atherosclerotic disease in patients with no symptoms of myocardial ischaemia and normal thallium and sestamibi stress tests. The four remaining participants with a low probability of ischaemic cardiomyopathy in the absence of cardiovascular risk factors (39–60 years of age, non‐smokers, no dyslipidaemia, no family history, no hypertension and no obesity) and without symptoms or signs of myocardial ischaemia did not undergo coronary angiography. Table 2 shows the placebo and statin effects on lipid parameters.
Table 2 Biochemical measurements after statin and placebo administration.
| Placebo | Statin treatment | p Value | |
|---|---|---|---|
| Cholesterol | |||
| Total (mmol/l) | 5.50 (0.21) | 3.47 (0.15) | <0.001 |
| LDL (mmol/l) | 3.56 (0.16) | 1.77 (0.11) | <0.001 |
| HDL (mmol/l) | 1.17 (0.07) | 1.18 (0.07) | 0.55 |
| Triglycerides (mmol/l) | 1.70 (0.20) | 1.15 (0.09) | <0.001 |
| Endothelial markers | |||
| vWF (%) | 126.14 (6.01) | 125.45 (6.40) | 0.89 |
| VCAM‐1 (ng/ml) | 584 (25) | 568 (28) | 0.24 |
| Endothelin 1 (pg/ml) | 1.57 (0.08) | 1.42 (0.09) | <0.05 |
| CNP (pmol/l) | 1.06 (0.05) | 0.99 (0.05) | 0.08 |
| Cardioendocrine hormones | |||
| ANP (pmol/l) | 51.25 (9.98) | 42.05 (7.47) | <0.05 |
| BNP (pmol/l) | 25.98 (4.35) | 21.56 (3.67) | 0.19 |
| N‐BNP (pmol/l) | 135.17 (24.73) | 116.05 (22.10) | 0.11 |
| Adrenomedullin (pmol/l) | 8.86 (0.70) | 8.26 (0.55) | 0.20 |
| Epinephrine (pmol/l) | 96.20 (21.56) | 91.82 (15.79) | 0.56 |
| Norepinephrine (pmol/l) | 3123.57 (391.39) | 2889.04 (245.52) | 0.43 |
Data presented as mean (SEM).
ANP, atrial natriuretic peptide; BNP, B‐type natriuretic peptide; CNP, C‐type natriuretic peptide; HDL, high density lipoprotein; LDL, low density lipoprotein; N‐BNP, N–terminal B‐type natriuretic peptide; UACR, urinary albumin creatinine ratio; VCAM, vascular cell adhesion molecule; vWF, von Willebrand factor.
Endothelial function
Table 3 summarises conduit vessel function after administration of the statin and placebo.
Table 3 Brachial artery function after placebo and statin administration.
| Placebo | Statin treatment | p Value | |
|---|---|---|---|
| Pulse rate (beats/min) | 70.5 (3.0) | 69.2 (2.9) | 0.34 |
| Mean arterial blood pressure (mm Hg) | 90.1 (2.3) | 90.2 (2.1) | 0.93 |
| Baseline blood flow (ml/min) | 66.6 (3.9) | 67.5 (3.1) | 0.76 |
| Peak hyperaemic flow (ml/min) | 138.1 (7.9) | 147.9 (9.6) | 0.30 |
| Baseline diameter (mm) | 3.57 (1.59) | 3.60 (1.60) | 0.49 |
| Maximum hyperaemic diameter (mm) | 3.75 (0.15) | 3.83 (1.60) | 0.12 |
| Flow‐mediated dilatation (%) | 5.64 (0.88) | 6.83 (0.97) | <0.05 |
| Endothelium‐independent dilatation (%) | 21.69 (2.94) | 20.87 (2.73) | 0.68 |
Data presented as mean (SEM).
