Abstract
Objective
To assess the haemodynamic effect of simultaneously adjusting atrioventricular (AV) and interventricular (VV) delays.
Method
35 different combinations of AV and VV delay were tested by using digital photoplethysmography (Finometer) with repeated alternations to measure relative change in systolic blood pressure (SBPrel) in 15 patients with cardiac resynchronisation devices for heart failure.
Results
Changing AV delay had a larger effect than changing VV delay (range of SBPrel 21 v 4.2 mm Hg, p < 0.001). Each had a curvilinear effect. The curve of response to AV delay fitted extremely closely to a parabola (average R2 = 0.99, average residual variance 0.8 mm Hg2). The response to VV delay was significantly less curved (quadratic coefficient 67 v 1194 mm Hg/s2, p = 0.003) and therefore, although the residual variance was equally small (0.8 mm Hg2), the R2 value was 0.7. Reproducibility at two months was good, with the SD of the difference between two measurements of SBPrel being 2.5 mm Hg for AV delay (2% of mean systolic blood pressure) and 1.5 mm Hg for VV delay (1% of mean systolic blood pressure).
Conclusions
Changing AV and VV delays results in a curvilinear acute blood pressure response. This shape fits very closely to a parabola, which may be valuable information in developing a streamlined clinical protocol. VV delay adjustment provides an additional, albeit smaller, haemodynamic benefit to AV optimisation.
Cardiac resynchronisation therapy improves haemodynamic status,1,2,3,4 with an increase in peak rate of rise of intraventricular pressure,5,6 an increase in stroke volume7 and consequently higher systemic arterial blood pressure.5,8,9 Additional haemodynamic improvements can be obtained by selecting the appropriate atrioventricular (AV) delay for an individual patient.1,8,10 Acute haemodynamic response can be improved yet further when interventricular (VV) delay is adjusted.6,11,12,13,14
Whereas the haemodynamic response to changes in AV delay has been seen, in example cases in the literature, to be curved around a peak,5 little is known about the characteristics of these curves, the magnitude of the response in typical stable ambulant patients, or the relative importance of AV and VV adjustments.
In this study, we used an algorithm based on beat‐by‐beat blood pressure, measured non‐invasively with the Finometer device, to assess the haemodynamic effect of simultaneously adjusting AV and VV delays.
The first objective of the study was to determine whether this technique can be used to quantify immediate differences in blood pressure efficiently for a matrix of different AV and VV delays.
The second objective was to establish whether the relationship across the AV and VV delays tested is curved, whether the characteristics of the curve differ between AV and VV delays, and whether this curve fits to a conveniently definable shape such as a parabola.
The third objective was to assess the reproducibility of this technique in terms both of individual measurements and of selection of haemodynamic optima for AV and VV delays.
METHODS
Participants
Fifteen outpatients with biventricular pacemakers or biventricular defibrillators implanted for clinical indications (New York Heart Association (NYHA) class III or IV heart failure, QRS > 120 ms, maximum drug treatment) were enrolled into this study between 3–30 months after implantation. In all patients the atrial lead was in the right atrial appendage. Twelve were men and three women, with age ranging from 54–79 years (mean 70 years). Cause of heart failure was ischaemic in 11 and idiopathic dilated in four. Mean systolic blood pressure (SBP) by sphygmomanometer was 122 (16) mm Hg. Mean ejection fraction of the patients at the time of the study was 33 (8)%. At the time of the study three patients were in NYHA class I, three were in NYHA II, six were in NYHA III and three were in NYHA IV. Patients gave informed consent for this study, which was approved by the local ethical committee.
Measurements
Data acquisition
Finger arterial pressure was measured non‐invasively with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). This technique, developed by Peňaz15 and Wesseling et al,16 uses a cuff that is placed around the finger, a built‐in photoelectric plethysmograph and a volume–clamp circuit that dynamically follows arterial pressure. This technique is well validated for measuring instantaneous changes in blood pressure.17,18,19,20,21
An ECG signal was also recorded. These signals were acquired through an analogue to digital card (National Instruments, Austin, Texas, USA) by custom software developed in our laboratory22 and analysed off line with further custom software based on the Matlab platform (MathWorks, Natick, Massachusetts, USA).
