Abstract
Objective
To examine the hypothesis that degenerative aortic stenosis (AS) is associated with the development of blood vessels and the expression of the secreted protein, acidic and rich in cysteine/osteonectin (SPARC), a matricellular protein that is involved in ossification, the modulation of angiogenesis and the production of metalloproteinases.
Methods
30 surgically excised AS valves and 20 normal aortic valves were studied.
Results
Blood vessels were detected in the aortic valves from patients with degenerative AS, whereas normal valves were avascular structures. Blood vessels in AS valves expressed endothelial nitric oxide synthase, CD34 and von Willebrand factor (vWF). Blood vessels were located in three distinct regions: near calcified nodules, under the leaflet border and in rich cellular areas forming cell islands. Blood vessels were predominantly present in early and intermediate grades of calcification. Cell islands were densely populated by CD45‐positive cells where endothelial cells (CD34+, vWF+) forming cord‐like structures were present. Immunoblotting detected SPARC only in AS valves and immunohistological analysis located SPARC in mature blood vessels. The proportion of blood vessels positive for SPARC was higher in valves with a lower grade of calcification. In cell islands, SPARC was distributed to mature blood vessels and to macrophages, where it co‐located with matrix metalloproteinase‐9, whereas no expression was detected in endothelial cells forming cord‐like structures.
Conclusion
The localisation of SPARC to mature blood vessels and its predominant expression in AS valves with a lower calcification grade suggest that the spatial and temporal distribution of this matricellular protein is tightly controlled to participate in the neovascularisation of AS valves.
Degenerative aortic stenosis (AS) is the most prevalent valve disease in developed countries. Its prevalence increases with age, and echocardiographic evidence of aortic valve calcification is detected in 13% of the population older than 75 years, whereas severe AS is found in up to 3%.1 Observations support a role for an atherosclerotic process in the pathophysiology leading to degenerative AS.2,3,4 Atherosclerosis is an inflammatory disease characterised by the expression of growth factors that promote neovascularisation.5,6 In AS valves, neovascularisation has been documented and has been correlated with the presence of inflammatory infiltrates and the degree of calcification.7 Neovascularisation is a complex process that requires the expression of many growth factors, extracellular matrix proteins, proteases and matricellular proteins that must have a coordinated spatiotemporal regulation.
The secreted protein, acidic and rich in cysteine/osteonectin (SPARC) is a matricellular protein that is secreted and interacts with cells, extracellular matrix, growth factors and proteases. It has been associated with embryological development, blood vessel formation and tissue remodelling.8 SPARC is expressed by newly formed blood vessels in chicken chorioallantoic membrane and by pulmonary arteries during rat lung development.9,10 In addition, SPARC has proinflammatory activity promoting the production metalloproteinases such as matrix metalloproteinase‐9 (MMP‐9), which has been shown to have a role in angiogenesis.11 Therefore, in relation to the multiple regulatory roles of SPARC during both embryological vasculogenesis and tissue remodelling, we have hypothesised that it would be expressed in AS valves and would be closely associated with valve neovascularisation.
PATIENTS AND METHODS
Patients
We examined 30 aortic valves from patients who underwent cardiac surgery for degenerative AS. All the patients had severe AS (valve area < 1.0 cm2) and an aortic regurgitation grade of ⩽ 2. Patients with a history of rheumatic disease, endocarditis or an inflammatory disease were not included in this study. At the time of surgery, valves with a commissural fusion or vegetations and bicuspid valves were not collected for this study. The mean age of patients was 67 (SD 9) years, and the ratio of male to female was 17:13. The most common co‐morbidities were dyslipidaemia (72.2%), high blood pressure (38.8%) and diabetes (33.3%). For controls, we selected 20 heart transplant recipients (mean age 50 (14) years; 15 men and five women; dyslipidaemia, 80%; high blood pressure, 29%; diabetes, 10%) who had no valve disease, from whom the aortic valve was obtained at the time of transplantation. Samples were taken at the time of surgery. One cusp was snap frozen in liquid nitrogen for later use in immunoblotting studies. The two remaining cusps were partially decalcified in Cal‐Ex (Fisher, Nepean, Ontario, Canada) for 24 h. Then one cusp was fixed in formaldehyde 10% for histological processing and one cusp was embedded in optimum cutting temperature compound (TissueTek, Miles Laboratories, Elkhart, Indiana, USA) and frozen in liquid nitrogen for immunohistological analysis.
