Abstract
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) is a genetically determined heart muscle disorder presenting clinically with even lethal ventricular arrhythmias, particularly in the young and athletes. It is reported familial with recessive and most commonly dominant inheritance. Disease‐causing genes are increasingly recognised among desmosomal proteins plakoglobin, desmoplakin, plakophilin2, and desmoglein2 displaying phenotypic heterogeneity. Mutations in the plakoglobin and desmoplakin genes have been identified to underlie recessive ARVC associated with woolly hair and palmoplantar keratoderma (Naxos disease), while mutations in plakophilin2, desmoglein2 as well as desmoplakin have been identified to underlie the dominant non‐syndromic form. Preliminary genotype–phenotype assessment indicates that mutations affecting the outer dense plaque of desmosome (desmoglein2, plakoglobin, plakophilin2 and the N‐terminal of desmoplakin) result in ARVC with the ordinary described phenotype. However, mutations at the inner dense plaque, particularly affecting the desmin‐binding site of desmoplakin, may result in ARVC with predominantly left ventricular involvement and clinical overlapping with dilated cardiomyopathy. The interesting finding of abnormal distribution of plakoglobin, independently of the primarily affected protein, might suggest a common pathway for plakoglobin in ARVC pathogenesis.
Keywords: arrhythmogenic right ventricular dysplasia/cardiomyopathy, Naxos disease, cell‐adhesions, desmosomal proteins, sudden death
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) is a genetically determined heart muscle disorder which presents clinically with ventricular arrhythmias of right ventricular origin.1 The pathological process is progressive loss of right ventricular myocardium with fibro‐fatty replacement which starts from right ventricular subepicardial layers towards the endocardium. Initial changes are in the “triangle of dysplasia” (outflow tract, inflow tract and apex) but there may be diffuse involvement of the right ventricle as well as the left ventricle. The diagnostic histological feature of dead or dying myocardial fibres, replaced by fibrosis, and adipose tissue is considered the substrate for slow conduction and re‐entrant ventricular arrhythmias.1 The prevalence of ARVC is estimated to be as high as 1:1000 and it is one of the most common causes of unexpected sudden death in the young, particularly athletes.2,3
ARVC is familial, usually with autosomal dominant inheritance. Early gene identification efforts were hampered by low penetrance, age related expression and difficulties making an accurate diagnosis, partly related to problems imaging the thin walled pyramidal shaped right ventricle which often appears draped around the more spherical left ventricle. The recognition of ARVC in recessive families with an associated hair and skin phenotype4 facilitated recognition of the first disease causing gene, a deletion mutation in plakoglobin in families from Aegean islands (Naxos disease) and the subsequent finding of a C‐terminal mutation in desmoplakin in Ecuadorian families (Carvajal syndrome).5,6 The finding of disease causing mutations in the desmosomal proteins plakoglobin and desmoplakin suggested that ARVC was a disease of cell adhesion and provided candidate genes for studies of autosomal dominant families.
CELL–CELL JUNCTIONS
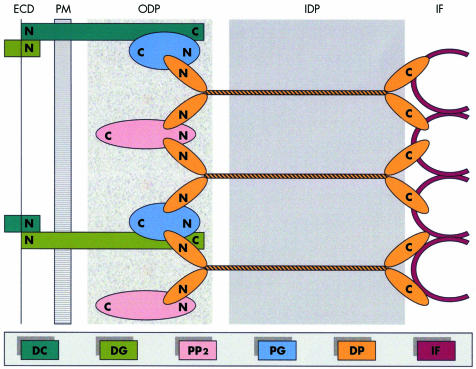
The structural and functional integrity of cardiac tissue is supported by desmosomes, adherens junctions and gap junctions located at intercalated disks.7 In desmosomes, plakoglobin, plakophilin2 and desmoplakin anchor desmin intermediate filaments to desmosomal cadherins, securing the mechanical cell–cell adhesion. Desmosomal cadherins (desmoglein and desmocollin) are transmembrane proteins forming an extracellular zipper‐like dimer with the corresponding part of desmosomal cadherins of the adjacent myocardial cell. Plakoglobin and plakophilin2 are armadillo proteins located at the outer dense plaque of desmosomes which bind with the N‐terminal of desmoplakin and with the C‐terminal of desmosomal cadherins. Desmoplakin is a larger dumbbell‐shaped molecule which makes up the inner dense plaque with its middle coiled‐coiled rod domain and binds via its C‐terminal with desmin intermediate filaments8 (fig 1). Plakoglobin is the only desmosomal protein which is also found at adherens junctions where it is involved in linking with the actin cytoskeleton of adjacent myocardial cells.9 Signalling to the nucleus and involvement in apoptotic mechanisms have also been attributed to plakoglobin.10 The integrity of desmosomes is necessary to maintain the normal function of gap junctions as intercellular channels responsible for the electrical coupling and signalling mechanisms in the regulation of cell growth, differentiation and development.11
Figure 1 A model of desmosomal plaque demonstrating the position of the major adhesive components. DP, desmoplakin; DC, desmocollin; DG, desmoglein; ECD, extracellular core domain; IDP, inner dense plaque; IF, intermediate filaments; ODP, outer dense plaque; PG, plakoglobin; PP2, plakophilin2; PM; plasma membrane.
