Abstract
Background
Levosimendan is a novel inodilator that improves central haemodynamics and symptoms of patients with decompensated chronic heart failure. The role, however, of repeated levosimendan infusions in the management of these patients has not yet been properly assessed.
Purpose
This randomised placebo‐controlled trial investigated the effects of serial levosimendan infusions on cardiac geometry and function, and on biomarkers of myocardial injury and neurohormonal and immune activation (troponin T, N‐terminal B‐type natriuretic pro‐peptide (NT‐proBNP), C reactive protein (CRP) and interleukin (IL) 6) in patients with advanced heart failure.
Methods
25 patients with decompensated chronic heart failure were randomised (2:1) to receive five serial 24‐h infusions (every 3 weeks) of either levosimendan (n = 17) or placebo (n = 8), and were evaluated echocardiographically and biochemically before and after each drug infusion and 30 days after the final infusion.
Results
Following treatment, cardiac end‐systolic and end‐diastolic dimension and volume indices were significantly reduced only in the levosimendan‐treated patients (p<0.01). A significant decrease in NT‐proBNP (p<0.01), high‐sensitivity CRP (p<0.01) and plasma IL6 (p = 0.05) was also observed in the levosimendan group, whereas these markers remained unchanged in the placebo group; similar changes were observed after each drug infusion. Although the number of patients with a positive troponin T (⩾0.01 ng/ml) was not different between the two groups at baseline, it was significantly higher in the placebo‐treated group during the final evaluation (p<0.05).
Conclusion
Serial levosimendan treatments improved left ventricular performance and modulated neurohormonal and immune activation beneficially in patients with advanced heart failure, without increasing myocardial injury.
Traditional inotropic agents, such as β‐adrenergic agonists, exert their positive inotropic effects by increasing intracellular calcium levels, which may, however, enhance myocardial energy consumption, promote cardiotoxicity and cardiomyocyte death, induce fatal arrhythmias and increase mortality.1,2 On the other hand, calcium sensitisers such as levosimendan may enhance myocardial performance and contractility, without promoting intracellular calcium overloading, increasing myocardial oxygen consumption or causing myocardial injury.3 Preliminary observations suggest that a single 24‐h infusion of levosimendan in patients with severe heart failure due to left ventricular systolic dysfunction results in beneficial haemodynamic changes, relief of symptoms and reduction in short‐term morbidity and mortality compared with placebo or dobutamine.4,5 A single levosimendan infusion also seems to have anti‐inflammatory and antiapoptotic properties in decompensated chronic heart failure, reducing circulating pro‐inflammatory cytokines and soluble apoptosis mediators.6 However, no sufficient data exist about the role of serial levosimendan infusions in the management of patients with advanced heart failure. In this randomised, placebo‐controlled study, we tested the hypothesis that serial levosimendan infusions improve left ventricular contractility and geometry, and modulate beneficially immune/neurohormonal activation in patients with advanced heart failure, without increasing markers of myocardial injury such as troponin T.
Patients and methods
Study population
Participants for the study were recruited from patients with systolic left ventricular dysfunction and New York Heart Association functional class III or IV symptoms of heart failure who were admitted to the Second Department of Cardiology and Heart Failure Clinic, Attikon University Hospital, Athens, Greece, for the management of advanced disease. Patients were screened for the study if they were currently taking angiotensin‐converting enzyme inhibitors and diuretics and had a documented left ventricular ejection fraction of ⩽30%. Exclusion criteria were acute or chronic infectious or inflammatory diseases, recent myocardial infarction (<8 weeks) or active myocardial ischaemia, hepatic or renal impairment (creatinine >221 μmol/l), use of immunosuppressive drugs, serious arrhythmias and supine systolic blood pressure <85 mm Hg. Twenty five patients were finally enrolled. The institutional ethics committee approved the study and all patients gave written consent.