Histological analysis
Partially decalcified and fixed tissues were processed for routine paraffin embedding. Valve samples were excised vertically to the base at the midpoint. Sections 5 μm thick were obtained perpendicular to the base and stained with haematoxylin–eosin and trichrome Masson. Histological sections were analysed and the degree of calcification was assessed by two independent observers blinded to the clinical data, using an adapted scale modified from Warren et al.12 Briefly, grade 1 valves maintain structural integrity of the cusps with mild fibrous thickening; grade 2 valves are thickened and present early nodular calcification with preservation of the fibrosa; grade 3 valves are grossly thickened and distorted with many calcified nodules and a distorted fibrosa; and grade 4 valves are grossly distorted with many calcified nodules and important fibrosis, and most of the structural components are altered or destroyed with disruption of elastic tissue. Surface ulcerations are often observed at this stage. There was excellent agreement between the two observers with regard to the Warren score (absolute difference 0.27, r = 0.91).
Immunohistological analysis and quantitative assessment of vascular density
Cryostat sections were examined immunohistologically. Cell markers were analysed immunohistologically with the following mouse monoclonal antibodies: anti‐von Willebrand factor (vWF) (Neo‐Marker, Freemont, California, USA), anti‐CD34 (BD Biosciences, Mississauga, Ontario, Canada), anti‐CD45 (Dako, Mississauga, Ontario, Canada) and anti‐monocyte/macrophage CD 68 (Cedarlane, Hornby, Ontario, Canada). The following mouse monoclonal antibodies were used: to detect endothelial nitric oxide synthase (eNOS) (Santa Cruz Biotechnology, Santa Cruz, California, USA), MMP‐9 (gelatinase B) (Chemicon, Temecula, California, USA) and SPARC (US Biological, Swampscott, Massachusetts, USA). Slides were then incubated with a biotin‐conjugated anti‐mouse immunoglobulin antibody (Jackson Immuno Research, Westgrove, Pennsylvania, USA), followed by horseradish peroxidase‐conjugated streptavidin and ABC substrate(Dako). Tissue sections were counterstained with haematoxylin. Mouse serum was used as a negative control in all immunohistology experiments.
Blood vessels were detected by immunostaining with vWF and a quantitative analysis was performed for each patient. Representative regions rich in blood vessels were detected in each section, the number of blood vessels was counted at 400× in triplicate and a mean value was attributed to each valve. Data were reported as the average number of blood vessels in each 400× field.
Western blot analysis
SPARC expression was documented from six normal valves and 12 AS valves by western immunoblotting. Aortic valve tissues were homogenised with a polytron at 4°C in lysis buffer (200 mM TRIS, pH 6.8, 2% sodium dodecyl sulphate) and centrifuged at 52 000 rpm at 4°C. Supernatants were used for western blot analysis. The concentration of proteins was determined with a DC protein assay (Biorad, Mississauga, Ontario, Canada). Fifteen micrograms of protein was boiled and loaded on to 12% sodium dodecyl sulphate‐polyacrylamide gels followed by electrophoresis and blotting on to nitrocellulose membranes. Membranes were blocked (1 h at room temperature) with Tris‐buffered saline containing 0.1% Tween‐20 and 5% non‐fat dry milk and incubated (2 h at room temperature) with a mouse anti‐SPARC (US Biological) and a mouse anti‐β actin (Sigma, St Louis, Missouri, USA). Membranes were then washed and incubated (1 h at room temperature) with a horseradish peroxidase‐labelled anti‐mouse IgG antibody (Amersham, Piscataway, New Jersey, USA) and detected with enhanced chemiluminescence (Amersham) on x ray films.