Measurement of relative change in blood pressure across different AV and VV delays
Beat‐to‐beat blood pressure was recorded during adjustment of the AV and VV delay of the participants' biventricular pacemaker while paced at a rate of 110 beats/min. Testing was performed at raised heart rates because previous work from this unit has shown that, at higher heart rates, altering AV delay had a more pronounced effect on blood pressure and that the peak AV delay became clearer at higher heart rates.23 We used systolic blood pressure as the haemodynamic target for optimisation because we reasoned that this would have two indications to increase if cardiac output rose: the rise in cardiac output would raise mean arterial pressure; and the rise in stroke volume would raise the pulsatility of blood pressure.
To minimise the effects of background variation in blood pressure we compared each tested AV and VV delay with an arbitrary fixed reference delay (AV 120 ms, VV 0 ms; fig 1A).23,24 This reference setting was chosen as a simple configuration attainable by all participants.
Figure 1 Example of the data acquired for measuring relative change in systolic blood pressure (BP) for the tested atrioventricular (AV) and interventricular (VV) delays. (A) Each tested AV and VV delay was compared with the reference AV and VV delays (AV 120 ms and VV 0 ms); this reference delay was returned to between each tested delay. (B) To reduce the effect of background noise the relative change in systolic blood pressure (SBPrel) (the mean of 10 beats before a change and the 10 beats immediately after a change) was calculated and the mean for at least six replicate transitions was established. (C) Thirty‐five different combinations of AV and VV delay were measured, each time returning to the reference AV and VV delay. (D) Constructing a surface plot of SBPrel for each patient tested was then possible. LV, left ventricle; RV, right ventricle.
We calculated the relative change in systolic blood pressure (SBPrel) by comparing the mean of the 10 beats immediately after a transition with the 10 beats immediately before. We used the immediate change in blood pressure because we believe that the steady state (for blood pressure) is achieved partly through vasodilatation and other peripheral responses, which dilute the effect on SBP. For SBP to reflect well the changes in cardiac function, we must make the recording before the peripheral responses have had time to fully come into effect. We therefore read the SBP for the 10 beats immediately after a transition. In this time (10 beats at 110 beats/min ≈ 5 s), the changes seen in SBP can essentially be due only to changes in cardiac function as a result of changes in pacing parameter.
We repeated the transitions, reversing the signs of SBPrel for reverse transitions, to obtain at least six replicate measurements for each SBPrel. The goal of performing reverse transitions was to cancel out any potential small drift in blood pressure trace. The Finometer is designed for long‐term monitoring of blood pressure with the aid of frequent automatic recalibration. We switched off auto recalibration to permit continuous uninterrupted blood pressure recording, and therefore the device was subject to a gradual drift in blood pressure measurement, of the order of 1–2 mm Hg/min. No recording ran for more than 5 min. We deliberately measured changes in blood pressure over a small number of beats (10 before a transition versus 10 after the transition). Therefore, slow gradual trends did not play a part in our measurements. Even the small contribution to our measurement made by these slow drifting trends were likely to be cancelled out because half our transitions were from nominal to tested AV/VV delay, and the other half were from tested to nominal; the effect of any secular drift in blood pressure is therefore self‐cancelling (fig 1B).
SBPrel was measured in the manner described above for a matrix of different AV and VV delays. Thirty‐five different combinations of AV and VV delay were tested (40, 80, 120, 140,160 and 200 ms for AV delay; left‐first 60, 40, 20 and 0, and right‐first 20 and 40 ms for VV delay; fig 1C). The tested AV and VV delays were changed simultaneously.
Reproducibility
Of these 15 patients, eight were enrolled into a reproducibility substudy, which involved an additional visit (mean interval two months) at which the matrix of 35 different combinations of AV and VV delays were retested. We also calculated the reproducibility of SBPrel in individual patients and the reproducibility of the AV delay identified as optimal.
Statistics
The SBPrel value was determined for each tested combination of AV and VV delay in relation to a reference AV delay (120 ms) and VV delay (0 ms) by taking the mean of observed blood pressure changes from at least six individual transitions. Pairs were compared by Student's paired t test. Proportions were compared by Fisher's exact test. A value of p < 0.05 was taken as significant. The statistical package Statview V.5.0 (SAS Institute Inc, Cary, North Carolina, USA) was used for analysis.