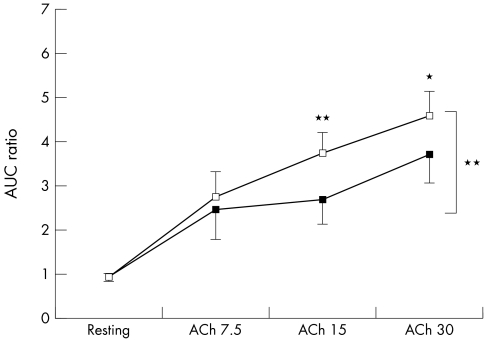
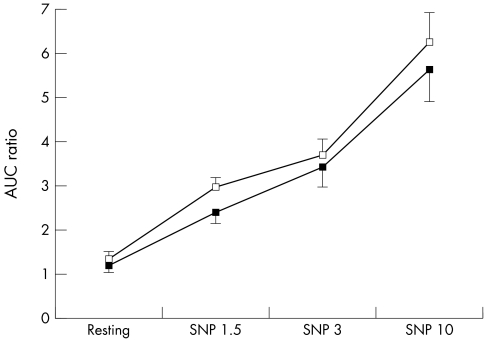
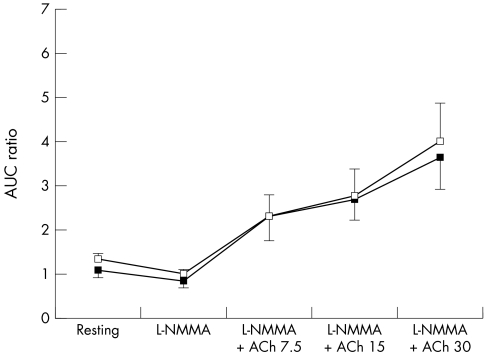
Compared with placebo, statin was associated with significantly greater endothelium‐dependent dilatation (FMD), but not EID. Figures 1–3 show forearm resistance vessel function during infusion of acetylcholine, sodium nitroprusside and l‐NMMA alone or in combination with acetylcholine after statin and placebo administration. After statin treatment, endothelium‐dependent vasodilatation during acetylcholine infusion was significantly greater than after placebo administration for the two highest infusion rates and when all infusion rates were analysed collectively (fig 1). This difference was obliterated during co‐infusion with the nitric oxide (NO) antagonist l‐NMMA (fig 3). Nitroprusside‐induced vasodilatation was similar after statin and placebo (p = 0.45) (fig 2). Mean blood pressure did not vary significantly between blood flow studies.
Figure 1 Effect of intra‐arterial acetylcholine (ACh; 7.5–30 μg/min) on forearm blood flow after placebo (black squares) and statin (white squares) application for six weeks. Data presented as mean (SEM). *p = 0.07; **p ⩽ 0.01. AUC, area under the curve.
Figure 2 Effect of intra‐arterial sodium nitroprusside (SNP; 1.5–10 μg/min) infusion on forearm blood flow after placebo (black squares) and after statin (white squares) administration for six weeks. Data presented as mean (SEM). AUC, area under the curve.
Figure 3 Effect of intra‐arterial infusion of NG‐monomethyl‐l‐arginine (l‐NMMA) alone and in combination with acetylcholine (ACh; 7.5–30 μg/min) on forearm blood flow after placebo (black squares) and after statin (white squares) administration for six weeks. Data presented as mean (SEM). AUC, area under the curve.
Endothelial markers and cardioendocrine hormones
All endothelial markers and cardioendocrine hormones measured in this study (table 2) were lower after six‐week treatment with statin than after placebo; however, this reached significance only for plasma concentrations of endothelin 1 and ANP. After both treatments γ glutamyl transferase, aspartate transferase and creatine kinase were similar (data not shown). Treatments did not differ in clinical measurements of heart failure (table 4).
Table 4 Clinical measurements of heart failure after placebo and statin administration.
| Placebo | Statin treatment | p Value | |
|---|---|---|---|
| Internal dimension in diastole (mm) | 68.0 (2.5) | 67.0 (2.6) | 0.41 |
| Internal dimension in systole (mm) | 59.1 (2.7) | 56.6 (3.6) | 0.20 |
| Left ventricular ejection fraction (%) | 29.7 (2.3) | 31.0 (2.4) | 0.59 |
| Peak early diastolic transmitral flow velocity (cm/s) | 76.4 (7.6) | 76.8 (8.1) | 0.37 |
| Peak transmitral flow velocity with atrial contraction (cm/s) | 83.4 (4.8) | 76.6 (4.3) | 0.20 |
| Deceleration time (ms) | 224.5 (26.8) | 204.5 (18.3) | 0.37 |
| Left ventricular mass (g) | 273.9 (23.5) | 306.0 (32.1) | 0.25 |
| 6 min walking test (m) | 466.2 (18.9) | 471.9 (20.2) | 0.44 |
| MLHFQL questionnaire score | 28.5 (4.6) | 29.9 (4.5) | 0.63 |
Data presented as mean (SEM).
MLHFQL, Minnesota Living with Heart Failure Quality of Life.