Statistical analysis
Quantitative analysis of blood vessel density was expressed as mean (SEM). Data were analysed for significance by using an unpaired Student's t test or a one‐way analysis of variance. A value of p < 0.05 was considered significant.
RESULTS
Macroscopic findings in AS valves show that all the valves were tricuspid, thickened, calcified and without commissural fusion. Control valves appeared normal without calcification and thickening. Histological observation of the control and AS valves confirmed that normal valves had a preserved architecture without calcification, whereas AS valves were thickened and showed multiple calcified nodules. By using the grading system of AS valves, which is based on the degree of calcification and concomitant structural damage, we found that most lesions were grade 3 and 4. We found the following distribution: grade 2 in eight valves; grade 3 in 12 valves; and grade 4 in 10 valves. Histological analysis with haematoxylin and eosin staining showed that blood vessels were present in AS valves (fig 1A), whereas normal valves were avascular structures. The number of blood vessels was higher in valves with a lower or an intermediate grade of calcification (grade 2 and 3). The mean numbers of blood vessels were 3.7 (0.5), 2.9 (0.8) and 1.2 (0.4) (blood vessels/400× field), respectively, for valves with a calcification grade of 2, 3 and 4 (p = 0.001) (fig 2). The blood vessels in AS valves were positive for eNOS, CD 34 and vWF, which were expressed in the endothelium of vascular structures (fig 1B–E). The endothelium lining the valve surface of normal and AS valves was positive for eNOS, CD34 and vWF as well (data not shown). The vascular structures in AS valves were located near calcified nodules and under the leaflet surface (fig 1D,E). In eight AS valves blood vessels were detected in rich cellular regions forming cell islands surrounded by foci of calcification (fig 3A). In cell islands, cells forming cellular clusters and endothelial cord‐like structures were detected and were positive for vWF and CD34, indicating active formation of blood vessels in these regions (fig 3B–C). These rich cellular areas were densely populated by cells that expressed the cell marker CD45 (fig 3E). Furthermore, cells positive for CD45 were found to form small clusters near mature blood vessels (fig 3F).
Figure 1 (A) Haematoxylin and eosin staining of aortic stenosis (AS) valves showing blood vessels. Immunostaining for (B) endothelial nitric oxide synthase (eNOS) localised to blood vessels of AS valves, (C) CD34 in blood vessels, (D) von Willebrand factor (vWF) near calcified nodules and (E) the leaflet surface (magnification 400×).
Figure 2 Quantitative analysis of vascular density in aortic stenosis (AS) valves determined and expressed as the number of blood vessels/400× field in relation to the calcification and remodelling grade; number of blood vessels was higher in grades 2 and 3 than in grade 4 (p = 0.001) (grey bar). The proportion of blood vessels expressing secreted protein, acidic and rich in cysteine/osteonectin (SPARC) (open bar) was higher in grade 2 than in grades 3 and 4 (p = 0.01). Data are mean (SEM).
Figure 3 (A) Haematoxylin and eosin staining showing blood vessels located in rich cellular areas forming cell islands (200×). (B) In cell islands immunostaining for von Willebrand factor (vWF) showed cell clusters (arrow) and the formation of endothelial cord‐like structures (inset) (200×). (C) High‐power magnification of inset (from fig 1B) showing endothelial (vWF) cord‐like structure (arrows) (400×). (D) Immunostaining for CD34 showed cell clusters (arrow) and cord‐like structures (open arrow) in cell islands (400×). (E) Cell island regions contained numerous cells positive for the CD45 cell marker (200×) that formed (F) cell clusters near blood vessels (600×).