GENOTYPE–PHENOTYPE
Plakoglobin mutation
A 2‐base‐pair deletion of the plakoglobin gene truncates the C‐terminal of the protein and causes Naxos disease, a recessive form of ARVC associated with skin and hair abnormalities.5 The clinical expression in autosomal recessive disease is more predictable and the phenotype less heterogeneous than in autosomal dominant ARVC. In Naxos disease the cutaneous abnormalities, apparent from early infancy, enabled the identification of children who would go on to develop ARVC.12 The increased mechanical stress (pressure, stretch, abrasion) which the hands and feet are exposed to from the first year of life influences the development of keratoderma on the palms and soles. In Naxos disease, the right ventricle is always involved, initially with localised kinetic abnormalities involving the triangle of dysplasia. With disease progression, the right ventricle becomes dilated and hypokinetic and there is left ventricular involvement; 10% of patients have left ventricular involvement by the second decade and 60% by the fifth decade of life. Clinical events are usually related to episodes of sustained ventricular tachycardia of left bundle branch block morphology. Arrhythmic storms are accompanied by stepwise structural progression. Involved myocardium reveals myocyte loss with fibrofatty replacement mainly at subepicardial and mediomural layers occasionally associated with an inflammatory infiltrate.13 In a child from Naxos, who was homozygous for the plakoglobin mutation, a detailed postmortem examination following a non‐cardiac death failed to reveal any macroscopic or histological cardiac abnormality. Immunohistochemical studies revealed that mutant plakoglobin failed to localise at intercellular junctions while desmoplakin, plakophilin2 and desmosomal cadherins appeared normal. Connexin43 was significantly reduced in both right and left ventricles with reduced number and size of gap junctions.11 This child had an abnormal ECG with 14 000 ventricular extrasystoles in the absence of myocyte death or fibro‐fatty replacement at postmortem examination, but with immunohistochemical evidence of altered gap junction expression of connexin43.11 This suggests that abnormalities in the mechanical junctions may modify the function of electrical coupling and cause arrhythmia in the absence of the classic pathology of myocyte loss and fibrofatty replacement.