Correlations and multivariate analysis
CNP and adrenomedullin were inversely correlated with endothelium‐dependent vasodilatation during acetylcholine infusion (with statin r = −0.606, p < 0.01 and r = −0.517, p < 0.05; with placebo r = −0.489, p < 0.05 and r = −0.581, p < 0.05, respectively). The endothelial makers CNP and N–terminal CNP correlated with systolic internal dimensions (with statin r = 0.486, p < 0.05 and r = 0.367, p = 0.111; with placebo r = 0.430, p = 0.058 and r = 0.583, p < 0.01) and diastolic internal dimensions (with statin r = 0.682, p < 0.001 and r = 0.494, p < 0.05; with placebo r = 0.483, p < 0.05 and r = 0.595, p < 0.01). Echocardiographic indices and lipoprotein fractions (specified in table 2) did not correlate with endothelium‐dependent responses in either forearm vessels or the brachial artery before or after statin treatment. Multivariate tests showed no significant influence of the functional heart failure class or the treatment order on endothelium‐dependent conduit or resistance vessel function.
DISCUSSION
Our findings suggest that in patients with non‐ischaemic heart failure short‐term statin treatment is associated with improvements in endothelium‐dependent vasodilatation in conduit and resistance vessels. We showed endothelial NO dependence of statin‐associated benefits in resistance vessels, as augmentation was seen only during NO‐stimulating acetylcholine infusion and reversed during co‐infusion of the NO‐blocking l‐NMMA. Nitroprusside‐induced, endothelium‐independent resistance vessel function was not augmented during statin treatment. Two comparable studies did not obtain statin‐induced improvements of resistance vessel function.27,28 In both of these studies non‐invasive plethysmography was used to assess resistance vessel function after ischaemia, a technique with a low specificity for endothelial function.29 In conduit vessels, our findings of improved endothelial function concur with the results of a study by Node et al30 in patients with idiopathic dilated cardiomyopathy also reporting FMD augmentation after 14 weeks of statin treatment. The more pronounced FMD improvement (from 8% to 13%) in this study may be due to the longer treatment period.
The endothelium‐specific benefits seen during statin treatment may be largely LDL cholesterol independent. Our participants exhibited serum LDL cholesterol concentrations below population average, a finding typical for CHF. Furthermore, neither absolute LDL cholesterol concentrations nor LDL cholesterol reductions determined the degree of endothelial amelioration. Cholesterol‐independent or pleiotropic statin effects have long been postulated,31 recently gaining more widespread recognition.32,33 Pleiotropic statin effects contribute to the reversal of two main pathogenic mechanisms leading to impaired vasodilatation in CHF: decreased vasodilating NO and increased vasoconstricting endothelin 1.34 Independent of LDL cholesterol reduction, statins upregulate and promote endothelial NO synthase35,36 and reduce endothelin 1 expression in endothelial cells.37 Compelling evidence that statin‐induced endothelial benefits in CHF are pleiotropic derives from a recent study by Landmesser et al,38 who reported FMD reductions in patients with CHF taking statins, but not with ezetimibe, despite almost identical LDL cholesterol reductions. It should be noted, however, that in this study ischaemic CHF was not an exclusion criterion.
In our study, treatment with a statin versus placebo was associated with lower concentrations of all measured cardioendocrine hormones, although the difference was significant only for ANP. Given the problems with cardioendocrine hormone measurement and the small patient population examined, the data are suggestive of statin‐derived benefits for the CHF typical neurohormonal imbalance. One other clinical study investigated the effect of statin on cardioendocrine hormones in non‐ischaemic CHF30 and reported BNP reductions. It is uncertain whether the stabilisation of the neurohormonal imbalance is secondary to improvements in endothelial dysfunction or to other statin effects with the potential to delay CHF progression. Such effects include the induction of neoangiogenesis, the downregulation of angiotensin II type 1 receptors, the restoration of autonomic dysfunction and the inhibition of proinflammatory cytokines.4 The absence of clear correlations between improvements in endothelium‐dependent vasodilatation and cardioendocrine hormone reductions in our study may emphasise direct, cardioprotective effects. However, endothelium‐ameliorating and other cardioprotective statin effects probably act synergistically.