In this open‐label study, patients were randomly allocated (2:1) to receive five repetitive infusions (every 3 weeks) with either levosimendan (n = 17) or placebo (n = 8). Levosimendan was given as a 10‐min bolus intravenous injection of 6 μg/kg followed by a continuous 24‐h infusion, initially at a rate of 0.1 μg/kg/min; in non‐responding patients, uptitration was carried out until a maximum rate of 0.4 μg/kg/min, as described previously.7 A 24‐h continuous infusion of dextrose 5% was given to the placebo‐treated group. Patients were clinically, echocardiographically and biochemically assessed before and 24 h after the end of each drug infusion, and re‐evaluated 30 days after the final infusion.
Echocardiographic and biochemical measurements
Left ventricular dimension and volume indices (dimensions or volumes/body surface area), ejection fraction and end‐systolic wall stress were assessed echocardiographically by a Vivid 7 computed sonography system (GE Medical Systems, Waukesha, Wisconsin, USA).8 Plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and cardiac troponin T levels were measured by electrochemiluminescence immunoassays with an Elecsys 2010 automatic analyser (Elecsys proBNP and troponin T; Roche Diagnostics, Mannheim, Germany). High‐sensitivity C reactive protein (CRP) level was measured by particle‐enhanced immunonephelometry with the Behring Nephelometer Analyzer using the relevant kit (Dade Behring, Marburg, Germany). Plasma samples were assayed in duplicate for pro‐inflammatory cytokine interleukin 6 (IL6) concentrations using commercially available enzyme‐linked immunosorbent assay kits (R&D systems, Minneapolis, Minnesota, USA). The intra‐assay and interassay coefficients of variation were <8% for ELISA in our laboratory. All laboratory tests were conducted by staff who was blinded to the treatment status of the individual patients.
Statistics
Data were statistically analysed using SPSS v.12.0. Continuous variables are expressed as mean (standard deviation (SD)). Categorical variables were compared using the χ2 test. Mean values were compared between groups using Student's t test or the Mann–Whitney U test, according to whether variables were normally distributed or not, as tested by the Kolmogorov–Smirnov test. Similarly, paired t test or Wilcoxon's paired test were used, respectively, to compare mean values before and after each therapeutic intervention as well as between baseline and final evaluation. Bivariate correlation was used to investigate potential relationships between variables. A value of p<0.05 was considered significant.
Results
Table 1 summarises the baseline features in the levosimendan‐treated and the placebo‐treated groups. The two groups were well matched regarding the demographics, clinical and echocardiographic characteristics, and various co‐treatments. Conventional treatment remained unchanged in the levosimendan‐treated patients during the whole study period. In contrast, six placebo‐treated patients needed a higher dosage of furosemide than at baseline because of worsening of symptoms.
Table 1 Baseline characteristics in the levosimendan‐treated and placebo‐treated groups.
| Levosimendan (n = 17) | Placebo (n = 8) | p Value* | |
|---|---|---|---|
| Age (years) | 67 (6) | 70 (8) | NS |
| Male:female | 16:1 | 7:1 | NS |
| NYHA class III/IV | |||
| 7/10 | 4/4 | NS | |
| BSA (m2) | 1.95 (0.18) | 1.97 (0.11) | NS |
| Cardiomyopathy | NS | ||
| Ischaemic/dilated | 14/3 | 7/1 | |
| Heart rate (beats/min) | 74 (9) | 73 (8) | NS |
| Systolic blood pressure (mm Hg) | 117 (14) | 110 (15) | NS |
| Diastolic blood pressure (mm Hg) | 73 (8) | 71 (8) | NS |
| LV end‐diastolic diameter index (mm/m2) | 36 (4) | 37 (4) | NS |
| LV end‐systolic diameter index (mm/m2) | 31 (5) | 31 (4) | NS |
| LV ejection fraction (%) | 22 (4) | 23 (4) | NS |
| LV end‐diastolic volume index (ml/m2) | 133 (25) | 144 (29) | NS |
| LV end‐systolic volume index (ml/m2) | 95 (28) | 98 (25) | NS |
| LV end‐systolic wall stress (g/cm2) | 859 (129) | 807 (107) | NS |
| Serum creatinine (μmol/l) | 115 (27) | 124 (18) | NS |
| Drugs | NS | ||
| ACE inhibitors | 17 | 8 | |
| Diuretics | 17 | 8 | |
| β‐blockers | 14 | 6 | |
| Aldosterone antagonists | 9 | 4 | |
| Amiodarone | 7 | 3 |
Values are mean (SD).