Plakophilin2 mutations
Mutations in the plakophilin2 gene are increasingly recognised as a common cause of ARVC. In two large series of dominant ARVC, mutations in plakophilin2 were detected in 26% and 11% of probands, respectively.14,15 Most of the mutations were detected in the C‐terminal half of the molecule. The described phenotype was typical for ARVC with predominant right ventricular involvement and fibrofatty replacement of myocardium.14,15 The increased incidence of plakophilin2 mutations in ARVC as compared to plakoglobin mutations, the other armadillo desmosomal protein, might be related to the ability of the latter to be partially compensated by other adhesion proteins.5
Desmoplakin mutations
To date, five dominant and two recessive mutations in the desmoplakin gene have been identified to cause ARVC truncating either the N or the C‐terminal of the protein at the outer dense plaque or inner dense plaque of desmosomes, resulting in distinctive cardiac phenotypes.6,16,17,18,19 They were identified in 16% of probands in a series of patients with dominant ARVC.18 In those patients in whom the N‐terminal of the desmoplakin (particularly at the plakoglobin binding site) was affected, the phenotype was typical for ARVC with respect to electrocardiographic and structural abnormalities;18 while mutations which cause truncation of the protein at the C‐terminal in the inner dense plaque resulted in a broader cardiac phenotype, occasionally with predominantly left ventricular involvement.6,19 These preliminary observations related to mutations in the desmoplakin gene remain to be confirmed. A left ventricular phenotype is more notable in Carvajal syndrome, a variant of Naxos disease, in which the mutation is predicted to disrupt the desmin‐binding site at the C‐terminal of desmoplakin.6 Homozygotes with a cutaneous phenotype similar to plakoglobin present with early left ventricular involvement leading to heart failure. Nevertheless, the one heart examined in detail also exhibited right ventricular aneurysms at the “triangle of dysplasia” with myocyte loss and replacement fibrosis at subepicardial and mediomural layers, although the fatty component of the usual right ventricular replacement process was absent.20 The immunohistochemical signal for plakoglobin, desmoplakin and desmin at the level of intercalated disks was diminished.20 The mutated desmin‐binding site of desmoplakin possibly affected the architecture of intermediate filaments resulting in a DCM‐like cardiac phenotype expressed in homozygous carriers of this mutation.21
Desmoglein2 mutations
Mutations in desmoglein2 gene (the only desmoglein isoform expressed in cardiomyocytes) have been reported recently in eight probands, representing 10% of screened index cases.22 The cardiac phenotype was characterised by typical ARVC features with predominantly right ventricular involvement and fibrofatty replacement of affected myocardium. In this series, 40% of ARVC probands had a mutation in genes encoding desmosomal proteins.
PATHOGENESIS OF ARVC
Preliminary genotype–phenotype analysis is providing insights into the mechanisms of myocyte cell death and arrhythmogenesis. In the cardiocutaneous syndromes caused by cell adhesion genes, the observation that physical stress causes palmar plantar keratoderma6,23 provides a working hypothesis for myocyte cell death in ARVC. The observation that the thin walled right ventricle and the thinnest segment of the left ventricle (posterior wall) are most often involved may reflect these areas being more vulnerable to physical stress or stretch. Genetic studies have also identified desmoplakin mutations in families with an arrhythmogenic left ventricular cardiomyopathy.19 Thus, the spectrum of cell‐adhesion disease is broader than our previous clinical view of ARVC as a disease of the right ventricle.13 The relation of a particular mutation and the clinical phenotype is of interest. The left ventricle is predominantly involved when mutations disrupt the cytoskeletal integrity, as with mutations affecting the desmin binding site at the inner dense plaque,6,19 whereas mutations in cell–cell adhesion proteins functioning at the outer dense plaque of desmosomes are expressed with a predominantly right ventricular phenotype.5,14,15,18
Plakoglobin seems to play an important role in ARVC development regardless of the underlying mutation. The failure of plakoglobin to localise at the intercalated disks, not only in myocardial tissue of patients with plakoglobin mutations but also in those with desmoplakin and plakophilin2 mutations, suggests a final common pathway role for plakoglobin in the pathogenesis of ARVC.11,20 The observation, however, that β‐catenin, an important structural protein of the adherens junctions, can compensate for the mechanical function of defective plakoglobin suggest that other mechanisms related to plakoglobin may be involved. In vitro studies reveal that impaired cell–cell contact via catenin interactions signals the nucleus to initiate apoptotic cell death and repair.9 It is suggested that plakoglobin is involved in a final common pathway of defects in the desmosomal mechanical junction via its signalling role. Anecdotal reports suggest that fibrous rather than fatty replacement occurs with rapidly progressive disease without being mutation specific.13,18 We speculate that fatty replacement requires time and may not develop if the disease is acute or rapidly progressive.
It has been considered that the surviving myocardial fibres embedded within fibrous and adipose tissue alter electrical properties and provide the substrate for slow conduction and re‐entrant ventricular arrhythmias.1 However, gap junction remodelling may be considered as an alternative pathway to intraventricular slow conduction enhancing the risk of ventricular arrhythmias.11 The described case of a child with Naxos disease presenting ventricular arrhythmias before the development of pathologic changes of myocardium provides further evidence to support this hypothesis.11
CONCLUSIONS
ARVC is a disease of cell–cell adhesion since mutations in genes encoding desmosomal proteins are increasingly identified in ARVC probands. The clinical heterogeneity may in part reflect the location of the genetic abnormality; mutations affecting the outer dense plaque cause typical ARVC, while predominantly left ventricular involvement develops with mutations affecting the inner dense plaque. Results of immunohistochemical studies suggest that plakoglobin is involved in a common final pathway in ARVC pathogenesis.