After six weeks of statin treatment, improvements in endothelial dysfunction and cardioendocrine imbalance had no measurable effect on clinical CHF indices. Longer treatment intervals are likely to yield measurable clinical benefits, however, considering the findings of two comparable studies that ejection fractions and quality of life scores improved after treatment intervals twice to thrice as long as in our study, despite lower dose equivalents.27,30
The coupling of endothelial and cardiac function can be illustrated by focusing on the endothelial hormones endothelin 1 and CNP. Blocking endothelin 1, excreted by the dysfunctional endothelium39 and typically raised in heart failure,40 with the endothelin receptor antagonist bosentan reduces ANP and BNP in sheep,41 stressing the concept that endothelial recovery may ameliorate neurohormonal imbalance. Statins decrease the endothelial synthesis of endothelin 1 in vitro37 and we showed this effect for the first time in patients with non‐ischaemic CHF without other major, endothelium‐harming conditions. CNP is considered a member of the natriuretic peptide family and structurally homologous to ANP and BNP,42 but is the only natriuretic peptide produced mainly in vascular endothelial cells43 and expressed proportional to endothelial stress.44 CNP may therefore be an important endothelial mediator of the haemodynamic disturbances found in CHF. In our study CNP reductions did not reach significance (p = 0.08). This is nevertheless remarkable, as peripheral plasma concentrations of CNP are close to assay detection limits and only minimally raised in heart failure,45 perhaps acting primarily in a paracrine fashion.
Statin treatment may potentially be unfavourable in patients with CHF. Lipoprotein reductions may negate statin‐specific pleiotropic benefits in CHF.3 Low cholesterol concentrations have been related to impaired survival from heart failure.46 Furthermore, statin treatment is associated with coenzyme Q10 reductions,47 which have been correlated with the severity of CHF.48 However, the cause–effect relationship of these observations has not been determined and we recently found that the beneficial effects of statin treatment on endothelium‐dependent vasodilatation in CHF are strongly associated with reductions in coenzyme Q10.49
Study limitations
Our study may have been underpowered, especially for highly variable measures such as cardioendocrine hormones or flow‐mediated brachial artery vasodilatation. The recorded improvements are subtle yet, if taken together, are consistent for statin‐associated benefits. In the absence of baseline data immediately before each treatment period, we are unable to exclude the influence of a time effect on our findings. However, the short‐term crossover design should have kept a time effect bias minimal. Focusing on patients with non‐ischaemic cardiomyopathy, we were unable to entirely exclude a potential component of ischaemic cardiomyopathy in eight participants.
Clinical significance
We showed for the first time that patients with non‐ischaemic heart failure have better endothelium‐dependent vasodilatation in resistance vessels and lower plasma endothelin 1 and ANP concentrations while being treated with a statin than when given a placebo. These benefits were detectable after only six weeks in participants already treated with endothelial and cardiac function‐ameliorating drugs. These benefits may translate into increased exercise capacity and improved peripheral perfusion when statins are given over longer time periods.2 Recent cohort studies show improved survival50,51,52,53 of patients with heart failure treated with statins. Our findings further strengthen the notion of using statins in heart failure regardless of its aetiology and in the absence of dyslipidaemia. However, the routine use of statins by patient with heart failure due to other causes than coronary artery disease can not be recommended while randomised controlled trials determining benefits versus risks are awaited.
Conclusions
Adding a statin to the treatment regimen of patients with CHF, optimised with standard treatment, results in improved endothelium‐dependent vasodilatation, reduced plasma endothelin 1 and ANP, and an overall trend for reduced cardioendocrine stress. These differences from placebo were present after six weeks of statin treatment in patients with non‐ischaemic CHF and below population‐average LDL concentrations.
ACKNOWLEDGEMENTS
We thank Mr Sinclair Bennett for developing blood flow analysis software and Christchurch Hospital Pharmacy staff for preparing intra‐arterial infusions.
Abbreviations
ANP - atrial natriuretic peptide
BNP - B‐type natriuretic peptide
CHF - chronic heart failure
CNP - C‐type natriuretic peptide
CV - coefficient of variation
EID - endothelium‐independent dilatation
FMD - flow‐mediated dilatation
LDL - low density lipoprotein
l‐NMMA - NG‐monomethyl‐l‐arginine
NO - nitric oxide
Footnotes
This study was funded entirely by a grant from the Health Research Council of New Zealand.
Competing interests: None declared.