ACE, angiotensin‐converting enzyme; BSA, body surface area; LV, left ventricular; NS, not significant; NYHA, New York Heart Association.
*Student's t test, Mann–Whitney U test or χ2 test, as appropriate.
Table 2 shows the echocardiographic measurements and plasma or serum biomarkers at baseline and at final evaluation. Levosimendan infusion was done at a rate of 0.1 μg/kg/min in all but two non‐responding patients, who were uptitrated to 0.2 μg/kg/min. Treatment was well tolerated by all patients, with maintenance of blood pressure and no significant increase in heart rate. At the final evaluation, 114 days after baseline assessment, an improvement in New York Heart Association functional status was observed only in the levosimendan‐treated patients. In the same group, left ventricular ejection fraction was also significantly improved, whereas left ventricular diameter and volume indices and left ventricular end‐systolic wall stress were all significantly reduced (all p<0.01). In the placebo‐treated patients, in contrast, left ventricular ejection fraction remained practically unaffected, whereas left ventricular end‐diastolic diameter index, left ventricular volume indices and left ventricular end‐systolic wall stress were all significantly higher at the final evaluation than the baseline values (all p<0.05). Accordingly, plasma NT‐proBNP level was significantly reduced after the serial levosimendan infusions (p<0.01), whereas it was significantly increased in the placebo group (p<0.01). Levosimendan treatment also induced a significant reduction in CRP and IL6 levels (p<0.01 and p = 0.05, respectively), whereas both inflammatory markers remained unaffected in the placebo group. Figure 1 shows the changes (%) between baseline and final evaluations in echocardiographic and biochemical markers in the two study arms. Finally, although the number of cases with a positive troponin T (⩾0.01 ng/ml) did not differ between the two groups at baseline, it was significantly higher in the placebo‐treated group during the final evaluation (p<0.05). It should be stated that “positive troponin T” represents only a mild elevation, not exceeding 0.1 ng/ml in most cases.
Table 2 Echocardiographic and biochemical measurements of treated patients at baseline and at final evaluation.
| Levosimendan (n = 17) | Placebo (n = 8) | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| NYHA class: II/III/IV | 0/7/10 | 10/7/0 | 0/4/4 | 0/3/5 |
| Heart rate (beats/min) | 74 (9) | 77 (10) | 73 (8) | 76 (10) |
| Systolic blood pressure (mm Hg) | 117 (14) | 114 (16) | 110 (15) | 110 (13) |
| Diastolic blood pressure (mm Hg) | 73 (8) | 70 (8) | 71 (8) | 69 (4) |
| LV end‐diastolic diameter (mm) | 36 (4) | 34 (4)* | 37 (4) | 39 (4)* |
| LV end‐systolic diameter (mm) | 31 (5) | 29 (6)* | 31 (4) | 32 (4) |
| LV ejection fraction (%) | 22 (4) | 26 (5)* | 23 (4) | 22 (4) |
| LV end‐diastolic volume index (ml/m2) | 133 (25) | 120 (28)* | 144 (29) | 156 (30)* |
| LV end‐systolic volume index (ml/m2) | 95 (28) | 80 (25)* | 98 (25) | 106 (22)* |
| LV end‐systolic wall stress (g/cm2) | 859 (129) | 748 (221)* | 807 (107) | 828 (105)* |
| NT‐proBNP (pg/ml) | 1547 (347) | 966 (363)* | 1302 (302) | 1529 (321)* |
| Serum creatinine (μmol/l) | 115 (27) | 115 (18) | 124 (18) | 133 (35) |
| hsCRP (ng/ml) | 9.3 (2.5) | 7.2 (4.1)* | 10.1 (3.1) | 10.5 (3.5) |
| Interleukin 6 (pg/ml) | 13.1 (3.8) | 10.8 (7.2)* | 18.1 (3.0) | 17.8 (3.0) |
| Troponin T: negative/positive† | 5/12 | 6/11 | 3/5 | 1/7 |
hsCRP, high‐sensitivity C reactive protein; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
*Significantly different versus the corresponding value before treatment in the same group (paired t test, Wilcoxon's paired test).