RESULTS
Identification of a haemodynamic peak
The individual AV delay corresponding to the maximum acute SBPrel was clearly identifiable in each of the patients tested. The AV delay corresponding to the peak blood pressure was individual to each patient (range 135–200 ms, mean 168 ms).
Adjustment of VV delay also resulted in a clearly identifiable peak in the majority of subjects (12 of 15). This peak was also individual to the patient (ranging from left‐first by 60 ms to right‐first by 40 ms; mean left‐first 8 ms). In only two of 15 patients was the optimal VV delay 0 ms. In the patients without a clear peak, changing VV delay had a significantly smaller effect on acute haemodynamic response (mean within‐patient range of SBPrel 2.3 mm Hg) than in patients with a clear peak (4.7 mm Hg, p = 0.04).
Size of effect on SBPrel caused by changes in AV and VV delay
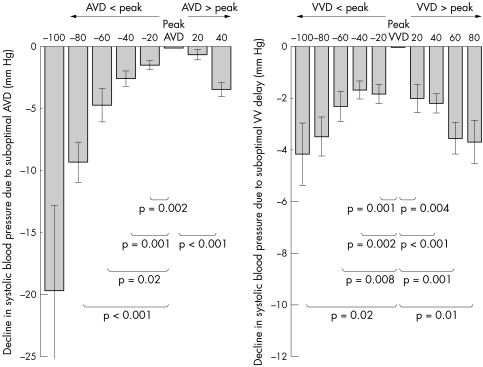
To compare the significance of non‐peak pacing parameters across all the patients, we aligned the individual patients' data to centre on the AV and VV delay giving the haemodynamic peak. This allowed us to quantify, in the overall group, the effect on blood pressure of deviating the pacemaker configuration away from the haemodynamic peak.
Even small changes in AV delay away from the patients' individual haemodynamic optimum had detectable effects on acute blood pressure (fig 2). By moving the delay just 40 ms away from peak, SBPrel fell by 3.4 (0.6) mm Hg (p < 0.001). The further away from peak, the greater the fall in blood pressure. At 60 ms away from peak, SBPrel fell by 4.7 (1.4) mm Hg (p = 0.02). At 80 ms, SBPrel fell by 9.2 (1.6) mm Hg (p < 0.001).
Figure 2 Haemodynamic consequence of non‐peak atrioventricular (AVD) and interventricular delays (VVD). Even small changes away from peak AVD or VVD have significant haemodynamic consequences. The results of all patients are shown after they have been aligned according to their individual peak AVD and VVD.
Similarly, VV delay had an important additional effect on acute haemodynamic response. Moving just 40 ms away from peak caused a 2.2 (0.4) mm Hg fall in SBPrel (p < 0.001), and at 60 ms SBPrel fell by 3.6 (0.6) mm Hg (p = 0.01).
The overall range of SBPrel was significantly less for adjustments in VV delay than for similar adjustments in AV delay. Comparison of the range of blood pressure, over the tested AV delays of 80–160 ms (this range of AV delay was selected so that it was a comparable range with VV delay), was 10 mm Hg, and the range for VV delay left‐first 60 ms to right‐first 40 ms was only 4 mm Hg (p < 0.001), even though the VV range was slightly broader in milliseconds. VV delay therefore appears to have an additional, but smaller, effect on acute haemodynamic response.
Parabolic shape of haemodynamic response
The effects of changing AV and VV delay on acute blood pressure are curvilinear rather than linear and fit closely to a quadratic equation. The curve of response to AV delay fitted extremely closely to a parabola. The example patient shown in fig 3 has an R2 for fitting to a parabola of 0.99, which is typical of the overall group, whose mean R2 for fitting to a parabola was 0.97. The average residual variance away from the parabola was only 0.8 mm Hg2.
Figure 3 Haemodynamic responses to atrioventricular (AV) and interventricular delays (VVD), with best‐fit parabolas superimposed. Effect on relative change in systolic blood pressure (SBPrel) of changes in AVD (left) and VVD (right) in a single patient. The best‐fit parabola for the patient is shown as the solid line (positive VVD denotes left ventricle paced first, negative denotes right ventricle paced first).