References
- 1.Kubo S H, Rector T S, Bank A J.et al Endothelium‐dependent vasodilation is attenuated in patients with heart failure. Circulation 1991841589–1596. [DOI] [PubMed] [Google Scholar]
- 2.Drexler H. Endothelium as a therapeutic target in heart failure. Circulation 1998982652–2655. [DOI] [PubMed] [Google Scholar]
- 3.Rauchhaus M, Coats A J, Anker S D. The endotoxin‐lipoprotein hypothesis. Lancet 2000356930–933. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, McMurray J J. Statins and chronic heart failure: do we need a large‐scale outcome trial? J Am Coll Cardiol 2002391567–1573. [DOI] [PubMed] [Google Scholar]
- 5.O'Driscoll G, Green D, Taylor R R. Simvastatin, an HMG‐coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 1997951126–1131. [DOI] [PubMed] [Google Scholar]
- 6.Vogel R A, Corretti M C, Plotnick G D. Changes in flow‐mediated brachial artery vasoactivity with lowering of desirable cholesterol levels in healthy middle‐aged men. Am J Cardiol 19967737–40. [DOI] [PubMed] [Google Scholar]
- 7.Vogel R A. Cholesterol lowering and endothelial function. Am J Med 1999107479–487. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi S, Lupattelli G, Siepi D.et al Short‐term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol 200036617–621. [DOI] [PubMed] [Google Scholar]
- 9.John S, Delles C, Jacobi J.et al Rapid improvement of nitric oxide bioavailability after lipid‐lowering therapy with cerivastatin within two weeks. J Am Coll Cardiol 2001371351–1358. [DOI] [PubMed] [Google Scholar]
- 10.Willenheimer R, Erhardt L R. Value of 6‐min‐walk test for assessment of severity and prognosis of heart failure. Lancet 2000355515–516. [DOI] [PubMed] [Google Scholar]
- 11.Rector T S, Cohn J N. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 19921241017–1025. [DOI] [PubMed] [Google Scholar]
- 12.Celermajer D S, Sorensen K E, Gooch V M.et al Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 19923401111–1115. [DOI] [PubMed] [Google Scholar]
- 13.Corretti M C, Anderson T J, Benjamin E J.et al Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 200239257–265. [DOI] [PubMed] [Google Scholar]
- 14.Pignoli P, Tremoli E, Poli A.et al Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986741399–1406. [DOI] [PubMed] [Google Scholar]
- 15.Watts G F, O'Brien S F, Silvester W.et al Impaired endothelium‐dependent and independent dilatation of forearm resistance arteries in men with diet‐treated non‐insulin‐dependent diabetes: role of dyslipidaemia. Clin Sci (Lond) 199691567–573. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin N, Calver A, Collier J.et al Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 199525918–923. [DOI] [PubMed] [Google Scholar]
- 17.Yandle T G, Espiner E A, Nicholls M G.et al Radioimmunoassay and characterization of atrial natriuretic peptide in human plasma. J Clin Endocrinol Metab 19866372–79. [DOI] [PubMed] [Google Scholar]
- 18.Yandle T G, Richards A M, Gilbert A.et al Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab 199376832–838. [DOI] [PubMed] [Google Scholar]
- 19.Davis M, Espiner E, Richards G.et al Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet 1994343440–444. [DOI] [PubMed] [Google Scholar]
- 20.Wright S P, Prickett T C, Doughty R N.et al Amino‐terminal pro‐C‐type natriuretic peptide in heart failure. Hypertension 20044394–100. [DOI] [PubMed] [Google Scholar]
- 21.Evans J J, Youssef A H, Yandle T G.et al Effects of endothelin‐1 on release of adrenomedullin and C‐type natriuretic peptide from individual human vascular endothelial cells. J Endocrinol 2002175225–232. [DOI] [PubMed] [Google Scholar]
- 22.Lewis L K, Smith M W, Yandle T G.et al Adrenomedullin(1–52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem 199844571–577. [PubMed] [Google Scholar]
- 23.Goldstein D S, Feuerstein G, Izzo J L., Jret al Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci 198128467–475. [DOI] [PubMed] [Google Scholar]
- 24.Sahn D J, DeMaria A, Kisslo J.et al Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978581072–1083. [DOI] [PubMed] [Google Scholar]
- 25.Devereux R B, Alonso D R, Lutas E M.et al Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 198657450–458. [DOI] [PubMed] [Google Scholar]
- 26.Schiller N B, Acquatella H, Ports T A.et al Left ventricular volume from paired biplane two‐dimensional echocardiography. Circulation 197960547–555. [DOI] [PubMed] [Google Scholar]
- 27.Laufs U, Wassmann S, Schackmann S.et al Beneficial effects of statins in patients with non‐ischemic heart failure. Z Kardiol 200493103–108. [DOI] [PubMed] [Google Scholar]
