Abstract
Objective
To evaluate a comprehensive multislice computed tomography (MSCT) protocol in patients with previous infarction, including assessment of coronary artery stenoses, left ventricular (LV) function and perfusion.
Patients and methods
16‐slice MSCT was performed in 21 patients with previous infarction; from the MSCT data, coronary artery stenoses, (regional and global) LV function and perfusion were assessed. Invasive coronary angiography and gated single‐photon emission computed tomography (SPECT) served as the reference standards for coronary artery stenoses and LV function/perfusion, respectively.
Results
236 of 241 (98%) coronary artery segments were interpretable on MSCT. The sensitivity and specificity for detection of stenoses were 91% and 97%. Pearson's correlation showed excellent agreement for assessment of LV ejection fraction between MSCT and SPECT (49 (13)% v 53 (12)%, respectively, r = 0.85). Agreement for assessment of regional wall motion was excellent (92%, κ = 0.77). In 68 of 73 (93%) segments, MSCT correctly identified a perfusion defect as compared with SPECT, whereas the absence of perfusion defects was correctly detected in 277 of 284 (98%) segments.
Conclusions
MSCT permits accurate, non‐invasive assessment of coronary artery stenoses, LV function and perfusion in patients with previous infarction. All parameters can be assessed from a single dataset.
The evaluation of patients with previous myocardial infarction is extensive but mandatory to allow optimal risk stratification. Coronary angiography is needed for precise assessment of the location and severity of obstructive coronary artery lesions. Information on systolic function (left ventricular ejection fraction (LVEF)) needs to be evaluated, as this is an important prognostic factor. LVEF can be assessed by two‐dimensional echocardiography, nuclear imaging with gated SPECT or invasively by left ventricular (LV) angiography. Assessment of perfusion in the infarct region is of importance; this can be performed by gated single‐photon emission computed tomography (SPECT). An imaging modality that can provide all this information during one acquisition would be preferred. Over the past few years, multislice computed tomography (MSCT) has emerged as a non‐invasive imaging modality that allows the acquisition of high resolution three‐dimensional images of the entire heart within 25 s. The technique allows non‐invasive coronary angiography, with sensitivities and specificities for the detection of significant stenoses ranging from 70–98%.1,2,3,4,5 As MSCT data acquisition is gated to the ECG, LV function can be derived from the same dataset.1,6 In particular, both global LV function (LVEF) and regional LV function (wall motion) can be assessed. Moreover, owing to the acquisition of images during the first pass of a contrast agent, hypoenhanced areas can be identified, indicating reduced perfusion. Accordingly, a single MSCT examination could potentially provide all this information, but no data are available on the value of MSCT on this topic. The purpose of the present study was to evaluate the feasibility of MSCT to assess all these parameters during a single acquisition. Conventional modalities were used as the reference standards to compare these different parameters: invasive coronary angiography to assess coronary stenoses and nuclear perfusion imaging with SPECT to assess resting perfusion. Moreover, as the SPECT studies were assessed in gated mode, global (LVEF) and regional LV function (wall motion) could also be derived from SPECT.
METHODS
Patients and study protocol
Twenty‐one patients with a history of myocardial infarction (> 3 months before the study) underwent MSCT and resting gated SPECT with technetium‐99m. Twenty patients also underwent invasive coronary angiography. Patients with atrial fibrillation were excluded, as well as patients with renal insufficiency (serum creatinine > 120 mmol/l), known allergy to iodine contrast media and severe claustrophobia, and pregnant patients. All patients provided informed consent to the study protocol, which was approved by the local ethics committee.
MSCT data acquisition
MSCT examinations were performed with a 16‐slice Toshiba multislice Aquilion 16 system (Toshiba Medical Systems, Otawara, Japan). Collimation was 16 × 0.5 mm (if arterial grafts were present, a collimation of 16 × 1.0 mm was used to decrease scan time) and rotation time was 400, 500 or 600 ms, depending on heart rate. Tube current and tube voltage were 250 mA and 120 kV, respectively. Total contrast dose for the scan varied from 120–150 ml, depending on total scan time, with an injection rate of 4 ml/s through the antecubital vein (Xenetix 300; Guerbet, Aulnay‐sous‐Bois, France), followed by a saline flush of 40 ml. To time the scan, automated detection of peak enhancement in the aortic root was used. All images were acquired during an inspiratory breath hold, while the ECG was registered simultaneously for retrospective gating of the data. With the aid of a segmental reconstruction algorithm, data of two or three consecutive heart beats were used to generate a single image. Estimated radiation dose was 8 mSv for men and 10 mSv for women.