ACKNOWLEDGEMENTS
This work was supported by the European Commission 5th Framework Program (ARVC/D Project, QLG1‐CT‐2000‐01091) and the British Heart Foundation.
Abbreviations
ARVC - arrhythmogenic right ventricular dysplasia/cardiomyopathy
DCM - dilated cardiomyopathy
References
- 1.Marcus F I, Fontaine G H, Guiraudon G.et al Right ventricular dysplasia: a report of 24 adult cases. Circulation 198265384–398. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D.et al Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988318129–133. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Thiene G, Corrado D.et al Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 199694983–991. [DOI] [PubMed] [Google Scholar]
- 4.Protonotarios N, Tsatsopoulou A, Patsourakos P.et al Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J 198656321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKoy G, Protonotarios N, Crosby A.et al Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 20003552119–2124. [DOI] [PubMed] [Google Scholar]
- 6.Norgett E E, Hatsell S J, Carvajal‐Huerta L.et al Recessive mutation in desmoplakin disrupts desmoplakin‐intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Molec Genet 200092761–2766. [DOI] [PubMed] [Google Scholar]
- 7.Perriard J C, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disk? Trends Cardiovasc Med 20031330–38. [DOI] [PubMed] [Google Scholar]
- 8.Green K J, Gaudry C A. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol 20001208–216. [DOI] [PubMed] [Google Scholar]
- 9.Zhurinsky J, Shtutman M, Ben‐Ze'ev A. Plakoglobin and β‐catenin: protein interactions, regulation and biological role. J Cell Sci 20001133127–3139. [DOI] [PubMed] [Google Scholar]
- 10.Brancolini C, Sgorbissa A, Schneider C. Proteolytic processing of the adherens junctions components β‐catenin and γ‐catenin/plakoglobin during apoptosis. Cell Death Differ 199851042–1050. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan S R, Gard J J, Protonotarios N.et al Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease). Heart Rhythm 200413–11. [DOI] [PubMed] [Google Scholar]
- 12.Protonotarios N, Tsatsopoulou A, Anastasakis A.et al Genotype‐phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol 2001381477–1484. [DOI] [PubMed] [Google Scholar]
- 13.Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Path 200413185–194. [DOI] [PubMed] [Google Scholar]
- 14.Gerull B, Heuser A, Wichter T.et al Mutations in the desmosomal protein plakophilin‐2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 2004361162–1164. [DOI] [PubMed] [Google Scholar]
- 15.Syrris P, Ward D, Asimaki A.et al Clinical expression of plakophilin‐2 mutations in familial arrhythmogenic right ventricular cardiomyopathy. Circulation 2006113356–364. [DOI] [PubMed] [Google Scholar]
- 16.Rampazzo A, Nava A, Malacrida S.et al Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 2002711200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcalai R, Metzger S, Rosenheck S.et al A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol 200342319–327. [DOI] [PubMed] [Google Scholar]
- 18.Bauce B, Basso C, Rampazzo A.et al Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J 2005161666–1675. [DOI] [PubMed] [Google Scholar]
- 19.Norman M, Simpson M, Mogensen J.et al Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005112636–642. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan S R, Gard J J, Carvajal‐Huerta L.et al Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc Pathol 20041326–32. [DOI] [PubMed] [Google Scholar]
- 21.Towbin J A, Bowles N E. The failing heart. Nature 2002415227–233. [DOI] [PubMed] [Google Scholar]
- 22.Pilichou K, Nava A, Basso C.et al Mutations in desmoglein‐2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 20061131171–1179. [DOI] [PubMed] [Google Scholar]
- 23.Coonar A S, Protonotarios N, Tsatsopoulou A.et al Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (Naxos disease) maps to 17q21. Circulation 1998972049–2058. [DOI] [PubMed] [Google Scholar]