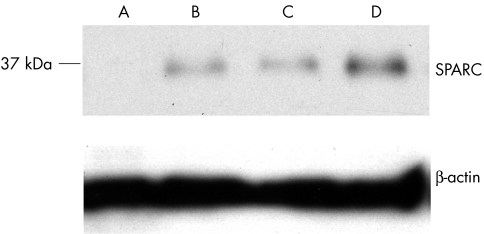
Immunohistological analysis showed that SPARC was not present in normal valves, whereas it was strongly expressed in AS valves. Further analysis showed that SPARC was present in different locations in AS valves. Near calcified nodules we found immunoreactivity for SPARC in fibroblasts and in the extracellular matrix (fig 4A). In three valves SPARC immunoreactivity was located at the endothelium lining of the valve surface, which it covered heterogeneously (fig 4B). In AS valves some mature blood vessels were found to be positive for SPARC, and the proportion of SPARC‐positive blood vessels was higher in valves with a lower or intermediate calcification grade (fig 2). In vascular structures SPARC immunoreactivity was located at the endothelium of medium‐sized blood vessels and capillaries that were located beneath the valve surface and in the stroma. (fig 4C–E). In cell islands SPARC was not found in cells forming clusters or endothelial cord‐like structures, whereas it was found in mature blood vessels and in macrophages present in these regions (fig 4F–H). The presence of SPARC in AS valves was confirmed with immunoblot analysis, which showed a protein band at ∼35 kDa (fig 5).
Figure 4 (A) Immunostaining for secreted protein, acidic and rich in cysteine/osteonectin (SPARC) showing localisation to fibroblasts and the extracellular matrix near calcified nodules (purple) (200×). SPARC expression (B) by the endothelium of the leaflet surface (arrow) (100×), (C) by the endothelium of blood vessels under the valve surface (200×), (D) by medium‐sized blood vessels (600×) and (E) by capillaries (1000×). (F) SPARC was expressed in cell islands by mature blood vessels (200 ×) and by (G) multinucleated cells (1000×) that were (H) CD68 positive (macrophages) (1000×). (I) macrophages in cell islands expressed matrix metalloproteinase‐9 (1000×).
Figure 5 Western blot analysis for secreted protein, acidic and rich in cysteine/osteonectin (SPARC) and β actin in (A) normal valve and (B–D) aortic stenosis (AS) valves, showing the expression of SPARC by calcified AS valves.
Analysis of immunoreactivity for MMP‐9 showed that it was highly expressed in AS valves but not in normal valves. Detailed analysis showed that MMP‐9 was present in macrophages (CD68‐positive cells) and, furthermore, in cell islands it was co‐localised with SPARC (fig 4H,I). It is significant to note that macrophages co‐expressing MMP‐9 and SPARC were detected only in rich cellular areas forming cell islands. MMP‐9 was also expressed in stromal cells dispersed throughout the leaflet tissue and in the vascular endothelium (fig 6).
Figure 6 (A) Immunostaining for matrix metalloproteinase‐9 (MMP‐9) showing strong expression throughout the leaflet (100×). High‐power magnification (600×) showing MMP‐9 localisation to (B) stromal cells and the vascular endothelium (C) at 400× and (D) at 1000×.
DISCUSSION
Our observations are in accordance with previous publications that neovascularisation is closely associated with the development of degenerative AS.7,13 We found that neovascularisation was not a random process in AS valves but rather that blood vessels develop in specific regions and at selected times during the course of the disease. Vascular density was higher in AS valves with a low or intermediate grade of calcification, suggesting that neovascularisation is temporally controlled in the development of AS. These observations are in accordance with previous evidence that vascular density is higher in patients with AS with an intermediate valve gradient.13 One can speculate that higher vascular density in valves with a lower calcification grade may be attributable to more cellular activity and turnover at this specific stage of the disease and that more advanced lesions would be fixed, with less tissue remodelling, which would be in accordance with the observation that the proportion of blood vessels expressing SPARC was higher in the lower calcification grades. Indeed, SPARC is expressed in tissue with a high cellular turnover. The observation that vascular density was lower in valves with a higher calcification grade suggests that blood vessels may be remodelled and disappear as a function of the metabolic rate of the tissue, analogous to what is seen during embryological development.14 However, factors that trigger blood vessels formation and regression at specific time points during the development of AS have not been studied.