†Positive troponin T is defined as ⩾0.01 ng/ml.
Figure 1 Changes (%) between baseline and final evaluation in echocardiographic variables and neurohormonal and inflammatory markers in the levosimendan‐treated and the placebo‐treated groups. EF, ejection fraction; IL6, inteleukin –6; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
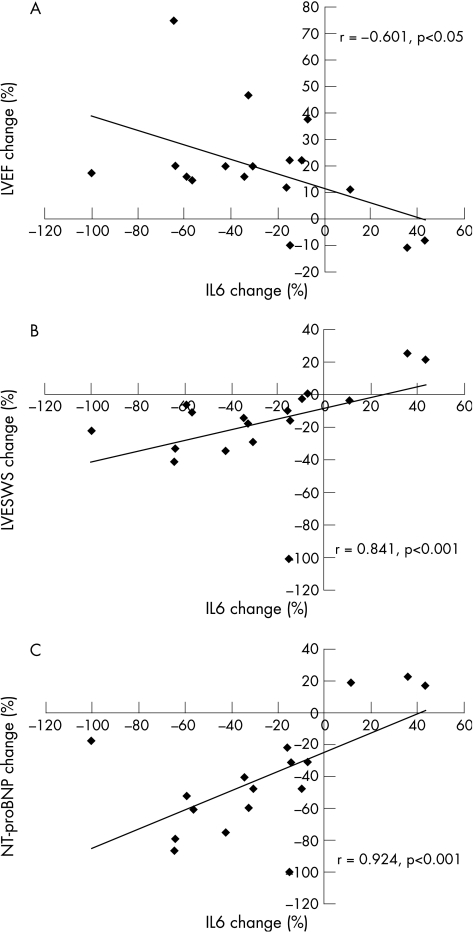
The observed changes in inflammatory markers between baseline and final evaluation were significantly correlated with those in echocardiographic and neurohormonal indices. More specifically, IL6 change (%) was significantly correlated with changes (%) in left ventricular ejection fraction (r = −0.601, p<0.05), left ventricular end‐systolic wall stress (r = 0.841, p<0.001) and NT‐proBNP (r = 0.924, p<0.001; fig 2A–C, respectively). Similarly, CRP change (%) was significantly correlated with changes (%) in left ventricular ejection fraction (r = −0.613, p<0.01), left ventricular end‐systolic wall stress (r = 0.673, p<0.01) and NT‐proBNP (r = 0.586, p<0.05).
Figure 2 Correlations between levosimendan‐induced reduction (%; baseline versus final evaluation) in plasma interleukin 6 (IL6) and respective changes in the (A) left ventricular ejection fraction (LVEF), (B) left ventricular end‐systolic wall stress (LV ESWS) and (C) plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP).