For evaluation of coronary artery stenoses, images with a slice thickness of 0.5 mm were reconstructed at typically 75% of the cardiac cycle. In case of motion artefacts, reconstructions at 40–50% were explored. Subsequently, images were transferred to a remote workstation (Vitrea2; Vital Images, Plymouth, Minnesota, USA) for post‐processing.
For evaluation of LV function and resting perfusion, 2.0 mm slices were reconstructed in the short‐axis orientation at 20 time points, starting at early systole (0% of cardiac cycle) to end diastole (95% of cardiac cycle) in steps of 5%. Images were transferred to a remote workstation with dedicated cardiac function analysis software (MR Analytical Software System; Medis, Leiden, The Netherlands).
MSCT data analysis
For the assessment of significant coronary artery stenoses, the MSCT angiograms were evaluated by two experienced observers blinded to the results of conventional coronary angiography. Coronary arteries were divided into 17 segments according to the guidelines of the American Heart Association/American College of Cardiology.7 Only segments with a diameter ⩾ 2.0 mm were included. Firstly, segments were classified as evaluable or not. Thereafter, the interpretable segments were evaluated for the presence of significant narrowing (⩾ 50% decrease in luminal diameter). In case of previous bypass surgery and patent bypass grafts, only segments distal to the anastomosis were evaluated.
To determine LV function, an independent observer outlined endocardial borders manually on the short‐axis cine images. The papillary muscles were regarded as being part of the LV cavity. The LV end systolic and end diastolic volumes were calculated and the LVEF was derived. One observer blinded to all other data visually assessed regional wall motion from the short‐axis slices in cine‐loop mode by using a 17‐segment model.8 A three‐point scale was used to assign to each segment a wall motion score (1, normokinesia; 2, hypokinesia; 3, akinesia or dyskinesia).9
To assess myocardial perfusion, the transmural extent of the LV wall showing reduced perfusion was evaluated for transmurality of decreased signal intensity. The same 17‐segment model as described for the assessment of regional wall motion was graded with a five‐point scoring system, with 0 indicating no perfusion defect; 1, a perfusion defect involving 1–25% of the LV wall; 2, a defect involving 26–50% of the LV wall; 3, a defect involving 51–75% of the LV wall; and 4, a defect involving > 75% of the LV wall.10
Resting gated SPECT
Myocardial perfusion SPECT images with 99mTc tetrofosmin (500 MBq, injected at rest) were recorded with a triple head SPECT camera system (GCA 9300/HG, Toshiba) equipped with low energy general‐purpose collimators. Around the 140 KeV energy peak of 99mTc tetrofosmin, a 20% window was used. A total of 90 projections (step and shoot mode, 35 s/projection, imaging time 23 min) were obtained over a 360° circular orbit. Data were stored in a 64 × 64, 16‐bit matrix. Resting perfusion was assessed by segmental tracer activity (0, ⩾ 75% of maximum tracer activity; 1, 50–75% of maximum tracer activity; 2, 25–50% of maximum tracer activity; and 3, ⩽ 25% of maximum tracer activity) in a similar 17‐segment model as described above.11
LV volumes were calculated from the gated short‐axis images by previously validated and commercially available automated software (quantitative gated SPECT; QGS, Cedars‐Sinai Medical Center, Los Angeles, California, USA).12 After segmentation of the LV, endocardial and epicardial surfaces are estimated and displayed, the LV end systolic and end diastolic volumes are calculated and the LVEF can be derived. Regional wall motion was evaluated in the same 17‐segment model and three‐point scale as described for MSCT.
Invasive coronary angiography
Conventional coronary angiography was performed according to the standard techniques. To obtain vascular access the femoral approach with the Seldinger technique was used. An experienced observer blinded to the MSCT data visually evaluated the coronary angiograms. Coronary arteries were divided into 17 segments according to the guidelines of the American Heart Association/American College of Cardiology.7 Consequently, the segments with a diameter ⩾2.0 mm were evaluated for the presence of significant narrowing (defined as ⩾ 50% decrease in luminal diameter). In case of previous bypass surgery and patent bypass grafts, only segments distal to the anastomosis were evaluated.