The response to VV delay adjustments was significantly less curved than that to AV delay adjustments (quadratic coefficient 67 v 1194 mm Hg/s2, p = 0.003). Although residual variance was equally small (0.8 mm Hg2), the R2 value for fitting to a parabola was therefore somewhat lower at 0.7.
This information may allow investigators developing haemodynamic optimisation protocols to improve the efficiency and speed of their protocols, as the close fit to a parabola means that testing a few points may provide almost all the information available for testing large numbers of points.
Three‐dimensional representation of acute changes in blood pressure
The AV delay parabolic curve displays the effect of changing AV delay alone. Similarly, the VV delay curve displays the effect of changing VV delay alone. Because a grid of different AV and VV delays was tested, the haemodynamic effect of simultaneous adjustment of AV and VV delays can be conveniently displayed as a surface plot (fig 4).
Figure 4 Haemodynamic response to testing a grid of atrioventricular (AV) and interventricular (VV) delays in four patients. These four panels show, for four different patients, the acute haemodynamic effects of a range of AV and VV delays. The three‐dimensional surfaces are obtained from the best‐fit quadratic equations for AV and VV delays in the individual patients. Each patient has the haemodynamic peak at a different position and different degrees of curvature in the AV and VV directions (positive VV delay denotes left ventricle paced first, negative denotes right ventricle paced first).
Reproducibility
Eight patients participated in a reproducibility substudy. The mean absolute difference between the two determinations, two months apart, of AV delay yielding peak acute SBP was 11 (SD 9) ms and for VV delay it was 9 (SD 8) ms.
The reproducibility, at two months, of SBPrel calculated for each tested AV and VV delay was also calculated (fig 5 shows an example). For AV delay the SD of the difference between two measurements of SBPrel, two months apart, was 2.5 mm Hg, which is 2% of the mean SBP. For VV delay this was 1.5 mm Hg, which is 1% of the mean SBP.
Figure 5 Example of reproducibility curves for an individual patient. The second test was two months after the first. AV, atrioventricular; SBPrel, relative change in systolic blood pressure; VV, interventricular.
The reproducibility of the six measures of SBPrel made for each tested pacing configuration, made at visit one, for individual patients was calculated. The mean SD of the difference, across all patients, of individual measurements of SBPrel was 3.9 mm Hg, which is 3.4 % of the mean SBP. With SBPrel calculated as a mean of these six values, the implied reproducibility expressed as the SD of difference between successive estimates of the mean is 3.9 / √6 = 1.6 mm Hg, which is 1.4% of mean SBP.
DISCUSSION
In this study we have found that changes in AV and VV delays are haemodynamically important and result in a curvilinear acute blood pressure response. We have found that the haemodynamic curves fit very closely to a parabola. Adjustment of VV delay produces an additional but smaller haemodynamic benefit to AV optimisation. The degree of curvature and resulting range of blood pressure are significantly less for the VV haemodynamic curves. Individual measurements and selection of peak values show good levels of reproducibility after two months.
Potential clinical implications of parabolic shape
Previous investigators using invasive haemodynamic measures have noted a curved distribution of the haemodynamic response around peak AV delays.1 However, the characteristics of these curves have not previously been specifically defined.
Our finding that both the AV and VV delay curves fit closely to a parabola may be very helpful in designing efficient algorithms for pacemaker optimisation. The knowledge that the curve is a parabola permits a limited number of data points to be acquired, saving time.
The reason many centres do not attempt to optimise resynchronisation devices is, most commonly, the time consuming nature of echocardiographic methods and the requirement for a skilled operator to perform prolonged data acquisition. The knowledge that a parabolic fit can be applied with a limited number of acquired data points would permit an optimisation protocol to be developed with shorter acquisition time. In principle this could be applied to any form of haemodynamic measure including echocardiography or invasive haemodynamic measurement, although the maximally efficient approach (with minimal patient risk, minimal operator skill required and maximal potential for automation) may be non‐invasive haemodynamic measurements.