Discussion
Cardiac remodelling is the currently accepted mechanism for the clinical development and deterioration of patients with chronic heart failure.9,10 Although several factors contribute to this process, activation of the deleterious neurohormonal systems and pro‐inflammatory cytokines has a key role.9,10 An essential cellular pathway associated with excessive neurohormonal activation that leads to progression of left ventricular remodelling is the loss of cardiomyocytes through apoptosis and necrosis.9,10 Plasma biomarkers such as cardiac troponin T and troponin I have been used to detect ongoing myocardial injury during the deterioration of patients with chronic heart failure, and to predict adverse cardiac events identifying patients at high risk.11,12 Additionally, circulating BNP, IL6 and serum CRP are established markers of neurohormonal/immune activation and have been used for risk stratification in chronic heart failure.13,14 More specifically, sustained high plasma levels of these factors (despite optimum medical treatment) are independent risk factors for morbidity and mortality in patients with advanced disease.13
The present study shows for the first time that serial levosimendan infusions lead to sustained improvement in left ventricular performance, as expressed by the chronic increase in the left ventricular ejection fraction and the reduction in cardiac wall stress and cardiac volumes. Previous observations have shown that a single bolus or 24‐h infusion of levosimendan causes a haemodynamic improvement by reducing cardiac wall stress and left ventricular filling pressures, and improving left ventricular contractility without increasing myocardial oxygen uptake.15,16 These findings are limited for a short period (about 10 days) after drug infusion. Recently, Nanas et al17 have reported that intermittent, long‐term, concomitant dobutamine and levosimendan infusions in severe heart failure refractory to dobutamine alone lead to clinical and haemodynamic improvement in patients, also increasing their 45‐day survival rates. We have extended these observations by showing that serial 24‐h levosimendan infusions alone every 3 weeks lead to chronic unloading of the failing heart and to the attenuation of cardiac dilatation, constituting a promising therapeutic approach for cardiac recovery in patients with advanced heart failure. This is consistent with the fact that the OR‐1896 metabolite of levosimendan, which is haemodynamically active with properties similar to those of the parent drug, has a half life of about 70–80 h, and thus the haemodynamic effects of levosimendan should theoretically persist for at least 1–2 weeks after the intravenous infusion.3
Moreover, cardiac unloading by levosimendan treatment seems to be related to the drug‐induced beneficial modulation of biochemical and metabolic substrate of the failing myocardium as expressed by the marked reduction in biomarkers of neurohormonal and immune activation, such as plasma NT‐proBNP, IL6 and CRP. These favourable biochemical properties of levosimendan seem to be maintained at the final evaluation (30 days after the final infusion), as compared with the previous studies,6,18,19 which have shown that drug immunomodulatory effects are limited to only 2–5 days after a single infusion.
Traditional inotropes acting through β‐adrenergic stimulation lead to intracellular calcium overloading and worsening of the cardiac metabolic status; thus, they may cause cardiomyocyte loss via the activation of apoptosis and necrosis.1,2,16 Both single and serial levosimendan infusions seem to exert positive inotropic action without increasing markers of myocardial injury such as troponin T. This is quite an important observation, since an increase in troponin T during the course of chronic heart failure predicts increased mortality,11,12 and may be explained by the fact that levosimendan does not cause intracellular calcium overloading and enhanced oxidative stress, adversely affecting the cardiac metabolic status. Additionally, serial levosimendan infusions seem to counteract effectively the deleterious effects of neurohormonal systems, which are responsible for the cardiomyocyte loss and myocardial injury in chronic heart failure.20
In conclusion, we have shown that serial levosimendan infusions induced sustained improvement in left ventricular volumes and contractile performance in patients with advanced heart failure, with a parallel beneficial modulation of activated neurohormonal systems, without causing myocardial injury. Although the long‐term effects of serial levosimendan infusions remain unknown, this therapeutic strategy may be a promising approach—as an alternative to interventional procedures—for the attenuation of progressive cardiac dysfunction in patients with advanced heart failure.
Abbreviations
CRP - C reactive protein
NT‐proBNP - N‐terminal pro‐B‐type natriuretic peptide
Footnotes
Competing interests: None declared.