Statistical analysis
Data are presented as mean (SD) or as number (%). Sensitivity, specificity, and positive and negative predictive values were calculated to detect significant coronary artery stenoses (⩾ 50% decrease in luminal diameter) on MSCT by using invasive angiography as the reference standard. Pearson's correlation coefficient (r) was calculated for the linear regression analysis of the LVEFs (MSCT versus gated SPECT). Bland–Altman analysis was performed for each pair of values of LVEF to calculate the limits of agreement and systematic error between the two modalities (MSCT versus gated SPECT).13 A value of p < 0.05 was considered significant.
A 3 × 3 table based on weighted κ statistics was applied to express the agreement for regional wall motion between MSCT and gated SPECT. A value of κ < 0.4 represents poor agreement, between 0.4 and 0.75 shows fair to good agreement, and > 0.75 states excellent agreement.14
RESULTS
MSCT was performed successfully in all 21 patients. The study population consisted of 20 men and one woman, with a mean (SD) age of 61 (13) years. A total of 17 (81%) patients used β blocking agents, and no additional β blockade was given before MSCT imaging. Mean (SD) heart rate was 64 (9) beats/min (range 51–78), duration of breath hold was about 20 s and success rate of obtaining adequate breath hold was 100%. Table 1 summarises clinical characteristics of the study population.
Table 1 Clinical characteristics of the study population (n = 21).
| Mean age (years) | 61 (13) |
| Men | 20 (95%) |
| Previous infarction | 21 (100%) |
| Location | |
| Anterior | 10 (48%) |
| Inferior | 10 (48%) |
| Both | 1 (4%) |
| Q wave on ECG | 13 (62%) |
| Previous CABG | 8 (38%) |
| Multivessel CAD | 13 (62%) |
| Angina pectoris (CCS class) | |
| I/II | 10 (48%) |
| III/IV | 11 (52%) |
| Heart failure (NYHA class) | |
| I/II | 17 (81%) |
| III/IV | 4 (19%) |
CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; NYHA, New York Heart Association.
Non‐invasive coronary angiography by MSCT
Conventional, invasive coronary angiography was performed in 20 patients. In these patients, a total of 241 segments were available for evaluation. Of these segments, 236 (98%) were of sufficient image quality on MSCT to assess the presence or absence of significant stenoses. During conventional coronary angiography, significant stenoses were observed in 46 segments, with 42 (91%) correctly identified on MSCT. In 185 of 190 (97%) segments, MSCT correctly ruled out the presence of significant stenoses. Accordingly, the sensitivity and specificity were 91% and 97%, respectively. Table 2 summarises the diagnostic accuracy of MSCT (for segments, vessels and patients). Figure 1 shows an example of MSCT coronary angiography and the corresponding conventional angiography.
Table 2 Diagnostic accuracy of MSCT for the detection of significant (⩾50% luminal narrowing) stenoses.
| Segment based | Vessel based | Patient based | |
|---|---|---|---|
| Evaluable (%) | 98 (236/241) | 99 (76/77)* | 100 (20/20) |
| Sensitivity (%) | 91 (42/46) | 92 (23/25) | 92 (12/13) |
| Specificity (%) | 97 (185/190) | 96 (49/51) | 86 (6/7) |
| PPV (%) | 89 (42/47) | 92 (23/25) | 92 (12/13) |
| NPV (%) | 98 (185/189) | 96 (49/51) | 86 (6/7) |
Data are percentages (segments).
*In one patient the left circumflex artery was uninterpretable due to its small size, whereas in three patients both the left anterior descending coronary artery and the left circumflex were supplied by patent grafts. In these patients, the left main artery was not evaluated.
MSCT, multislice computed tomography; NPV, negative predictive value; PPV, positive predictive value.
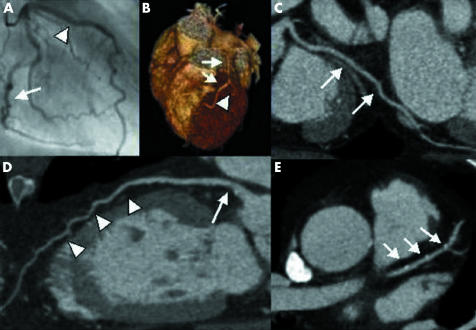
Figure 1 Example of multislice computed tomography (MSCT) coronary angiography. (A) Conventional angiography showing the left circumflex artery (LCX) (arrow) and the left anterior descending coronary artery (LAD) (arrow head). Corresponding MSCT images: (B) MSCT three‐dimensional volume‐rendered reconstruction depicting the LAD (arrows) and the first diagonal (arrowhead). (C) MPR of the LCX (white arrows). (D) MPR of the LAD (arrow heads) with soft plaque (arrow). (E) Transaxial image of the LAD (arrows) showing small calcifications.