The origin of blood vessels in AS valves is not clearly established. In adults, vasculogenesis has been shown to develop from bone marrow‐derived endothelial progenitor cells (EPC), which are circulating cells that are recruited to regions where neovascularisation is taking place. Whereas the contribution of angiogenesis to pathological neovascularisation is well documented, the occurrence of vasculogenesis during the formation of new blood vessels in adults is a recent concept. Bone marrow‐derived EPC were identified to be positive for CD34, a marker known to be expressed on some haematopoietic stem cells and mature endothelial cells.15 Furthermore, EPC expressed the leucocyte common antigen CD45, which is not expressed by mature endothelial cells.16 We have observed that cell islands were densely populated by CD45‐positive cells and on occasion formed clusters near blood vessels. Furthermore, in cell islands we observed that CD34‐positive and vWF‐positive cells formed clusters and cord‐like structures. The endothelial cord‐like structures were seen as isolated structures in blood islands and were not seen to originate from mature blood vessels. These observations suggest that vasculogenesis may occur in these particular regions of AS valves. Indeed, Tepper et al17 have shown in an experimental model that the formation of blood vessels in response to ischaemia was associated with the recruitment of EPC, formation of cellular clusters, with the concomitant acquisition of mature endothelial markers such as vWF, and subsequent formation of endothelial cord‐like structures before the formation of mature blood vessels.17 Although the exact origin of endothelial cells that form vascular structures in AS valves has not been clearly determined, this is the first study to show vascular cord‐like structures in AS valves in vivo, indicating that specific rich cellular areas are present in at least some valves and are actively participating in the development of new blood vessels.
Intact SPARC protein inhibits cellular proliferation and has antiangiogenic activity in vitro. However, fragments of SPARC with the KGHK motif strongly induce angiogenic activity both in vitro and in vivo.18 From the intact protein, proteases such as serine proteases, cathepsins and stromelysins release SPARC fragments with the KGHK motif.9 Although we cannot conclude from our studies that SPARC has direct pro‐ or antiangiogenic activity in AS valves, we have found a close association with its expression and the presence of blood vessels. We have observed that SPARC was expressed by endothelial cells in blood vessels, and the proportion of vascular structures positive for SPARC was increased in valves with a low grade of calcification, suggesting that the distribution of SPARC was temporally controlled and distributed to newly formed blood vessels. In cell islands, we did not observe the expression of SPARC in cord‐like structures but it was present in fully formed blood vessels, suggesting that SPARC is not expressed during rapid cellular division and alignment of cells in cord‐like structures but rather after the formation of well‐formed blood vessels. These observations are in accordance with a previous publication showing that during chicken chorioallantoic membrane development SPARC was located in newly well‐formed blood vessels.9 Thus, the expression pattern of SPARC in AS valves strongly supports a highly regulated process in which the protein is involved in the formation of new blood vessels.
Monocytes/macrophages have been implicated in neovascularisation through the production of mediators influencing angiogenesis, direct transdifferentiation into endothelial cells or the creation of proteases‐positive tunnels in tissues, which can subsequently be colonised by endothelial cells.19,20 In AS valves macrophages co‐expressing SPARC and MMP‐9 were detected only in rich cellular areas forming cell islands. In addition, MMP‐9 was localised to the endothelium of blood vessels, indicating that endothelial cells are activated. In vitro, SPARC has proinflammatory effects on human peripheral blood monocytes inducing the expression of MMP‐9.21 Macrophages co‐expressing SPARC and MMP‐9 were restricted to cell islands, where developing vascular cords were found, suggesting that macrophages may be involved in the formation of blood vessels in these areas. However, at this stage SPARC expression by macrophages and its potential relationship with angiogenic activity in AS valves remains to be established.
This study is the first, to our knowledge, to underline that (1) neovascularisation in AS valves is closely associated with SPARC expression, which has a regulated spatiotemporal distribution, and (2) blood vessels with vascular cord‐like structures are actively formed in specific rich cellular areas. However, precise mechanisms related to either angiogenesis or vasculogenesis with the expression of different growth factors and their interactions with SPARC, as well as their specific contributions to the neovascularisation of AS valves, remain to be studied. Control and modulation of neovascularisation during the development of degenerative AS may provide new targets for treatment to inhibit or decrease the progression of the disease.