- 28.Tousoulis D, Antoniades C, Bosinakou E.et al Effects of atorvastatin on reactive hyperaemia and the thrombosis‐fibrinolysis system in patients with heart failure. Heart 20059127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelke K A, Halliwill J R, Proctor D N.et al Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 1996811807–1814. [DOI] [PubMed] [Google Scholar]
- 30.Node K, Fujita M, Kitakaze M.et al Short‐term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation 2003108839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan C J, Murphy M B, Buckley B M. Statins do more than just lower cholesterol. Lancet 19963481079–1082. [DOI] [PubMed] [Google Scholar]
- 32.Davidson M H. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 20051112280–2281. [DOI] [PubMed] [Google Scholar]
- 33.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004109III39–III43. [DOI] [PubMed] [Google Scholar]
- 34.Von Haehling S, Anker S D, Bassenge E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail Rev 2003899–106. [DOI] [PubMed] [Google Scholar]
- 35.Laufs U, La Fata V, Plutzky J.et al Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998971129–1135. [DOI] [PubMed] [Google Scholar]
- 36.Feron O, Dessy C, Desager J P.et al Hydroxy‐methylglutaryl‐coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation 2001103113–118. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez‐Perera O, Perez‐Sala D, Navarro‐Antolin J.et al Effects of the 3‐hydroxy‐3‐methylglutaryl‐CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin‐1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 19981012711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landmesser U, Bahlmann F, Mueller M.et al Simvastatin versus ezetimibe: pleiotropic and lipid‐lowering effects on endothelial function in humans. Circulation 20051112356–2363. [DOI] [PubMed] [Google Scholar]
- 39.Levin E R. Endothelins. N Engl J Med 1995333356–363. [DOI] [PubMed] [Google Scholar]
- 40.Parker J D, Thiessen J J. Increased endothelin‐1 production in patients with chronic heart failure. Am J Physiol Heart Circ Physiol 2004286H1141–H1145. [DOI] [PubMed] [Google Scholar]
- 41.Rademaker M T, Charles C J, Espiner E A.et al Combined inhibition of angiotensin II and endothelin suppresses the brain natriuretic peptide response to developing heart failure. Clin Sci (Lond) 2004106569–576. [DOI] [PubMed] [Google Scholar]
- 42.Wilkins M R, Redondo J, Brown L A. The natriuretic‐peptide family. Lancet 19973491307–1310. [DOI] [PubMed] [Google Scholar]
- 43.Chen H H, Burnett J C., Jr C‐type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol 199832(Suppl 3)S22–S28. [PubMed] [Google Scholar]
- 44.Chun T H, Itoh H, Ogawa Y.et al Shear stress augments expression of C‐type natriuretic peptide and adrenomedullin. Hypertension 1997291296–1302. [DOI] [PubMed] [Google Scholar]
- 45.Totsune K, Takahashi K, Murakami O.et al Elevated plasma C‐type natriuretic peptide concentrations in patients with chronic renal failure. Clin Sci (Lond) 199487319–322. [DOI] [PubMed] [Google Scholar]
- 46.Rauchhaus M, Clark A L, Doehner W.et al The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol 2003421933–1940. [DOI] [PubMed] [Google Scholar]
- 47.Folkers K, Langsjoen P, Willis R.et al Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci USA 1990878931–8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortensen SA Vadhanavikit S, Folkers K. Deficiency of coenzyme Q10 in myocardial failure. Drugs Exp Clin Res 198410497–502. [PubMed] [Google Scholar]
- 49.Strey C H, Young J M, Molyneux S L.et al Endothelium‐ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis 2005179201–206. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D, Nye R, Levy W C. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol 2004931124–1129. [DOI] [PubMed] [Google Scholar]
- 51.Horwich T B, MacLellan W R, Fonarow G C. Statin therapy is associated with improved survival in ischemic and non‐ischemic heart failure. J Am Coll Cardiol 200443642–648. [DOI] [PubMed] [Google Scholar]
- 52.Sola S, Mir M Q, Rajagopalan S.et al Statin therapy is associated with improved cardiovascular outcomes and levels of inflammatory markers in patients with heart failure. J Card Fail 200511607–612. [DOI] [PubMed] [Google Scholar]
- 53.Fukuta H, Sane D C, Brucks S.et al Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation 2005112357–363. [DOI] [PubMed] [Google Scholar]