Development of an efficient optimisation technique may increase the number of centres that routinely optimise pacemaker settings. It could become part of clinical routine and be performed, potentially in an automated fashion, during a routine pacemaker check.
Relative importance of AV and VV delay adjustment
Previous investigators have identified incremental haemodynamic benefits, in addition to AV optimisation, when VV delay is optimised.6,11 VV and total dyssynchrony are also reduced.25 However, the relative size of the effect on acute haemodynamic response and degree of curvature have not previously been studied. Our finding that adjusting AV delay has a significantly larger haemodynamic effect than VV delay underlines the importance of selecting an appropriate AV delay.
We found that, for most patients, the VV delay giving a haemodynamic peak is not zero. This agrees with previous studies that used invasive haemodynamic measurements.11 This phenomenon may be because lead positions and underlying conduction abnormalities vary between patients, and even normal people have some differences in time of activation between myocardial regions.
Blood pressure as a haemodynamic marker for effect of changing pacing parameters
Acute changes in blood pressure are potentially a good measure of the effect of changing pacing parameters on cardiac function. Whereas one may be concerned, in principle, that a change in blood pressure would instead be a result of a change in peripheral vascular tone, it is reassuring to remember that the stimulus for such a change in vascular tone must be a change in cardiac performance. Additionally, addressing the early few seconds after a change in pacing configuration allows us to focus on the primary cardiac effects rather than any secondary (vascular) effects. Even if secondary vascular effects do occur, they are likely to attenuate, rather than augment, the changes in blood pressure, and so this approach cannot overestimate the immediate effects on cardiac function.
Size of effect
At first glance, the magnitude of the change in blood pressure may seem small when small changes are made in AV or VV delay from their haemodynamic peak (40 ms away from peak AV delay reduces blood pressure by 3.4 mm Hg; 40 ms away from peak VV delay reduces blood pressure by 2.2 mm Hg). However, even small differences in blood pressure are universally recognised to predict substantial differences in outcome in patients with chronic heart failure. For example, cross sectional observational data suggest a 4% (95% confidence interval (CI) 3% to 5%) relative increase in mortality per mm Hg decline in SBP in outpatients with chronic heart failure.26 The landmark trials with cardiac resynchronisation therapy showed an increase in blood pressure, paralleling the improvements in symptoms and survival, for patients entered into the device arm. For example, in the COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in Chronic Heart Failure) trial, patients in the resynchronisation arm initially gained about 2 mm Hg (CI not published) in SBP (in comparison with the control arm) and went on to have an 18% relative reduction (95% CI 1% increase to 42% reduction) in the combined end point of morbidity and mortality.27 Similarly, the CARE‐HF (Cardiac Resynchronization‐Heart Failure) trial showed, at three months, that the increment in blood pressure attributable to being in the device arm was 5.8 mm Hg (95% CI 3.5 to 8.2), and the mortality reduction was 37% (95% CI 23% to 49%).28
Thus, although causality between the increase in SBP in the landmark trials and clinical effect is not established, and chronic changes in blood pressure (as measured in the trials) are probably smaller than the acute ones measured in this study, it does indicate that even small differences in blood pressure can have significant clinical effects in patients with heart failure.
Study limitations
This study had a small number of patients relative to trials with event end points. However, its purpose was not to assess events or symptoms but to assess in detail the characteristics, size and reproducibility of the haemodynamic responses that can be elicited by non‐invasive monitoring during adjustment of pacing parameters.
Throughout this study we paced our patients atrially at raised heart rates. It is important to remember that the effective left atrial AV delay obtained by pacing from the right atrial appendage is likely to be considerably shorter than the value that is programmed. Also, the sensed and paced AV delays are likely to result in different effective AV delays and therefore cannot be directly compared. In particular, the optimal sensed AV delay may well differ from the optimal paced AV delay. However, we believe that the nature of the response to changing AV delay (the parabolic shape) would be similar even though it may be centred on a different point.
In this study we examined the shape of the haemodynamic response but in clinical practice it is important to recognise the potential distinction between sensed AV delay optimum and paced AV delay optimum.
Another consequence of using right atrial appendage stimulation is that it may blur what is happening to real VV delay. If left atrial activation is delayed then the benefit from activating the right ventricle first may actually be from the left ventricle being activated later.