ACKNOWLEDGEMENTS
The authors thank Brigitte Dionne, Stephanie Dionne and Martine Fleury for their technical assistance.
Abbreviations
AS - aortic stenosis
eNOS - endothelial nitric oxide synthase
EPS - endothelial progenitor cells
MMP‐9 - matrix metalloproteinase‐9
SPARC - secreted protein, acidic and rich in cysteine/osteonectin
vWF - von Willebrand factor
Footnotes
This work was supported by the Quebec Heart Institute Foundation
References
- 1.Lindroos M, Kupari M, Heika J.et al Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993211220–1225. [DOI] [PubMed] [Google Scholar]
- 2.Otto C M, Kuusisto J, Reichenbach D D.et al Characterization of the early lesion of degenerative valvular aortic stenosis histological and immunohistochemical studies. Circulation 199490844–853. [DOI] [PubMed] [Google Scholar]
- 3.Rajamannan N M, Subramaniam M, Rickard D.et al Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 20031072181–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fendly B S, Siscovick D, Lind B K.et al Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol 199729630–634. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 6.Moreno P R, Purushothaman R, Fuster V. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta implications for plaque vulnerability. Circulation 20041102032–2038. [DOI] [PubMed] [Google Scholar]
- 7.Mazzone A, Epistolato M C, Caterina R D.et al Neoangiogenesis, T Lymphocyte infiltration, and heat shock protein‐60 are biological hallmarks of an inflammatory process in end‐stage calcified aortic valve stenosis. J Am Coll Cardiol 2004431670–1676. [DOI] [PubMed] [Google Scholar]
- 8.Sage H E. Regulation of interactions between cells and extracellular matrix: a command performance on several stages. J Clin Invest 2001107781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iruela‐Arispe M L, Lane T F, Redmond D.et al Expression of SPARC during development of the chicken chorioallantoic membrane: evidence for regulated proteolysis in vivo. Mol Biol Cell 19956327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strandjord T P, Sage H E, Clark J G. SPARC participates in the branching morphogenesis of developing foetal rat lung. Am J Respir Cell Mol Biol 199513279–287. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw A D, Sage H E. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 20011071049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren B A, Yong J L C. Calcification of the aortic valve: its progression and grading. Pathology 199729360–368. [DOI] [PubMed] [Google Scholar]
- 13.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol 200334756–763. [DOI] [PubMed] [Google Scholar]
- 14.Risau W, Flamme I. Vaculogenesis. Annu Rev Cell Dev Biol 19951173–91. [DOI] [PubMed] [Google Scholar]
- 15.Khakoo A Y, Finkel T. Endothelial progenitor cells. Ann Rev Med 20055679–101. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Murohara T, Sullivan A.et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997275964–967. [DOI] [PubMed] [Google Scholar]
- 17.Tepper O M, Capla J M, Galiano R D.et al Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow‐derived cells. Blood 20051051068–1077. [DOI] [PubMed] [Google Scholar]
- 18.Sage E H. Pieces of eight: bioactive fragments of extracellular proteins as regulators of angiogenesis. Trends Cell Biol 19977182–186. [DOI] [PubMed] [Google Scholar]
- 19.Havemann K, Pujol B F, Adamkievicz J. In vitro transformation of monocytes and dendritic cells into endothelial like cells. In: Moldovan NI, ed. Novel angiogenic mechanisms: role of circulating progenitor endothelial cells. New York: Kluwer Academic/Plenum, 200347–57. [DOI] [PubMed]
- 20.Moldovan N I, Goldschmidt‐Clermont P J, Parker‐Thornburg J.et al Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloprotease‐positive tunnels in ischemic myocardium. Circ Res 200087378–384. [DOI] [PubMed] [Google Scholar]
- 21.Shankavaram U T, DeWitt D L, Funk S E.et al Regulation of human monocyte matrix metalloproteinases by SPARC. J Cell Physiol 1997173327–334. [DOI] [PubMed] [Google Scholar]