LV function: MSCT versus gated SPECT
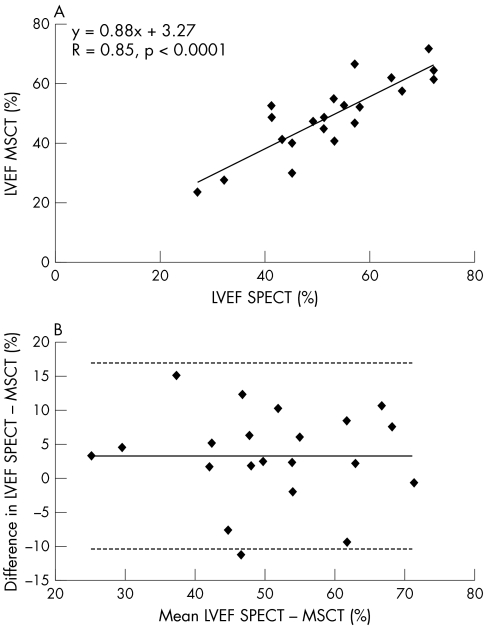
The average LVEF was 53 (12)% (range 27–72%) on gated SPECT, as compared with 49 (13)% (range 24–72%) on MSCT. Correlation by linear regression analysis was excellent (r = 0.85, p < 0.0001) (fig 2A). Bland–Altman analysis (fig 2B) showed a mean difference (2 SD) of 3.3 (13.6)%, which was not statistically different from zero.
Figure 2 (A) Linear regression plot shows correlation between left ventricular ejection fraction (LVEF) as measured by single‐photon emission tomography (SPECT) and multislice computed tomography (MSCT). (B) Bland–Altman plot of LVEF shows the difference between each pair plotted against the average value of the same pair—that is, mean value of differences (solid line) and mean value of differences (2 SD) (dotted lines).
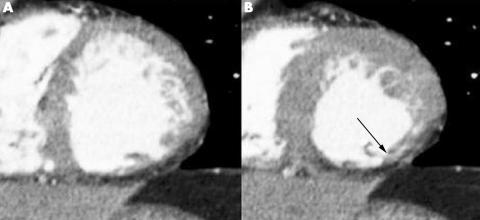
On resting gated SPECT, regional wall motion abnormalities were detected in 75 (21%) of 357 segments, with 40 (53%) showing hypokinesia and 35 (47%) akinesia or dyskinesia. In 69 (92%) of the dysfunctional segments, decreased systolic wall motion was also observed on the MSCT images (table 3). Agreement between the two techniques was excellent, with 92% of segments scored identically on both modalities (κ = 0.77). Agreements for the individual gradings (1 to 3) were 96%, 75% and 74%, respectively. Figure 3 shows an example of decreased wall motion on MSCT.
Table 3 Agreement on assessment of regional wall motion between MSCT and resting gated SPECT.
| SPECT | MSCT | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| 1 | 271 | 10 | 1 | 282 |
| 2 | 6 | 30 | 4 | 40 |
| 3 | 0 | 9 | 26 | 35 |
| Total | 277 | 49 | 31 | 357 |
Agreement was 92%, κ = 0.77.
1, normokinesia; 2, hypokinesia; 3, akinesia or dyskinesia; MSCT, multislice computed tomography; SPECT, single‐photon emission computed tomography.
Figure 3 Example of wall motion analysis with multislice computed tomography of a patient with an inferior myocardial infarction. Short‐axis images in (A) end diastole and (B) end systole show reduced wall thickening (arrow) in the inferior wall.
Resting perfusion: MSCT versus SPECT
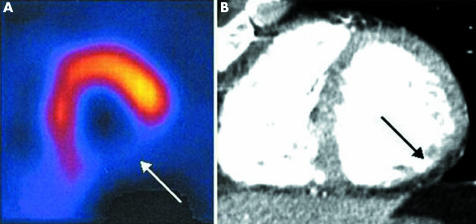
On the resting SPECT images, tracer uptake was reduced in 73 (20%) of 357 segments. Of these 73 segments, perfusion defects were detected on MSCT in 68 (93%) segments. In 277 of 284 (98%) segments with normal perfusion on SPECT, no perfusion defects were observed on MSCT as well. The degree of transmurality of the perfusion defects on MSCT paralleled the relative reduction in tracer activity on SPECT. Figure 4 shows an example of a large perfusion defect on MSCT.