References
- 1.Chatterjee K, Wolfe C L, De Marco T. Nonglycoside inotropes in congestive heart failure: are they beneficial or harmful? Cardiol Clin 19941263–72. [PubMed] [Google Scholar]
- 2.Remondino A, Kwon S H, Communal C.et al Beta‐adrenergic receptor‐stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c‐Jun NH2‐terminal kinase‐dependent activation of the mitochondrial pathway. Circ Res 200392136–138. [DOI] [PubMed] [Google Scholar]
- 3.McBride B, White M. Levosimendan: implications for clinicians. J Clin Pharmacol 2003431071–1081. [DOI] [PubMed] [Google Scholar]
- 4.Moiseyev V S, Poder P, Andrejevs N.et al Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo‐controlled, double‐blind study (RUSSLAN). Eur Heart J 2002231422–1432. [DOI] [PubMed] [Google Scholar]
- 5.Follath F, Cleland J G, Just H.et al Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial, Lancet 2002360196–202. [DOI] [PubMed] [Google Scholar]
- 6.Parissis J, Adamopoulos S, Antoniades C.et al Effects of levosimendan on circulating pro‐inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol 2004931309–1312. [DOI] [PubMed] [Google Scholar]
- 7.Slawsky M T, Colucci W S, Gottlieb S S.et al Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 20001022222–2227. [DOI] [PubMed] [Google Scholar]
- 8.Gould K L, Lipscomb K, Hamilton G W.et al Relation of left ventricular shape, function and wall stress in man. Am J Cardiol 197434627–634. [DOI] [PubMed] [Google Scholar]
- 9.Francis G. Pathophysiology of chronic heat failure. Am J Med 2001110S37–S46. [Google Scholar]
- 10.Mann D L. Mechanisms and models in heart failure: a combinatorial approach. Circulation 1999100999–1008. [DOI] [PubMed] [Google Scholar]
- 11.Perna E, Macin S, Cimbaro C.et al Ongoing myocardial injury in stable severe heart failure: value of cardiac troponin T monitoring for high‐risk patient identification. Circulation 20041102376–2382. [DOI] [PubMed] [Google Scholar]
- 12.Del Carlo C, Pereira‐Barreto A, Cassaro‐Strunz C.et al Serial measure of cardiac troponin T levels for prediction of clinical events in decompensated heart failure. J Card Fail 20041043–48. [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Tsutamoto T, Wada A.et al High levels of plasma brain natriuretic peptide and interleukin‐6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000361587–1593. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel A, Martinsson A, Wretlind B.et al IL‐6 levels in acute and post myocardial infarction: their relation to CRP levels infarction size, left ventricular systolic function, and heart failure. Eur J Intern Med 200415523–528. [DOI] [PubMed] [Google Scholar]
- 15.Michaels A D, McKeown B, Kostal M.et al Effects of intravenous levosimendan on human coronary vasomotor regulation, left ventricular wall stress, and myocardial oxygen uptake. Circulation 20051111504–1509. [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G, Pieske B, Castell M.et al Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation 1998982141–2147. [DOI] [PubMed] [Google Scholar]
- 17.Nanas J, Papazoglou P, Tsagalou E.et al Efficacy and safety of intermittent, long‐term, concomitant dobutamine and levosimendan infusions in severe heart failure refractory to dobutamine alone. Am J Cardiol 200595768–771. [DOI] [PubMed] [Google Scholar]
- 18.Kyrzopoulos S, Adamopoulos S, Parissis J.et al Levosimendan reduces plasma B‐type natriuretic peptide and interleukin‐6, and improves central hemodynamics in severe heart failure patients. Int J Cardiol 200599409–413. [DOI] [PubMed] [Google Scholar]
- 19.Avgeropoulou C, Andreadou I, Markantonis‐Kyroudis S.et al The Ca(2+)‐sensitizer levosimendan improves oxidative damage, BNP and pro‐inflammatory cytokine levels in patients with advanced decompensated heart failure in comparison to dobutamine. Eur J Heart Fail 20057882–887. [DOI] [PubMed] [Google Scholar]
- 20.Adamopoulos S, Parissis J, Kremastinos D. A glossary of circulating cytokines in chronic heart failure. Eur J Heart Fail 20013517–526. [DOI] [PubMed] [Google Scholar]