We believe it is important to optimise delay at higher heart rates because it is during exercise with associated higher heart rates when patients with heart failure become most symptomatic. We feel that it is under these conditions, when the time available for each cardiac cycle is shorter than at resting rates, that it is crucial to establish the optimal AV delay.
In this study we did not record optimisation during exercise. Exercise is of course associated with many additional physiological changes in addition to increased heart rates. Previous investigators have shown that, in contrast to normal patients with pacemakers, in cardiac resynchronisation therapy the relatively short baseline AV delay should be prolonged at increased heart rates during exercise.29 However, the objective of this study was to investigate the characteristics of the haemodynamic curves, in particular their size, shape and the relative importance of changes in AV and VV delay. Although the AV delay determined as optimal may vary, the shape of the haemodynamic response curve (parabola) will probably be similar regardless of the method used to raise the heart rate.
In principle, we could have conducted this study at a variety of heart rates, but because of the large number of pacing configurations needing to be tested for each heart rate, we decided to limit the study to examine only one heart rate.
We decided to use a raised heart rate because in previous studies we examined in detail the effect of heart rate on acute changes in blood pressure.23 We found that at higher heart rates, altering AV delay had a more pronounced effect on blood pressure and that the peak AV delay became clearer at higher heart rates. At a range of raised heart rates (90, 110 and 130 beats/min) the response to changing AV delay is parabolic.
Conclusions
Changing AV and VV delays results in a curvilinear and reproducible acute blood pressure response. This shape fits very closely to a parabola, which may be helpful in designing a streamlined clinical protocol to select AV and VV delays corresponding to peak acute haemodynamic responses. Adjustment of VV delay provides an additional, albeit smaller, haemodynamic benefit to AV optimisation.
Abbreviations
AV - atrioventricular
CARE‐HF - Cardiac Resynchronization‐Heart Failure
COMPANION - Comparison of Medical Therapy, Pacing and Defibrillation in Chronic Heart Failure
NYHA - New York Heart Association
SBP - systolic blood pressure
SBPrel - relative change in systolic blood pressure
VV - interventricular
Footnotes
Competing interests: We acknowledge support from the British Heart Foundation, Medtronic and the Coronary Flow Trust. Dr Whinnett (FS/05/068), Dr Davies (FS/05/006) and Dr Francis (FS/04/079) are British Heart Foundation fellows. Our institution has filed a patent involving some of the techniques described in this paper.
References
- 1.Auricchio A, Stellbrink C, Block M.et al Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation 1999992993–3001. [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Kramer A, Spinelli J.et al Biventricular mechanical asynchrony predicts hemodynamic effect of uni‐ and biventricular pacing. Am J Physiol Heart Circ Physiol 2003285H2788–H2796. [DOI] [PubMed] [Google Scholar]
- 3.Butter C D, Auricchio A, Stellbrink C.et al Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 20011043026–3029. [DOI] [PubMed] [Google Scholar]
- 4.Breithardt O, Stellbrink C, Franke A.et al Acute effects of cardiac resynchronization therapy on left ventricular Doppler indices in patients with congestive heart failure. Am Heart J 200214334–44. [DOI] [PubMed] [Google Scholar]
- 5.Auricchio A, Ding J, Spinelli J C.et al Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol 2002108929–932. [DOI] [PubMed] [Google Scholar]
- 6.Van Gelder B M, Bracke F A, Meijer A.et al Effect of optimizing the VV interval on left ventricular contractility in cardiac resynchronisation therapy. Am J Cardiol 2004931500–1503. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G S, Berger R D, Fetics B J.et al Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle‐branch block. Circulation 20001023053–3059. [DOI] [PubMed] [Google Scholar]