Figure 4 Example of a large perfusion defect on single‐photon emission computed tomography (SPECT) and multislice computed tomography (MSCT) of the same patient as in fig 3. (A) SPECT short‐axis image showing a perfusion defect in the inferior wall (arrow) at rest. (B) Corresponding MSCT short‐axis image in end diastole showing a hypoenhanced area (arrow) in the inferior wall, indicating a perfusion defect.
DISCUSSION
The evaluation of patients with previous infarction is extensive and different imaging modalities are needed to provide the necessary information. For efficient patient management, a comprehensive non‐invasive cardiac examination would be ideal. The current study was designed to evaluate the possibility of deriving additional information on LV function and perfusion from cardiac MSCT, apart from the information on coronary arteries.
Conventional coronary angiography is considered the diagnostic standard for determining the presence of coronary artery stenoses and MSCT has recently been shown to assess coronary artery stenoses with high accuracy.1,2,3,4,5 The current findings show that, also in patients with previous myocardial infarction, non‐invasive coronary angiography with MSCT has a high sensitivity and specificity (91% and 97%, respectively) for the detection of coronary artery stenoses, in line with previous observations in patients with known or suspected coronary artery disease.1,2,3,4,5 Other parameters can also be obtained from the same dataset that is used for evaluation of the coronary arteries, including LV function and perfusion. In particular, LVEF is an important prognostic parameter after myocardial infarction.15 The current results show excellent agreement between MSCT and gated SPECT for the assessment of LVEF. Previous studies have shown similar results with other imaging modalities, including magnetic resonance imaging (MRI) and two‐dimensional echocardiography, although these studies were not specifically evaluating a selected study population after myocardial infarction and focused mainly on patients with relatively preserved LVEF.1,16,17,18
Besides assessment of global LV function, MSCT can also be used to evaluate regional wall motion. Agreement between MSCT and gated SPECT (κ = 0.77) was good in the present study. No direct comparisons between gated SPECT and MSCT for assessment of regional wall motion have been reported, but Mahnken et al16 reported good agreement for assessment of regional wall motion between 16‐slice MSCT and MRI (κ = 0.79).
Lastly, MSCT was used in the present study to assess resting perfusion. The results showed that MSCT detected perfusion defects correctly in 93% of the segments with a perfusion defect on SPECT imaging. On the other hand, MSCT did not detect any perfusion abnormalities in 98% of the segments with normal perfusion on SPECT. In addition, the extent of transmurality of the perfusion defects on MSCT corresponded to the relative reduction in tracer activity as assessed on SPECT. Most segments with ⩾ 75% tracer activity had no or minimal perfusion abnormalities on MSCT, whereas segments with ⩽ 25% tracer activity had predominantly extensive, transmural perfusion defects on MSCT. Minimal information on assessment of perfusion with MSCT is currently available. Two recent studies have focused on assessment of perfusion defects (indicating scar tissue) with MSCT in comparison to MRI. Nikolaou et al19 compared 16‐slice MSCT with MRI for assessment of myocardial infarction; they reported a sensitivity and specificity of 91% and 79%, respectively. In the second study, both early‐phase MSCT and late‐enhancement MSCT were performed and compared with MRI for the evaluation of perfusion defects and infarct size in 28 patients with reperfused myocardial infarction.20 Agreement between MRI and MSCT for the detection of scar tissue was good. MRI is regarded as the reference standard for the assessment of LV function, and scar tissue due to myocardial infarction can be evaluated by delayed enhancement on MRI as well. A major advantage of this technique is the lack of radiation exposure.
Several limitations of the present study need attention. The study population was relatively small, and larger studies are needed to confirm the current observations. A drawback of MSCT remains the radiation dose (8–10 mSv in this study, which is higher than with conventional diagnostic angiography) and future developments are needed to reduce radiation. Furthermore, most MSCT parameters were evaluated qualitatively, and software allowing quantitative analysis needs to be developed. In addition, MSCT at present provides information only on resting perfusion. Additional imaging during stress conditions would allow assessment of stress perfusion and the combination of the stress and rest images would potentially allow detection of ischaemia and scar tissue. However, in view of the radiation burden, such a protocol may currently not be feasible for routine clinical practice.
In conclusion, the present study showed the feasibility of integrated assessment of coronary anatomy, regional and global LV function, and perfusion with MSCT in patients with previous infarction. Agreement with invasive coronary angiography and gated SPECT (for assessment of function and perfusion) was excellent. In patients with previous infarction, the coronary arteries can be evaluated non‐invasively for the detection or exclusion of possible new significant stenoses. In patients with previous bypass surgery, non‐invasive evaluation with MSCT can provide information on graft patency. The additional data on LV function as well as extent of (possibly) infarcted myocardium can be taken into account in the risk stratification of these patients; this concept needs further testing in future studies.