- 8.Kass D A, Chen C H, Curry C.et al Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999991567–1573. [DOI] [PubMed] [Google Scholar]
- 9.Blanc J J, Etienne Y, Gilard M.et al Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation 1997963273–3277. [DOI] [PubMed] [Google Scholar]
- 10.Sawhney N S, Waggoner A D, Garhwal S.et al Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm 20045562–567. [DOI] [PubMed] [Google Scholar]
- 11.Perego G B, Chianca R, Facchini M.et al Simultaneous vs. sequential biventricular pacing in dilated cardiomyopathy: an acute hemodynamic study, Eur J Heart Fail 20035305–313. [DOI] [PubMed] [Google Scholar]
- 12.Bordachar P, Lafitte S, Reuter S.et al Echocardiographic parameters of ventricular dyssynchrony validation in patients with heart failure using sequential biventricular pacing. J Am Coll Cardiol 2004442157–2165. [DOI] [PubMed] [Google Scholar]
- 13.Porciani M C, Dondina C, Macioce R.et al Echocardiographic examination of atrioventricular and interventricular delay optimization in cardiac resynchronization therapy. Am J Cardiol 2005951108–1110. [DOI] [PubMed] [Google Scholar]
- 14.Sogaard P, Egeblad H, Pedersen A K.et al Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation 20021062078–2084. [DOI] [PubMed] [Google Scholar]
- 15.Peňaz J. Photoelectric measurement of blood pressure, volume and flow in the finger. In: Albert R, Vogt WS, Helberg W, eds. Digest of the International Conference on Medicine and Biological Engineering. Dresden: Conference Committee of the Xth International Conference on Medicine and Biological Engineering, 1973104
- 16.Wesseling K H, De Wit B, Van der Hoeven G M.et al Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis 19953667–82. [Google Scholar]
- 17.Smith N T, Wesseling K H, de Wit B. Evaluation of two prototype devices producing non‐invasive, pulsatile, calibrated blood pressure measurement from a finger. J Clin Monit 1985117–29. [DOI] [PubMed] [Google Scholar]
- 18.Van Egmond J, Hasenbos M, Crul J F. Invasive v. non‐invasive measurement of arterial pressure: comparison of two automatic methods and simultaneously measures direct intra‐arterial pressure, Br J Anaesth 198557434–444. [DOI] [PubMed] [Google Scholar]
- 19.Petersen M E, Williams T R, Sutton R. A comparison of non‐invasive continuous finger blood pressure measurement (Finapres) with intra‐arterial pressure during prolonged head‐up tilt. Eur Heart J 1995161647–1654. [PubMed] [Google Scholar]
- 20.Jellema W T, Imholz P M, van Goudoever J.et al Finger arterial versus intrabrachial pressure and continuous cardiac output during head‐up tilt testing in healthy subjects. Clin Sci 199691193–200. [DOI] [PubMed] [Google Scholar]
- 21.Imholz B P M, Weiling W, Montfrans G A.et al Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 199838605–616. [DOI] [PubMed] [Google Scholar]
- 22.Davies L C, Francis D P, Jurak P.et al Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clin Sci(Lond) 199997515–522. [PubMed] [Google Scholar]
- 23.Whinnett Z I, Davies J E R, Willson K.et al Determination of optimal atrioventricular delay for cardiac resynchronization therapy using acute non‐invasive blood pressure. Europace 20068358–366. [DOI] [PubMed] [Google Scholar]
- 24.Whinnett Z I, Davies J E R, Willson K.et al Continuous non‐invasive hemodynamic monitoring to optimize atrioventricular delay in cardiac resychronization therapy. J Am Coll Cardiol 20054599A [Google Scholar]
- 25.Vanderheyden M, De Backer T, Rivero‐Ayerza M.et al Tailored echocardiographic interventricular delay programming further optimizes left ventricular performance after cardiac resynchronization therapy. Heart Rhythm 200521066–1072. [DOI] [PubMed] [Google Scholar]
- 26.Shamim W, Francis D P, Yousufuddin M.et al Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol 199970171–178. [DOI] [PubMed] [Google Scholar]
- 27.Bristow M R, Saxon L A, Boehmer J.et al Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 20043502140–2150. [DOI] [PubMed] [Google Scholar]
- 28.Cleland J G, Daubert J C, Erdmann E.et al The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 20053521539–1549. [DOI] [PubMed] [Google Scholar]
- 29.Scharf C, Li P, Muntwyler J.et al Rate‐dependent AV delay optimization in cardiac resynchronization therapy. PACE 200528279–284. [DOI] [PubMed] [Google Scholar]