Abbreviations
LV - left ventricular
LVEF - left ventricular ejection fraction
MRI - magnetic resonance imaging
MSCT - multislice computed tomography
SPECT - single‐photon emission computed tomography
Footnotes
JDS is financially supported by The Netherlands Heart Foundation, The Hague, The Netherlands, grant number 2002B105. JWJ is financially supported by The Netherlands Heart Foundation, grant number 2001D032.
Competing interests: None declared.
References
- 1.Schuijf J D, Bax J J, Salm L P.et al Noninvasive coronary imaging and assessment of left ventricular function using 16‐slice computed tomography. Am J Cardiol 200595571–574. [DOI] [PubMed] [Google Scholar]
- 2.Achenbach S, Giesler T, Ropers D.et al Detection of coronary artery stenoses by contrast‐enhanced, retrospectively electrocardiographically‐gated, multislice spiral computed tomography. Circulation 20011032535–2538. [DOI] [PubMed] [Google Scholar]
- 3.Nieman K, Rensing B J, van Geuns R J.et al Non‐invasive coronary angiography with multislice spiral computed tomography: impact of heart rate. Heart 200288470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieman K, Cademartiri F, Lemos P A.et al Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation 20021062051–2054. [DOI] [PubMed] [Google Scholar]
- 5.Nieman K, Pattynama P M, Rensing B J.et al Evaluation of patients after coronary artery bypass surgery: CT angiographic assessment of grafts and coronary arteries. Radiology 2003229749–756. [DOI] [PubMed] [Google Scholar]
- 6.Schuijf J D, Bax J J, Jukema J W.et al Noninvasive angiography and assessment of left ventricular function using multislice computed tomography in patients with type 2 diabetes. Diabetes Care 2004272905–2910. [DOI] [PubMed] [Google Scholar]
- 7.Austen W G, Edwards J E, Frye R L.et al A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975515–40. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira M D, Weissman N J, Dilsizian V.et al Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002105539–542. [DOI] [PubMed] [Google Scholar]
- 9.Pace L, Cuocolo A, Marzullo P.et al Reverse redistribution in resting thallium‐201 myocardial scintigraphy in chronic coronary artery disease: an index of myocardial viability. J Nucl Med 1995361968–1973. [PubMed] [Google Scholar]
- 10.Wu E, Judd R M, Vargas J D.et al Visualisation of presence, location, and transmural extent of healed Q‐wave and non‐Q‐wave myocardial infarction. Lancet 200135721–28. [DOI] [PubMed] [Google Scholar]
- 11.Schinkel A F, Bax J J, Sozzi F B.et al Prevalence of myocardial viability assessed by single photon emission computed tomography in patients with chronic ischaemic left ventricular dysfunction. Heart 200288125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germano G, Kiat H, Kavanagh P B.et al Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995362138–2147. [PubMed] [Google Scholar]
- 13.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986i307–310. [PubMed]
- 14.Fleiss J L.Statistical methods for rates and proportions. 2nd ed. New York: Wiley, 1981218
- 15.White H D, Norris R M, Brown M A.et al Left ventricular end‐systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 19877644–51. [DOI] [PubMed] [Google Scholar]
- 16.Mahnken A H, Koos R, Katoh M.et al Sixteen‐slice spiral CT versus MR imaging for the assessment of left ventricular function in acute myocardial infarction. Eur Radiol 200515714–720. [DOI] [PubMed] [Google Scholar]
- 17.Juergens K U, Grude M, Maintz D.et al Multi‐detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology 2004230403–410. [DOI] [PubMed] [Google Scholar]
- 18.Yamamuro M, Tadamura E, Kubo S.et al Cardiac functional analysis with multi‐detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology 2005234381–390. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaou K, Sanz J, Poon M.et al Assessment of myocardial perfusion and viability from routine contrast‐enhanced 16‐detector‐row computed tomography of the heart: preliminary results. Eur Radiol 200515864–871. [DOI] [PubMed] [Google Scholar]
- 20.Mahnken A H, Koos R, Katoh M.et al Assessment of myocardial viability in reperfused acute myocardial infarction using 16‐slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol 2005452042–2047. [DOI] [PubMed] [Google Scholar]